无机材料学报 ›› 2021, Vol. 36 ›› Issue (11): 1208-1216.DOI: 10.15541/jim20210124
所属专题: 【生物材料】肿瘤治疗
收稿日期:2021-03-01
修回日期:2021-03-29
出版日期:2021-11-20
网络出版日期:2021-05-10
通讯作者:
张 伟, 教授, E-mail: wei.zhang@dlut.edu.cn
作者简介:魏成雄(1992-), 男, 博士研究生. E-mail: weicx@mail.dlut.edu.cn
基金资助:
WEI Chengxiong( ), JIN Xin, YIN Peinan, WU Chengwei, ZHANG Wei(
), JIN Xin, YIN Peinan, WU Chengwei, ZHANG Wei( )
)
Received:2021-03-01
Revised:2021-03-29
Published:2021-11-20
Online:2021-05-10
Contact:
ZHANG Wei, professor. E-mail: wei.zhang@dlut.edu.cn
About author:WEI Chengxiong(1992-), male, PhD candidate. E-mail: weicx@mail.dlut.edu.cn
Supported by:摘要:
光热治疗是一种非侵入式的新型肿瘤治疗手段, 可弥补传统治疗方式的不足。碳纳米材料作为一种高效的光热剂, 在肿瘤光热治疗中表现出巨大的应用潜力。本研究采用超声辅助法使邻苯三酚与甲醛5 min快速聚合, 经煅烧处理制备了单分散、粒径均一的碳球。