无机材料学报 ›› 2021, Vol. 36 ›› Issue (11): 1199-1207.DOI: 10.15541/jim20210056
所属专题: 【虚拟专辑】药物递送(2020~2021)
收稿日期:2021-01-28
修回日期:2021-03-24
出版日期:2021-11-20
网络出版日期:2021-04-05
通讯作者:
常 江, 研究员. E-mail: jchang@mail.sic.ac.cn
作者简介:包 峰(1991-), 男, 博士研究生. E-mail: baofeng@student.sic.ac.cn
基金资助:
BAO Feng1,2( ), CHANG Jiang1,2(
), CHANG Jiang1,2( )
)
Received:2021-01-28
Revised:2021-03-24
Published:2021-11-20
Online:2021-04-05
Contact:
CHANG Jiang, professor. E-mail: jchang@mail.sic.ac.cn
About author:BAO Feng(1991-), male, PhD candidate. E-mail: baofeng@student.sic.ac.cn
Supported by:摘要:
电纺丝支架已被广泛用于组织工程领域, 其中硅酸钙等生物活性陶瓷复合的电纺丝支架, 在应用中展现出了优异的生物活性。硅酸钙复合电纺丝支架中硅酸钙降解释放的硅酸根离子(SiO32-)已被证实具有促进成血管性能, 但其有效活性离子浓度范围比较窄, 仅在0.79~1.8 μg/mL之间。因此精确控制组织工程材料的离子释放浓度, 使材料释放的离子能较长时间保持在有效活性浓度范围, 对于组织工程应用具有重要意义。本研究通过调节电纺丝孔径大小及硅酸钙纳米线的不同复合方式, 制备了多种硅酸钙复合电纺丝纤维支架, 并比较了其在体外环境下的离子释放模式及对人脐静脉内皮细胞的增殖促进作用。实验结果表明, 混纺及同时电喷-电纺复合方式的小孔径硅酸钙复合电纺支架由于高分子的疏水作用和小孔径结构对离子扩散的阻碍, 可以实现离子缓释。通过体外细胞实验发现, 具有离子缓释效果的支架可以更好地促进人脐静脉内皮细胞的增殖, 说明通过调控支架离子缓释, 可以有效调控其生物活性, 获得最佳组织工程应用效果。