该碳球兼具优良的细胞生物相容性和高光热转换效率。在808 nm近红外光照射下, 碳球呈现良好的光热效应和光热稳定性, 光热转换效率达到41.4%。细胞实验表明, 碳球无明显细胞毒性, 对肿瘤细胞具有显著的光热杀伤效果。制备的高光热效应碳球光热剂有望用于肿瘤光热治疗。
中图分类号:
魏成雄, 金鑫, 殷培楠, 吴承伟, 张伟. 高光热效应碳球的快速制备及肿瘤细胞光热治疗[J]. 无机材料学报, 2021, 36(11): 1208-1216.
WEI Chengxiong, JIN Xin, YIN Peinan, WU Chengwei, ZHANG Wei. Carbon Spheres for Photothermal Therapy of Tumor Cells: Rapid Preparation and High Photothermal Effect[J]. Journal of Inorganic Materials, 2021, 36(11): 1208-1216.
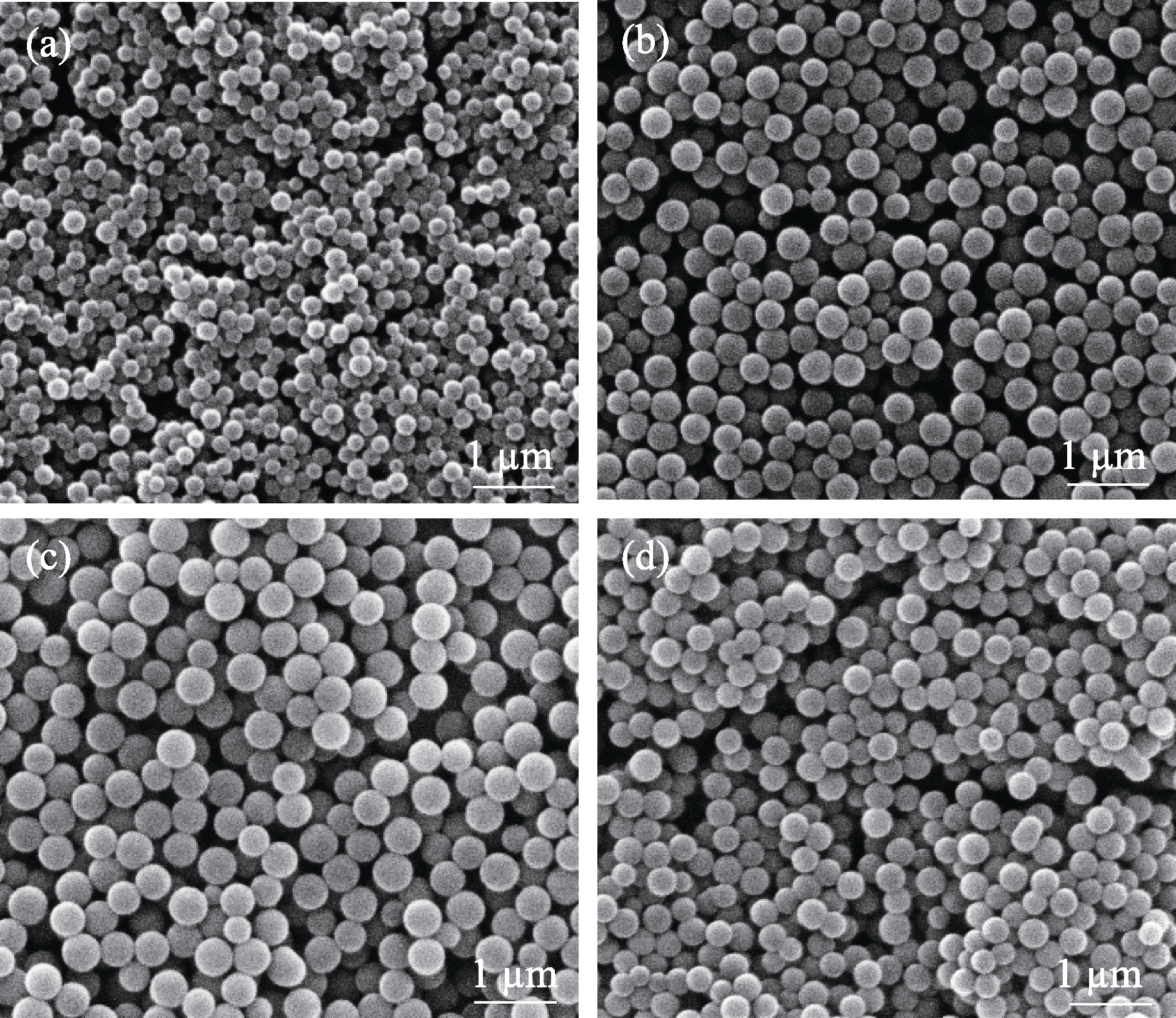
图2 邻苯三酚与甲醛摩尔比为(a)2:1、(b)1:1、(c)1:2和(d)1:4时对酚醛树脂微球形貌的影响
Fig. 2 Effects of molar ratios of pyrogallic acid to formaldehyde at (a) 2:1, (b) 1:1, (c) 1:2, and (d) 1:4 on morphologies of phenolic resin spheres
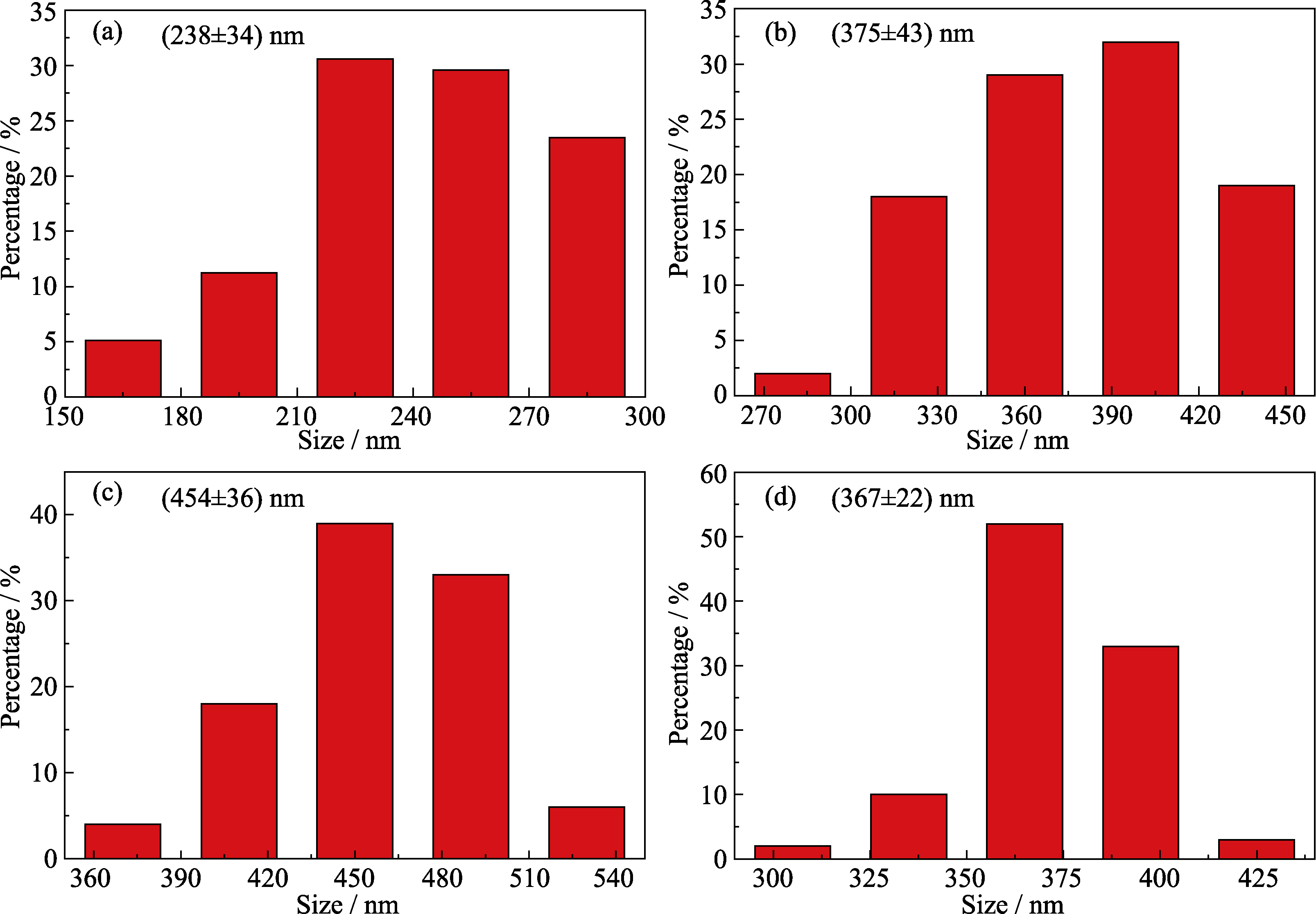
图3 邻苯三酚与甲醛摩尔比为(a)2 : 1、(b)1 : 1、(c)1 : 2和(d)1 : 4时对酚醛树脂微球直径的影响
Fig. 3 Effects of molar ratios of pyrogallol to formaldehyde at (a) 2 : 1, (b) 1 : 1, (c) 1 : 2, and (d) 1 : 4 on particle sizes of phenolic resin spheres
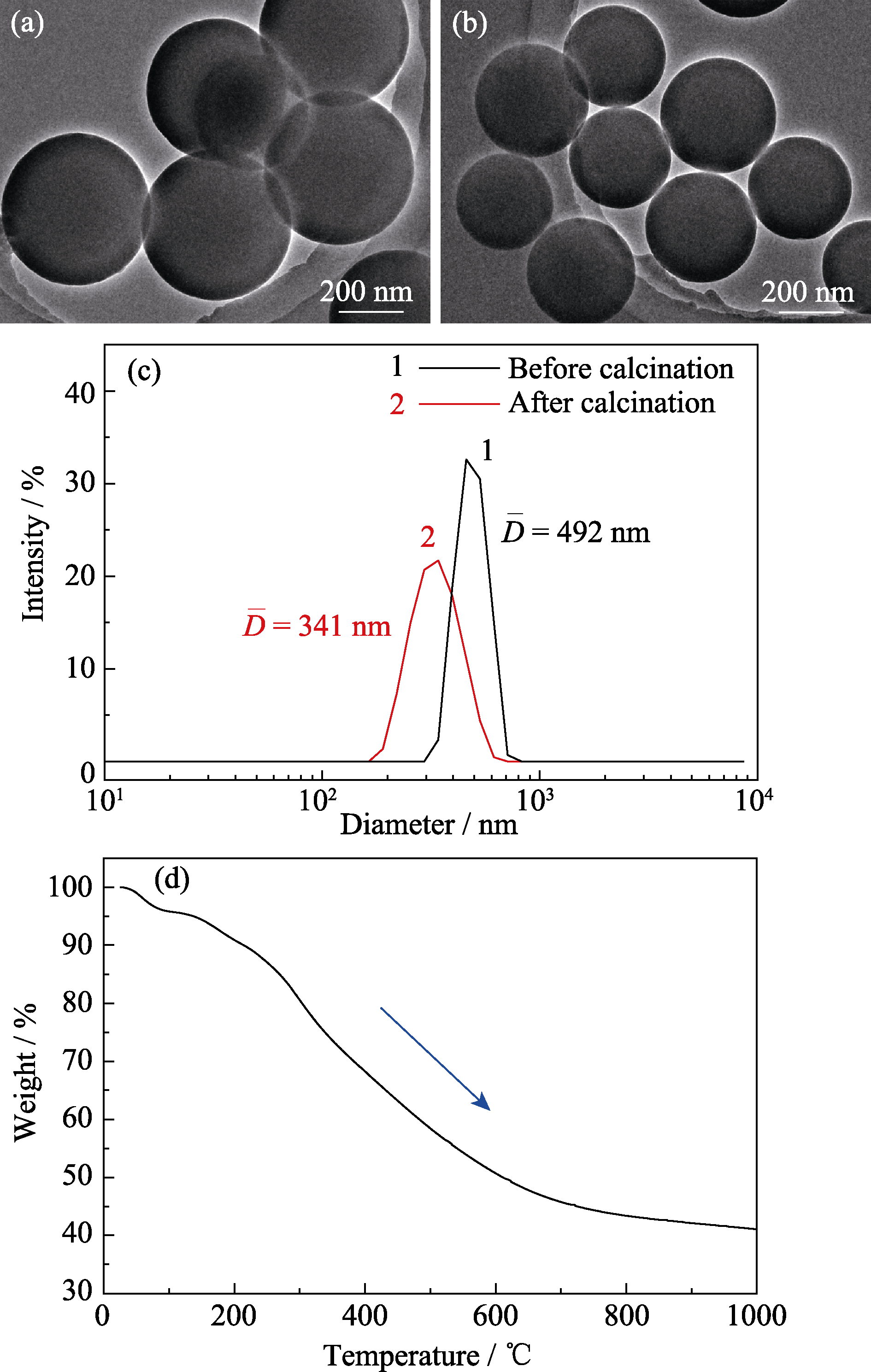
图4 酚醛树脂微球(a)煅烧前与(b)煅烧后的TEM照片及其(c)动态光散射粒度分析曲线和(d)煅烧后的碳球在N2气氛下的热重分析曲线
Fig. 4 TEM morphologies of phenolic resin spheres (a) before and (b) after calcination, their (c) particle size analysis curves of dynamic light scattering and (d) thermogravimetric analysis curve of carbon spheres after calcination in N2 atmosphere
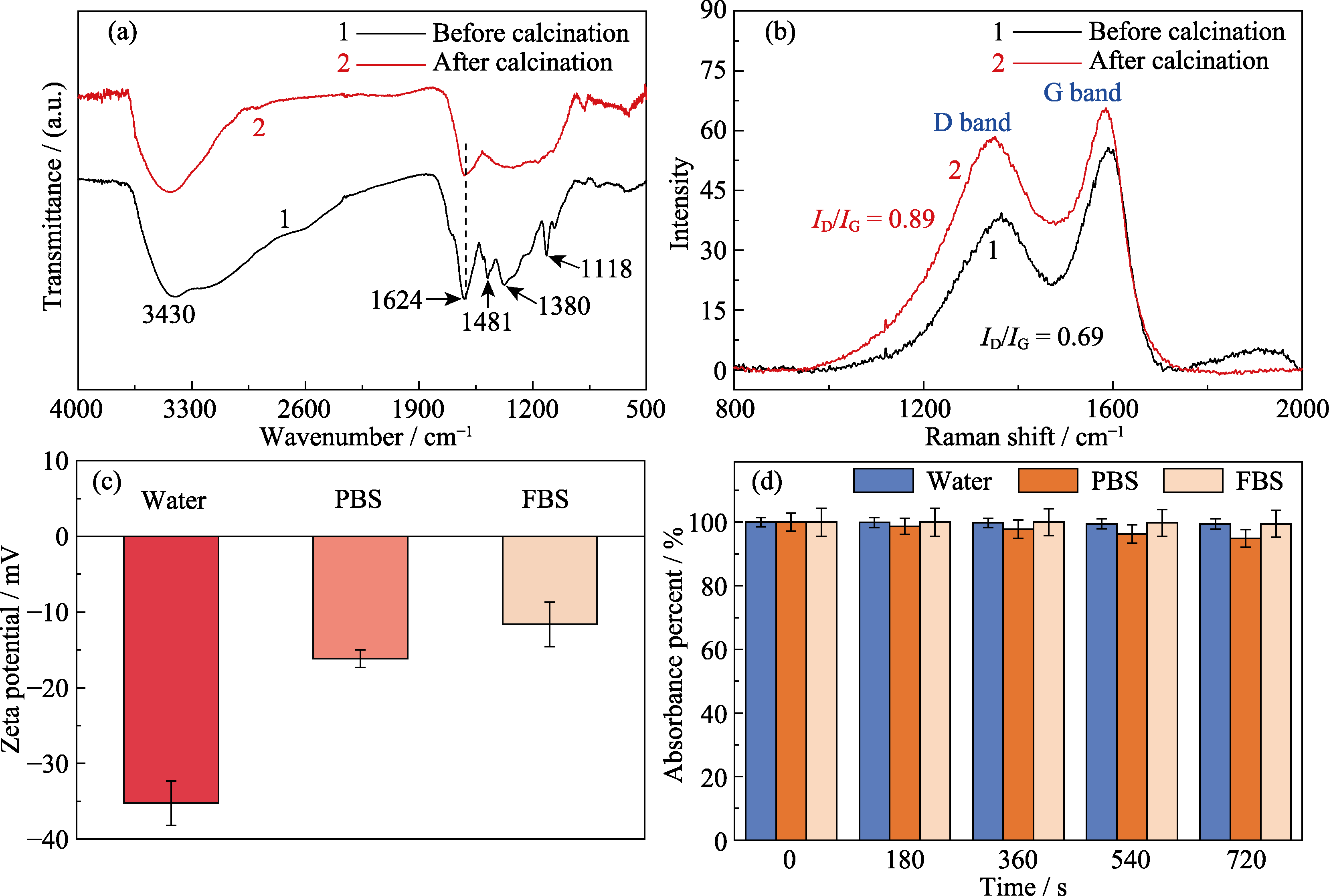
图5 微球煅烧前后的(a)傅里叶红外光谱图和(b)拉曼光谱图及其(c)碳球分散到不同介质中的Zeta电位和(d)在808 nm处吸光度随时间变化的百分比