中图分类号:
包峰, 常江. 硅酸钙纳米线复合电纺丝支架的制备及离子释放研究[J]. 无机材料学报, 2021, 36(11): 1199-1207.
BAO Feng, CHANG Jiang. Calcium Silicate Nanowires Based Composite Electrospun Scaffolds: Preparation, Ion Release and Cytocompatibility[J]. Journal of Inorganic Materials, 2021, 36(11): 1199-1207.
| Sample | Mass of PCL/g | Mass of PLA/g | Mass of gelatin/g | Volume of HFIP/mL |
|---|---|---|---|---|
| 5ES | 0.175 | 0.175 | 0.150 | 10 |
| 10ES | 0.350 | 0.350 | 0.300 | 10 |
| 18ES | 0.630 | 0.630 | 0.540 | 10 |
| 22ES | 0.770 | 0.770 | 0.660 | 10 |
表1 不同孔径电纺丝溶液的配制
Table 1 Preparation of electrospun solution with various pore size
| Sample | Mass of PCL/g | Mass of PLA/g | Mass of gelatin/g | Volume of HFIP/mL |
|---|---|---|---|---|
| 5ES | 0.175 | 0.175 | 0.150 | 10 |
| 10ES | 0.350 | 0.350 | 0.300 | 10 |
| 18ES | 0.630 | 0.630 | 0.540 | 10 |
| 22ES | 0.770 | 0.770 | 0.660 | 10 |
| Sample | Voltage/kV | Flow rate / (mL·min-1) | Needle-to-collector distance/cm |
|---|---|---|---|
| 5ES | 10 | 0.02 | 8 |
| 10ES | 12 | 0.05 | 10 |
| 18ES | 15 | 0.08 | 15 |
| 22ES | 18 | 0.20 | 20 |
表2 不同孔径电纺丝的制备参数
Table 2 Preparation parameters of electrospun membranes with various pore size
| Sample | Voltage/kV | Flow rate / (mL·min-1) | Needle-to-collector distance/cm |
|---|---|---|---|
| 5ES | 10 | 0.02 | 8 |
| 10ES | 12 | 0.05 | 10 |
| 18ES | 15 | 0.08 | 15 |
| 22ES | 18 | 0.20 | 20 |

图1 多种形式硅酸钙纳米线复合电纺丝纤维支架制备示意图
Fig. 1 Schematic illustration of different preparation methods for calcium silicate composite electrospun scaffolds with different forms (a) CSH@ES: mixed electrospun of CSH-contained blended electrospun solution; (b) CSH&ES: simultaneously electrospraying CSH particles with elctrospun fibers; (c) CSH/ES: electrospraying CSH particles on the surface of as-spun electrospun membranes; (d) ES/CSH/ES: electrospraying CSH particles on as-spun electrospun membranes, then electrospun fibers on the surface to construct an ES/CSH/ES sandwich structure
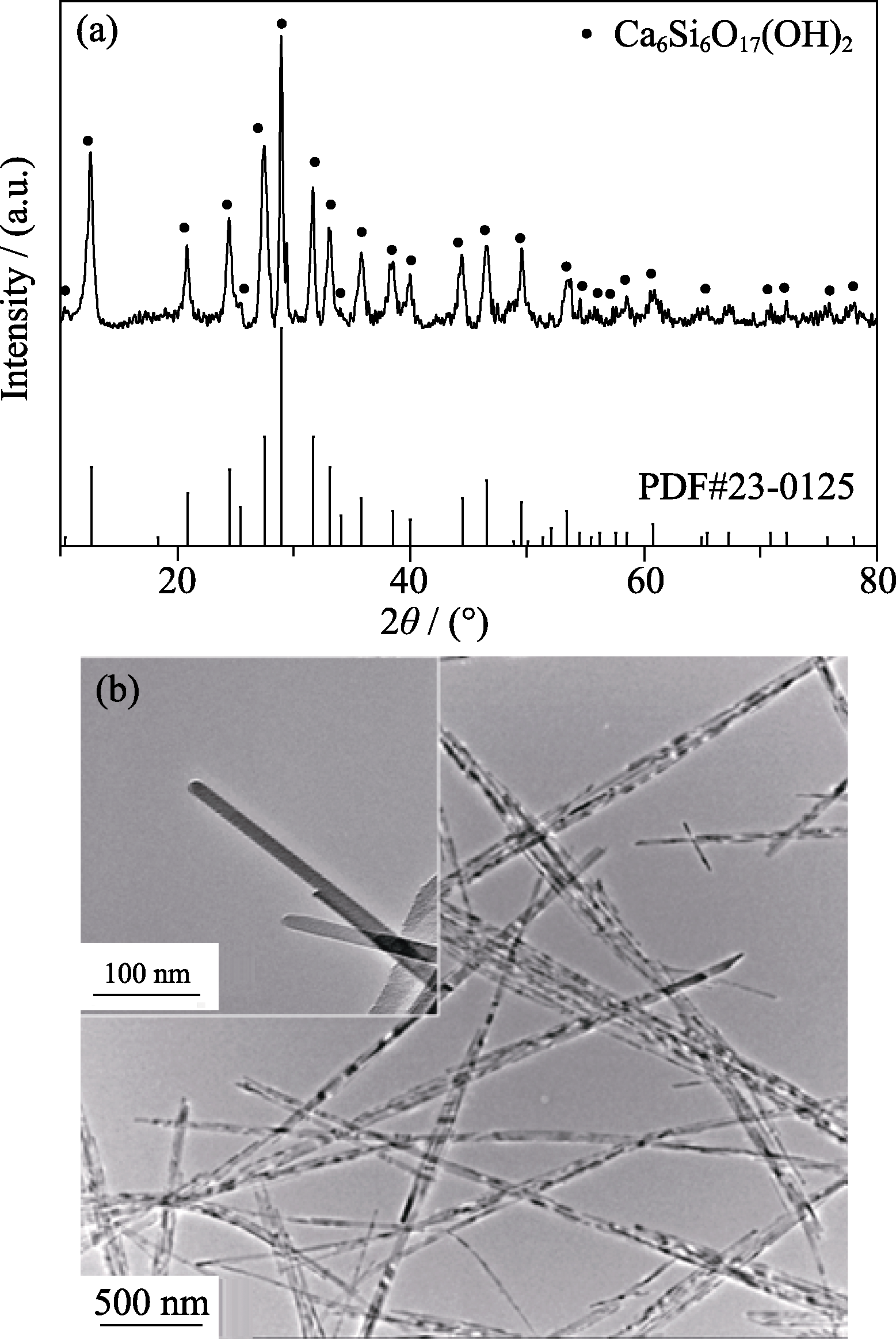
图2 硅酸钙纳米线的(a)XRD图谱和(b)TEM照片
Fig. 2 (a) XRD patterns and (b) TEM images of calcium silicate nanowires with inset in (b) showing magnified TEM image of CSH

图3 不同孔径未复合CSH纳米线的电纺丝纤维支架的SEM照片
Fig. 3 SEM images of electrospun scaffolds contained no CSH nanowires with different pore sizes (a) 5ES; (b) 10ES; (c) 15ES; (d) 22ES
| Sample | Fiber diameter/μm | Pore diameter/μm |
|---|---|---|
| 5ES | (0.24±0.05) | (0.68±0.13) |
| 10ES | (0.82±0.16) | (3.45±0.73) |
| 18ES | (4.112±0.81) | (17.73±3.53) |
| 22ES | (10.27±2.04) | (50.46±10.87) |
表3 电纺支架的纤维直径及孔径统计结果
Table 3 Statistical results of fiber diameter and pore diameter of electrospun scaffolds