Fig. 5 (a) FT-IR and (b) Raman spectra of phenolic resin spheres before and after calcination, (c) Zeta potential of carbon spheres in different dispersion medium and (d) absorbance percent of carbon spheres in different dispersion medium at 808 nm
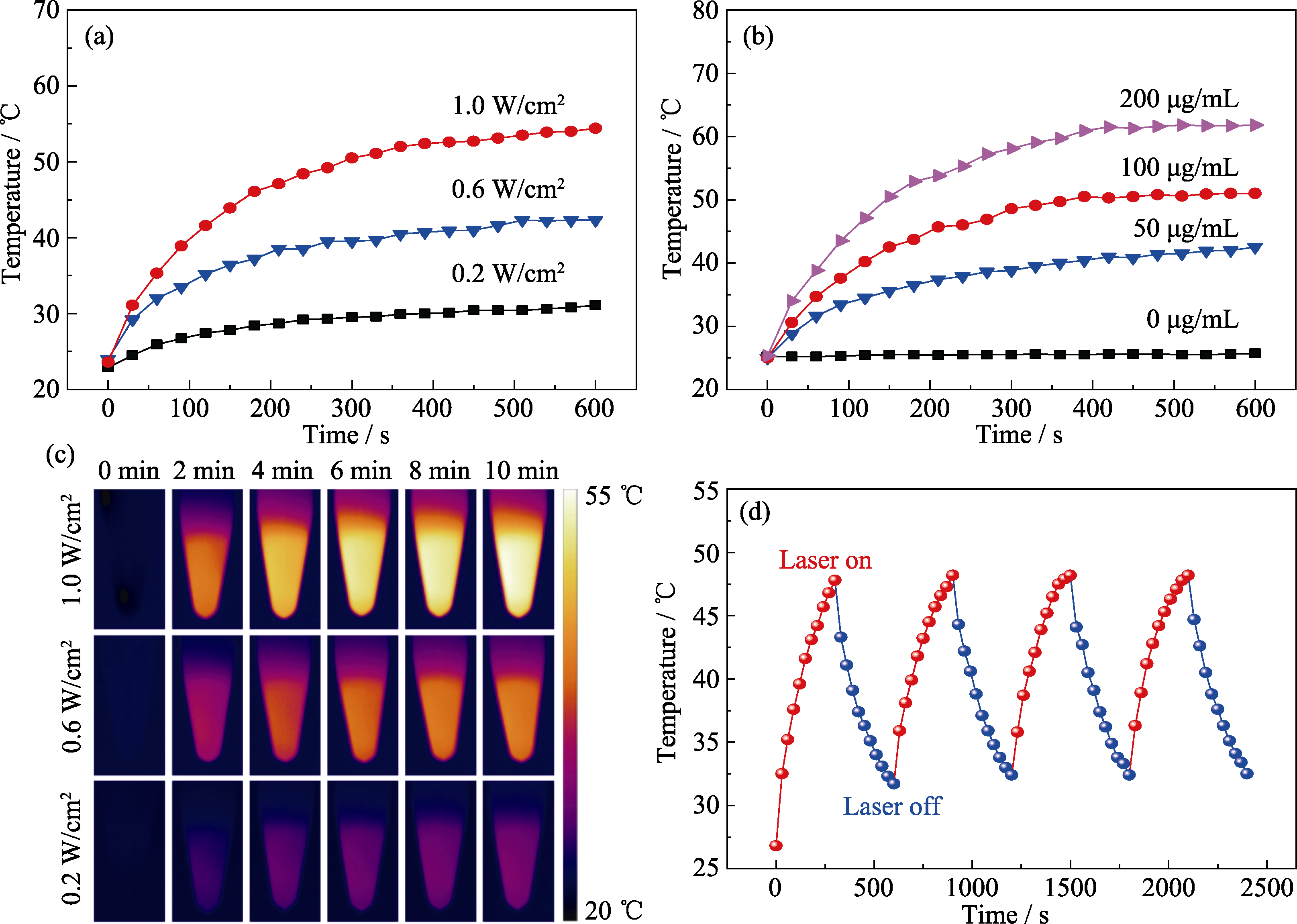
图6 碳球的光热性能测试
Fig. 6 Evaluation of photothermal effect of carbon spheres Influence of (a) laser power density (concentration: 100 μg/mL) and (b) concentration of carbon spheres (laser power density: 0.8 W/cm2) on photothermal effect; (c) Cloud images of temperature changes; (d) Measurement of the photothermal stability (concentration: 100 μg/mL, laser power density: 0.8 W/cm2)
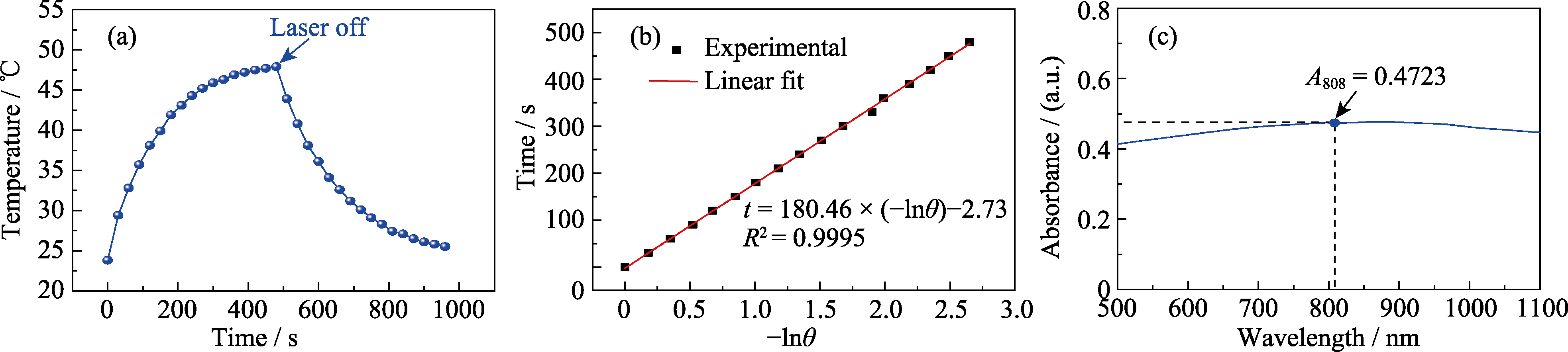
图7 碳球的光热转换效率测试
Fig. 7 Measurement of the photothermal conversion efficiency of carbon spheres (a) Heating and cooling curves of carbon