| Sample | Fiber diameter/μm | Pore diameter/μm |
|---|---|---|
| 5ES | (0.24±0.05) | (0.68±0.13) |
| 10ES | (0.82±0.16) | (3.45±0.73) |
| 18ES | (4.112±0.81) | (17.73±3.53) |
| 22ES | (10.27±2.04) | (50.46±10.87) |
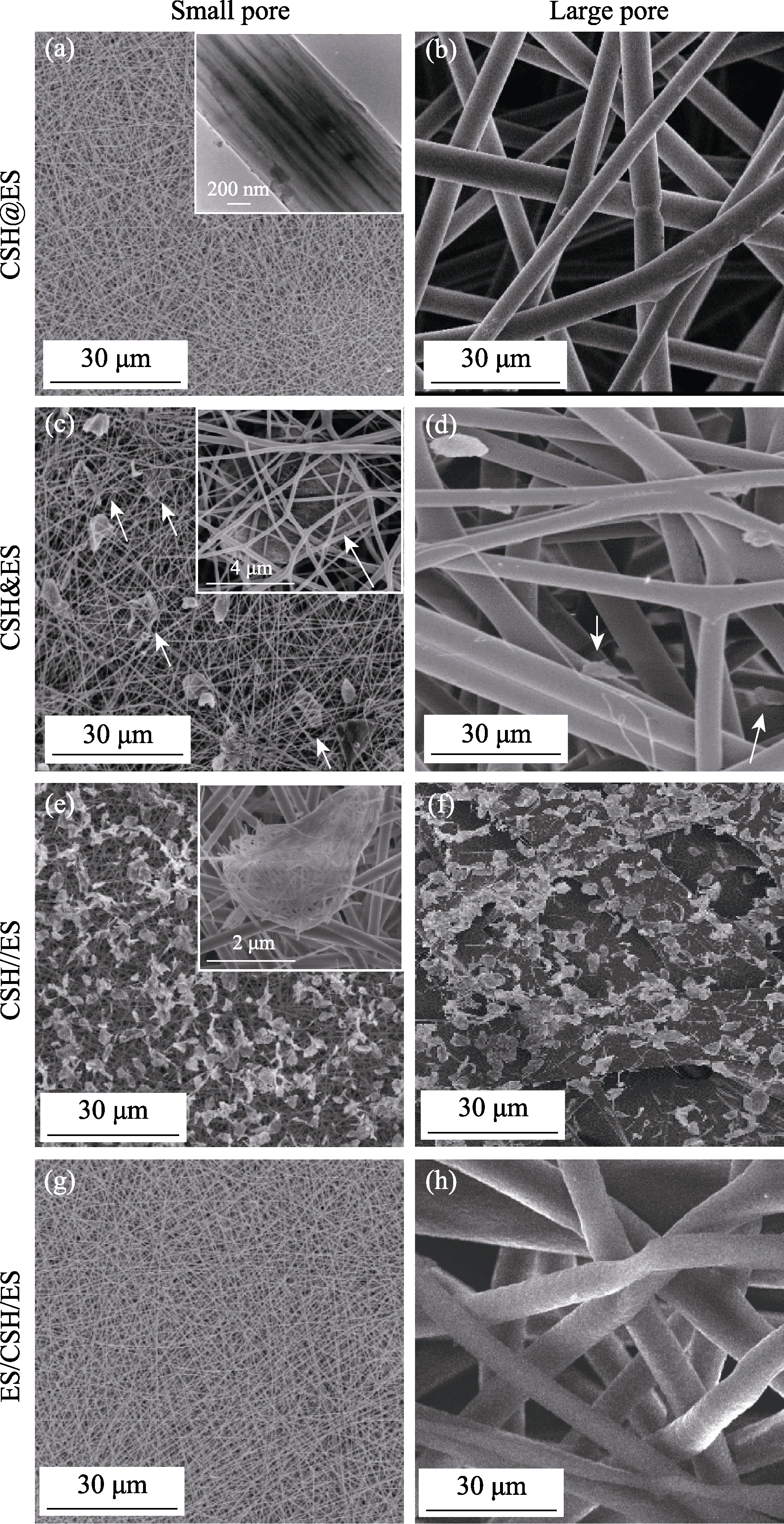
图4 多种复合形式的硅酸钙纳米线不同孔径电纺丝支架的SEM照片及CSH颗粒在支架内的分布
Fig. 4 SEM images of various forms of calcium silicate composite electrospun scaffolds with different pore sizes and CSH particle distributions in the scaffolds (a, b) CSH@ES; (c, d) CSH&ES; (e, f) CSH//ES; (g, h) ES/CS/ES; (a, c, e, g) Small pore size; (b, d, f, h) Large pore size. The inset in (a) shows CSH nanowires being uniformly distributed inside the fibers. The inset in (c) reveals agglomerated CSH particles being embedded in the scaffolds with white arrows indicating the CSH particles embedded in scaffolds. The inset in (e) shows the CSH nanowires formed agglomerates on the surface of the scaffolds