sphere suspension (concentration: 100 μg/mL, laser power density: 0.8 W/cm2); (b) Linear fitting curve of t and -lnθ at cooling phase; (c) UV-visible absorption curve of carbon sphere suspension
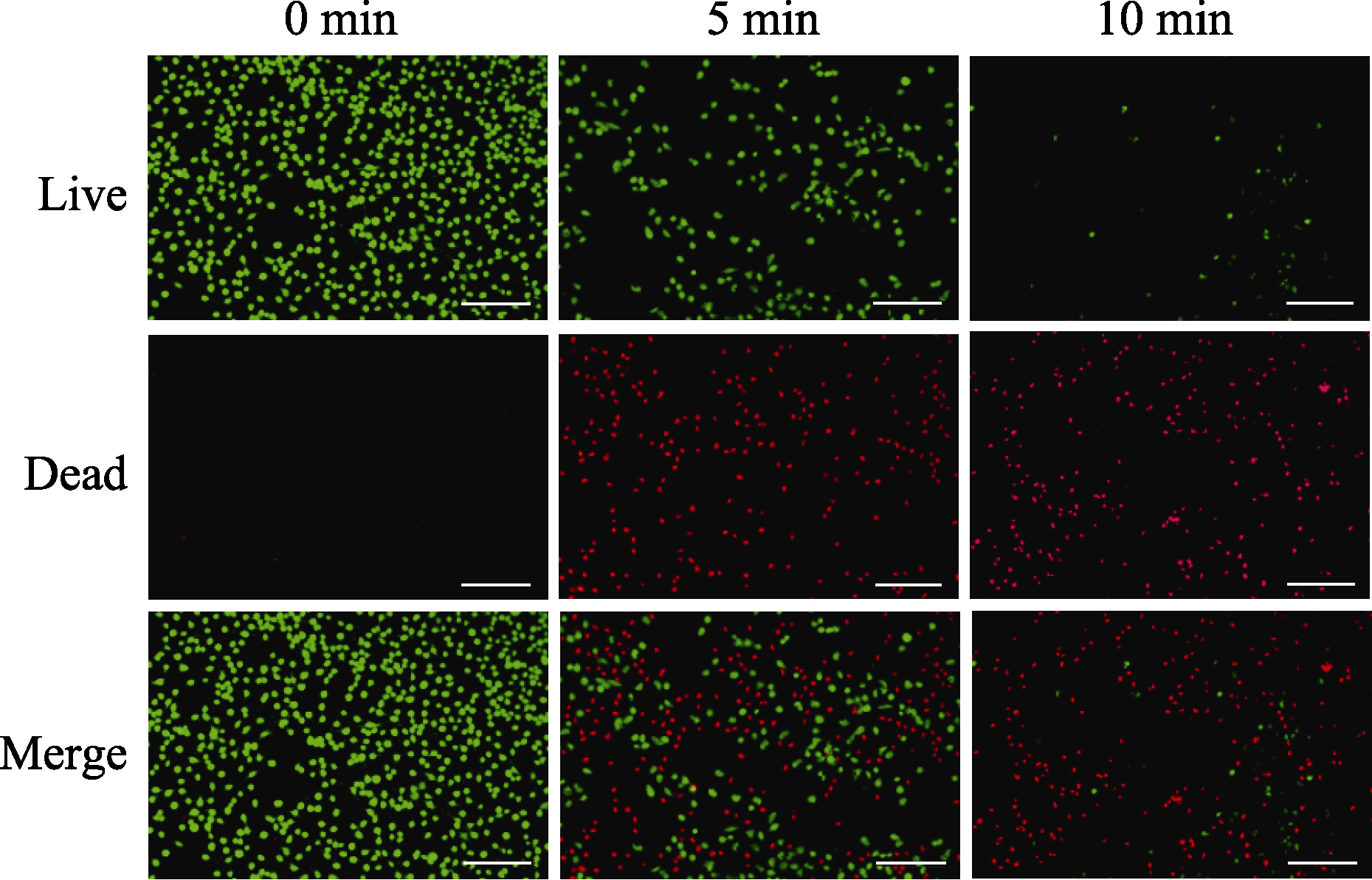
图10 含碳球的肿瘤细胞悬液经不同时长近红外光照射后的活、死细胞荧光照片
Fig. 10 Live/dead fluorescence images of tumor cells co-culcured with carbon spheres by different laser irradiation time (Green: live cells; Red: dead cells; Scale bar=200 μm)
| [1] |
SCHROEDER A, HELLER D A, WINSLOW M M, et al. Treating metastatic cancer with nanotechnology. Nature Reviews: Cancer, 2012, 12(1):39-50.
DOI URL |
| [2] |
LUETKE A, MEYERS P A, LEWIS I, et al. Osteosarcoma treatment-where do we stand? A state of the art review. Cancer Treatment Reviews, 2014, 40(4):523-532.
DOI URL |
| [3] |
ZOU L L, WANG H, HE B, et al. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics, 2016, 6(6):762-772.
DOI URL |
| [4] |
ZHI D F, YANG T, O'HAGAN J, et al. Photothermal therapy. Journal of Controlled Release, 2020, 325:52-71.
DOI URL |
| [5] |
LIU S, PAN X T, LIU H Y. Two-dimensional nanomaterials for photothermal therapy. Angewandte Chemie International Edition, 2020, 59(15):5890-5900.
DOI URL |
| [6] |
KIM H, CHUNG K, LEE S, et al. Near-infrared light-responsive nanomaterials for cancer theranostics. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology, 2016, 8(1):23-45.
DOI URL |
| [7] |
JAQUE D, MARTINEZ MAESTRO L, DEL ROSAL B, et al. Nanoparticles for photothermal therapies. Nanoscale, 2014, 6(16):9494-9530.
DOI URL |
| [8] | PAN H Y, ZHANG C, WANG T T, et al. In situ fabrication of intelligent photothermal indocyanine green-alginate hydrogel for localized tumor ablation. ACS Applied Materials & Interfaces, 2019, 11(3):2782-2789. |
| [9] |
LIU B, SUN J, ZHU J J, et al. Injectable and NIR-responsive DNA-inorganic hybrid hydrogels with outstanding photothermal therapy. Advanced Materials, 2020, 32(39):2004460.
DOI URL |
| [10] |
FERNANDES N, RODRIGUES C F, MOREIRA A F, et al. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomaterials Science, 2020, 8(11):2990-3020.
DOI URL |
| [11] |
SONG X J, CHEN Q, LIU Z. Recent advances in the development of organic photothermal nano-agents. Nano Research, 2014, 8(2):340-354.
DOI URL |
| [12] |
JIN R M, YANG J, ZHAO D H, et al. Hollow gold nanoshells- incorporated injectable genetically engineered hydrogel for sustained chemo-photothermal therapy of tumor. Journal of Nanobiotechnology, 2019, 17(1):99.
DOI URL |
| [13] |
ZHU X M, WAN H Y, JIA H L, et al. Porous Pt nanoparticles with high near-infrared photothermal conversion efficiencies for photothermal therapy. Advanced Healthcare Materials, 2016, 5(24):3165-3172.
DOI URL |
| [14] |
WENG Y Z W, GUAN S Y, WANG L, et al. Hollow carbon nanospheres derived from biomass by-product okara for imaging-guided photothermal therapy of cancers. Journal of Materials Chemistry B, 2019, 7(11):1920-1925.
DOI URL |
| [15] |
LIU W, ZHANG X Y, ZHOU L, et al. Reduced graphene oxide (rGO) hybridized hydrogel as a near-infrared (NIR)/pH dual- responsive platform for combined chemo-photothermal therapy. Journal of Colloid and Interface Science, 2019, 536:160-170.
DOI URL |
| [16] |
WANG J X, YAO C J, SHEN B, et al. Upconversion-magnetic carbon sphere for near infrared light-triggered bioimaging and photothermal therapy. Theranostics, 2019, 9(2):608-619.
DOI URL |
| [17] |
XIE H H, LI Z B, SUN Z B, et al. Metabolizable ultrathin Bi2Se3 nanosheets in imaging-guided photothermal therapy. Small, 2016, 12(30):4136-4145.
DOI URL |
| [18] |
JIANG G B, HUA Q, WEN S W, et al. Carbon dot/WS2 heterojunctions for NIR-II enhanced photothermal therapy of osteosarcoma and bone regeneration. Chemical Engineering Journal, 2020, 383:123102.
DOI URL |
| [19] |
DONG L L, JI G M, LIU Y, et al. Multifunctional Cu-Ag2S nanoparticles with high photothermal conversion efficiency for photoacoustic imaging-guided photothermal therapy in vivo. Nanoscale, 2018, 10(2):825-831.
DOI URL |
| [20] |
NEELGUND G M, OKI A R. Influence of carbon nanotubes and graphene nanosheets on photothermal effect of hydroxyapatite. Journal of Colloid and Interface Science, 2016, 484:135-145.
DOI URL |
| [21] |
ZHANG M, WANG W T, WU F, et al. Magnetic and fluorescent carbon nanotubes for dual modal imaging and photothermal and chemo-therapy of cancer cells in living mice. Carbon, 2017, 123:70-83.
DOI URL |
| [22] |
LI P, YAN Y, CHEN B L, et al. Lanthanide-doped upconversion nanoparticles complexed with nano-oxide graphene used for upconversion fluorescence imaging and photothermal therapy. Biomaterials Science, 2018, 6(4):877-884.
DOI URL |
| [23] |
LIU H J, LI C W, QIAN Y, et al. Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window. Biomaterials, 2020, 232:119700.
DOI URL |
| [24] |
ZHOU L, DONG K, CHEN Z W, et al. Near-infrared absorbing mesoporous carbon nanoparticle as an intelligent drug carrier for dual-triggered synergistic cancer therapy. Carbon, 2015, 82:479-488.
DOI URL |
| [25] |
GE J C, JIA Q Y, LIU W M, et al. Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Advanced Materials, 2015, 27(28):4169-4177.
DOI URL |
| [26] |
LIU Y L, AI K L, LIU J H, et al. Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Advanced Materials, 2013, 25(9):1353-1359.
DOI URL |
| [27] |
WANG S J, WANG Y, BIAN C, et al. The thermal stability and pyrolysis mechanism of boron-containing phenolic resins: the effect of phenyl borates on the char formation. Applied Surface Science, 2015, 331:519-529.
DOI URL |
| [28] | POL V G, SHRESTHA L K, ARIGA K. Tunable, functional carbon spheres derived from rapid synthesis of resorcinol-formaldehyde resins. ACS Applied Materials & Interfaces, 2014, 6(13):10649-10655. |
| [29] |
ZHAO X, ZHANG M, SUN X D, et al. Comprehensive understanding of the formation process on monodisperse resorcinol-formaldehyde polymer and carbon spheres and their use as substrates for surface-enhanced Raman spectroscopy. Applied Surface Science, 2020, 506:144591.