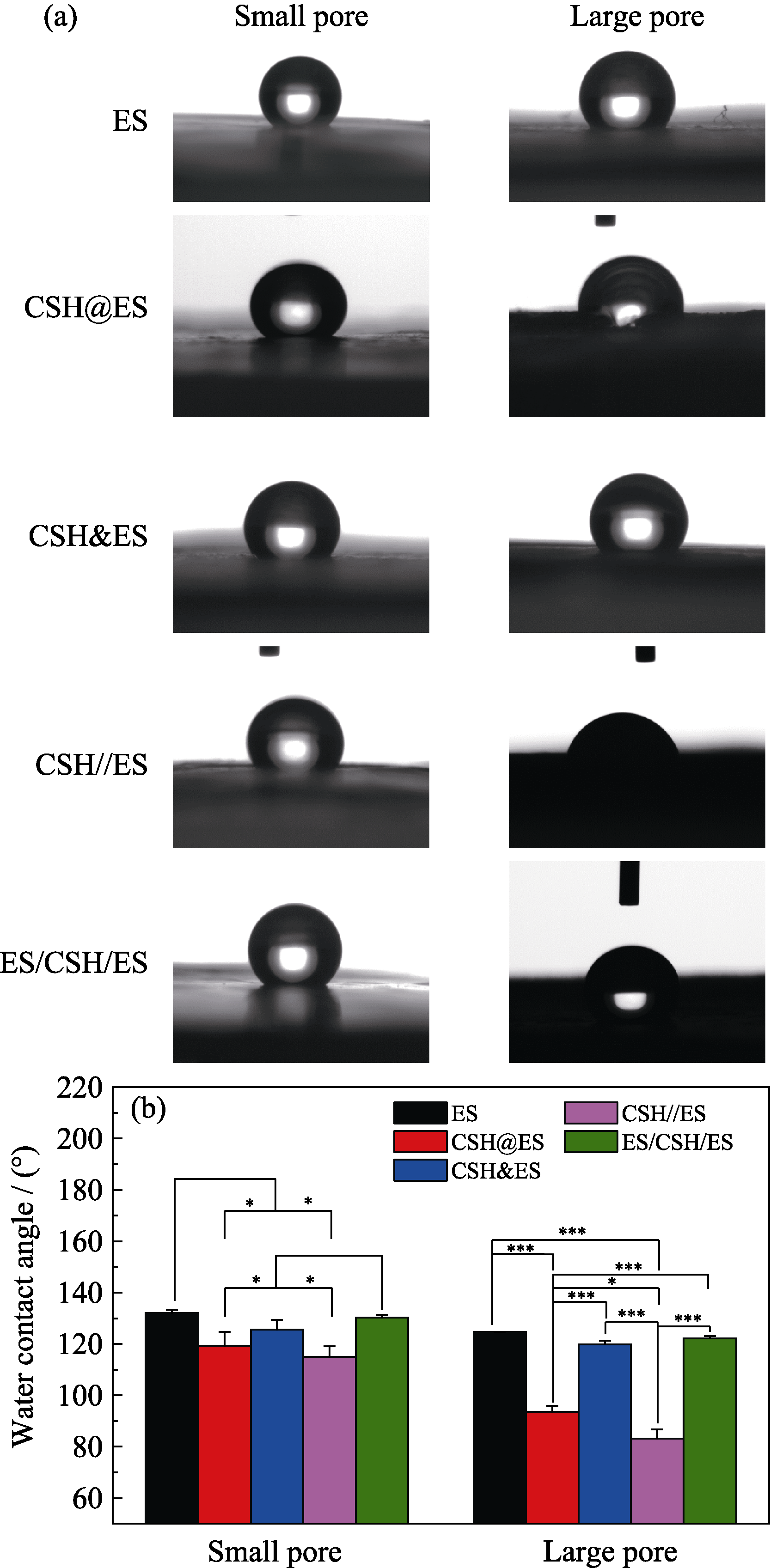
图5 不同孔径的复合硅酸钙纳米线电纺丝支架的接触角
Fig. 5 Water contact angle measurements of various forms of calcium silicate composite electrospun scaffolds with different pore sizes (a) Photographs of water contact angle on electrospun scaffolds; (b) Statistical results of water contact angle. *p<0.05, ** p<0.01, ***p<0.001
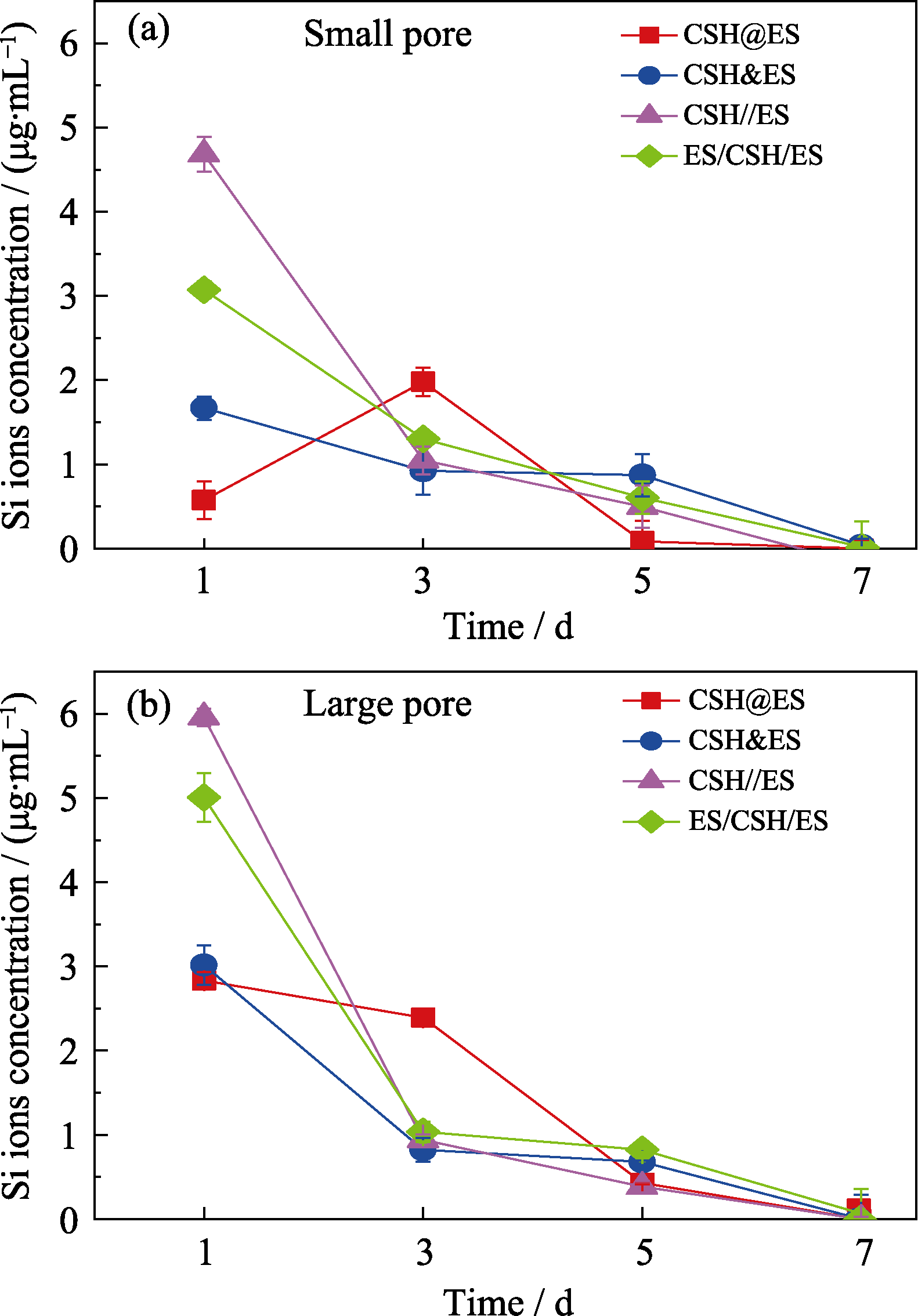
图6 复合方式对硅酸钙纳米线电纺丝支架释放SiO32-行为的影响
Fig. 6 Effects of the different composite forms on release behavior of SiO32- in calcium silicate nanowires in composite electrospun scaffolds (a) Scaffolds with small pore size; (b) Scaffolds with large pore size
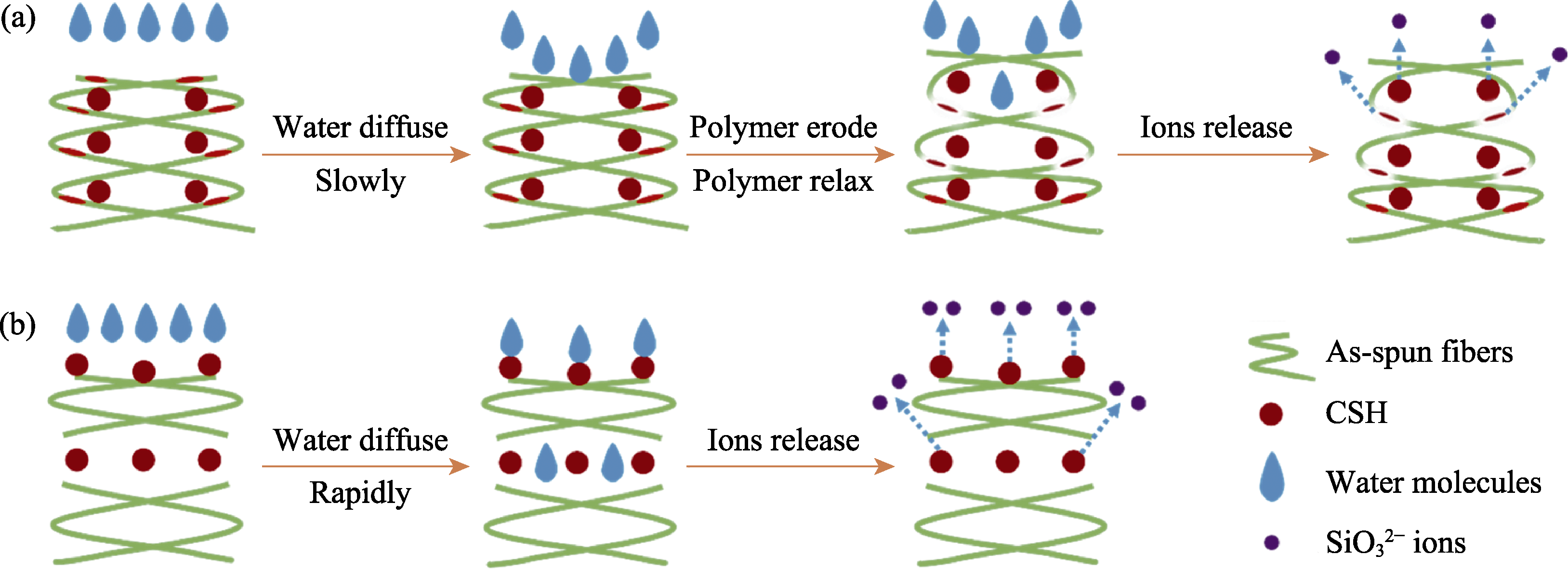
图7 硅酸钙复合电纺丝纤维支架离子释放过程示意图
Fig. 7 Schematic illustrations of ions release behaviors from calcium silicate composite in electrospun scaffolds (a) Ions controlled release of CSH@ES and CHS&ES groups; (b) Ions burst release process of CSH//ES and ES/CSH/ES groups
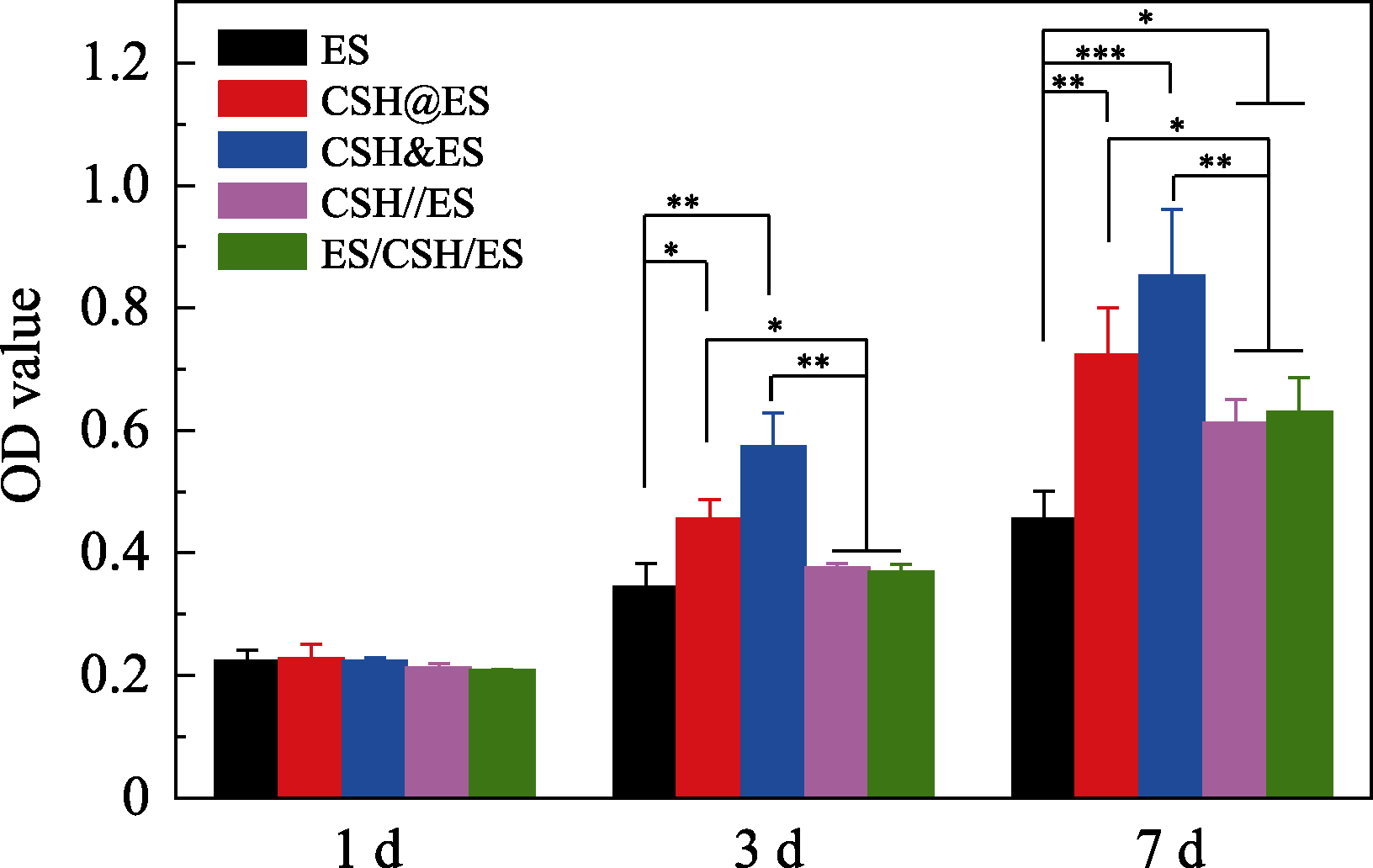
图8 人脐静脉内皮细胞在小孔径不同复合方式电纺丝纤维支架上培养1、3、7 d时的增殖数据
Fig. 8 Proliferation of HUVECs after being cultured on various composite electrospun scaffolds with small pore *p<0.05, ** p< 0.01, ***p<0.001
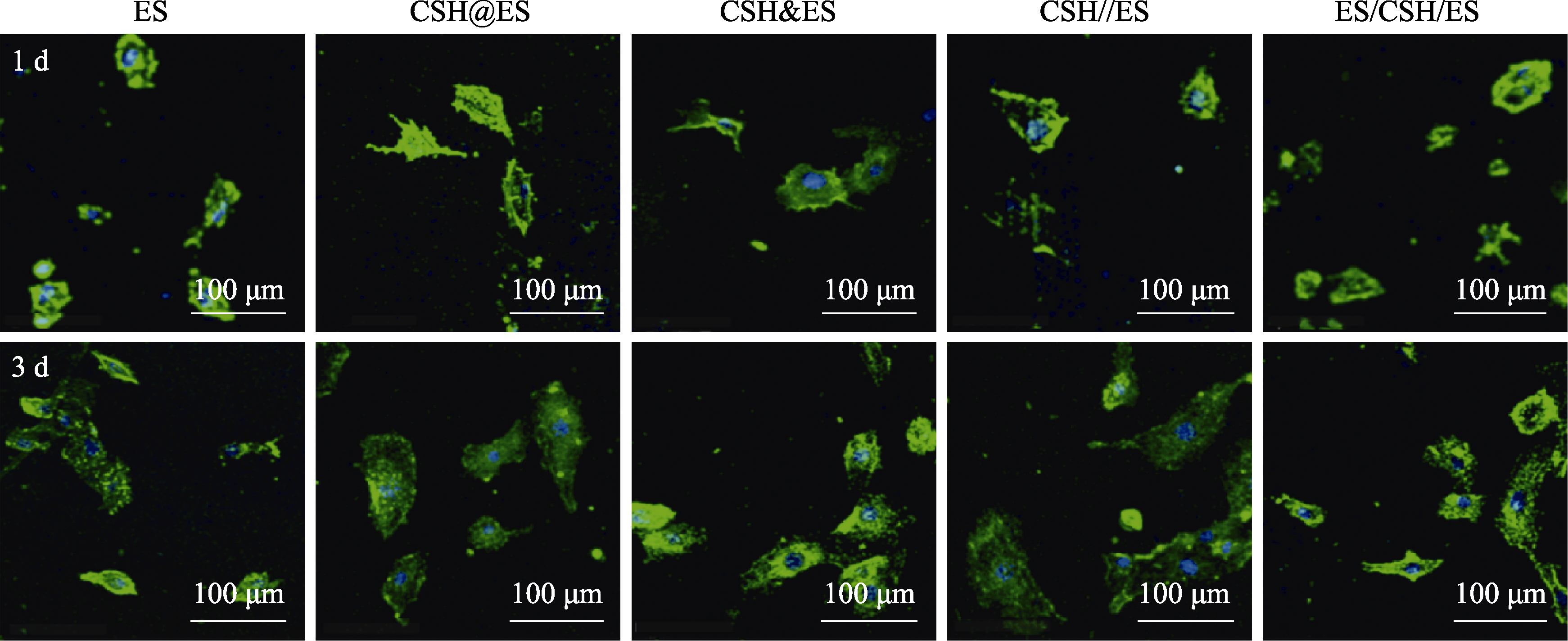
图9 人脐静脉内皮细胞在小孔径不同方式复合电纺丝纤维支架上培养1和3 d时的粘附形态
Fig. 9 HUVEC morphologies after being cultured on various composite electrospun scaffolds with small pore for 1 and 3 d
| [1] | XIE X, CHEN Y, WANG X, et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. Journal of Materials Science & Technology, 2020, 59:243-261. |
| [2] | ZHANG Y, DOU L, MA N, et al. Biomedical applications of electrospun nanofibers. Surface Review and Letters, 2020, 27(11):1-26. |
| [3] |
NIEMCZYK-SOCZYNSKA B, GRADYS A, SAJKIEWICZ P. Hydrophilic surface functionalization of electrospun nanofibrous scaffolds in tissue engineering. Polymers, 2020, 12(11):2636.