DOI URL |
| [30] |
CAN M, BULUT E, ÖZACAR M. Synthesis and characterization of pyrogallol-formaldehyde nano resin and its usage as an adsorbent. Journal of Chemical and Engineering Data, 2012, 57(10):2710-2717.
DOI URL |
| [31] |
LIU M M, CAI C, LI J, et al. Stober synthesis of tannic acid-formaldehyde resin polymer spheres and their derived carbon nanospheres and nanocomposites for oxygen reduction reaction. Journal of Colloid and Interface Science, 2018, 528:1-9.
DOI URL |
| [32] |
GUAN Q, ZHOU L L, ZHOU L N, et al. A carbon nanomaterial derived from a nanoscale covalent organic framework for photothermal therapy in the NIR-II biowindow. Chemical Communications, 2020, 56:7793-7796.
DOI URL |
| [33] |
LIANG Z G, XIA H, ZHANG L M, et al. One-pot synthesis of monodisperse phenolic resin spheres with high thermal stability and its derived carbon spheres as supercapacitor electrodes. Results in Physics, 2020, 16:102912.
DOI URL |
| [34] |
NEELGUND G M, OKI A. Advancement in photothermal effect of carbon nanotubes by grafting of poly(amidoamine) and deposition of CdS nanocrystallites. Industrial & Engineering Chemistry Research, 2018, 57(23):7826-7833.
DOI URL |
| [35] |
ZHOU L B, JING Y, LIU Y B, et al. Mesoporous carbon nanospheres as a multifunctional carrier for cancer theranostics. Theranostics, 2018, 8(3):663-675.
DOI URL |
| [36] | WENG Y Z W, GUAN S Y, WANG L, et al. Defective porous carbon polyhedra decorated with copper nanoparticles for enhanced NIR-driven photothermal cancer therapy. Small, 2020, 16(1):e1905184. |
| [37] |
ZHU J, MU S. Defect engineering in carbon-based electrocatalysts: insight into intrinsic carbon defects. Advanced Functional Materials, 2020, 30:2001097.
DOI URL |
| [38] |
WANG W, SHANG L, CHANG G J, et al. Intrinsic carbon- defect-driven electrocatalytic reduction of carbon dioxide. Advanced Materials, 2019, 31:1808276.
DOI URL |
| [39] |
ZHAO P, ZHU L L. Dispersibility of carbon dots in aqueous and/or organic solvents. Chemical Communications, 2018, 54:5401-5406.
DOI URL |
| [1] | 白志强, 赵璐, 白云峰, 冯锋. MXenes的制备、性质及其在肿瘤诊疗中的研究进展[J]. 无机材料学报, 2022, 37(4): 361-375. |
| [2] | 张文君, 赵雪莹, 吕江维, 曲有鹏. 中空有序介孔有机硅的研究进展: 制备及在肿瘤治疗中的应用[J]. 无机材料学报, 2022, 37(11): 1192-1202. |
| [3] | 郑燕宁, 季军荣, 梁雪玲, 赖正杰, 陈启帆, 廖丹葵. 氮掺杂中空碳球氧化物模拟酶性能研究[J]. 无机材料学报, 2021, 36(5): 527-534. |
| [4] | 王玉伟, 陈佳杰, 田正芳, 朱敏, 朱钰方. 卟啉基金属有机框架负载高铁酸钾: 光-化学动力学联合治疗肿瘤性能研究[J]. 无机材料学报, 2021, 36(12): 1305-1315. |
| [5] | 程晓昆, 张越, 吕海军, 刘歆颖, 侯森林, 陈爱兵. 多孔碳纳米材料构建抗肿瘤药物靶向传递系统的研究进展[J]. 无机材料学报, 2021, 36(1): 9-24. |
| [6] | 谢雪, 吴建荣, 蔡晓军, 郝俊年, 郑元义. 光热/pH响应B-CuS-DOX纳米药物用于化疗-光热协同治疗肿瘤[J]. 无机材料学报, 2021, 36(1): 81-87. |
| [7] | 杜娟, 刘磊, 于奕峰, 张越, 吕海军, 陈爱兵. 封装热解同步沉积法制备结构可调的中空碳球及其在头孢氨苄吸附中的应用[J]. 无机材料学报, 2020, 35(5): 608-616. |
| [8] | 古毓康, 曹 磊, 万 勇, 高建国. 铝合金基底微碳球作为润滑油添加剂的摩擦学性能及其润滑机理[J]. 无机材料学报, 2017, 32(6): 625-630. |
| [9] | 胡枫香, 李 玲, 林 奎, 崔 兰, 石彩静, Sayyar Ali Shah, 崔 屾. 氮掺杂空心碳球的制备及其光学性质研究[J]. 无机材料学报, 2016, 31(8): 827-833. |
| [10] | 翟云刚, 董文杰, 高勇平, 牛德超, 陈健壮, 顾金楼, 李永生, 施剑林. 超顺磁金纳米壳复合颗粒的粒径调控及其诊疗应用[J]. 无机材料学报, 2015, 30(9): 950-956. |
| [11] | 杨 涛, 祝迎春, 钱霍飞, 袁建辉, 许钫钫. 微米空心碳球串珠结构的制备与形成机理[J]. 无机材料学报, 2011, 26(2): 139-144. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||