DOI URL |
| [4] |
XU F, FAN Y. Electrostatic self-assemble modified electrospun poly-L-lactic acid/poly-vinylpyrrolidone composite polymer and its potential applications in small-diameter artificial blood vessels. Journal of Biomedical Nanotechnology, 2020, 16(1):101-110.
DOI URL |
| [5] |
AWAD N K, NIU H, ALI U, et al. Electrospun fibrous scaffolds for small-diameter blood vessels: a review. Membranes, 2018, 8(1):15.
DOI URL |
| [6] |
HAN J, WU Q, XIA Y, et al. Cell alignment induced by anisotropic electrospun fibrous scaffolds alone has limited effect on cardiomyocyte maturation. Stem Cell Research, 2016, 16(3):740-750.
DOI URL |
| [7] |
SUH T C, AMANAH A Y, GLUCK J M. Electrospun scaffolds and induced pluripotent stem cell-derived cardiomyocytes for cardiac tissue engineering applications. Bioengineering, 2020, 7(3):105.
DOI URL |
| [8] |
AMINI S, SAUDI A, AMIRPOUR N, et al. Application of electrospun polycaprolactone fibers embedding lignin nanoparticle for peripheral nerve regeneration: in vitro and in vivo study. International Journal of Biological Macromolecules, 2020, 159:154-173.
DOI URL |
| [9] |
ZHA F, CHEN W, HAO L, et al. Electrospun cellulose-based conductive polymer nanofibrous mats: composite scaffolds and their influence on cell behavior with electrical stimulation for nerve tissue engineering. Soft Matter, 2020, 16(28):6591-6598.
DOI URL |
| [10] | BAO F, PEI G, WU Z, et al. Bioactive self-pumping composite wound dressings with micropore array modified Janus membrane for enhanced diabetic wound healing. Advanced Functional Materials, 2020, 30(49):1-11. |
| [11] |
REN X Z, HAN Y M, WANG J, et al. An aligned porous electrospun fibrous membrane with controlled drug delivery - an efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomaterialia, 2018, 70:140-153.
DOI URL |
| [12] |
WANG X C, LV F, Li T, et al. Electrospun micropatterned nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing. ACS Nano, 2017, 11(11):11337-11349.
DOI URL |
| [13] |
XIA L, YIN Z, MAO L, et al. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Scientific Reports, 2016, 6:22005.
DOI URL |
| [14] |
ZHANG Y, HUANG C, CHANG J. Ca-doped mesoporous SiO2/ dental resin composites with enhanced mechanical properties, bioactivity and antibacterial properties. Journal of Materials Chemistry B, 2018, 6(3):477-486.
DOI URL |
| [15] |
LI J, ZHAI D, LV F, et al. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomaterialia, 2016, 36:254-66.
DOI URL |
| [16] |
WANG X, GAO L, HAN Y, et al. Silicon-enhanced adipogenesis and angiogenesis for vascularized adipose tissue engineering. Advanced Science, 2018, 5(11):1800776.
DOI URL |
| [17] |
YU Q, HAN Y, WANG X, et al. Copper silicate hollow microspheres-incorporated scaffolds for chemo-photothermal therapy of melanoma and tissue healing. ACS Nano, 2018, 12(3):2695-2707.
DOI URL |
| [18] | ZHANG Z, DAI Q, ZHANG Y, et al. Design of a multifunctional biomaterial inspired by ancient Chinese medicine for hair regeneration in burned skin. ACS Applied Materials & Interfaces, 2020, 12(11):12489-12499. |
| [19] | WANG X, WANG L, WU Q, et al. Chitosan/calcium silicate cardiac patch stimulates cardiomyocyte activity and myocardial performance after infarction by synergistic effect of bioactive ions and aligned nanostructure. ACS Applied Materials & Interfaces, 2019, 11(1):1449-1468. |
| [20] |
LI J Y, LV F, XU H, et al. A patterned nanocomposite membrane for high-efficiency healing of diabetic wound. Journal of Materials Chemistry B, 2017, 5(10):1926-1934.
DOI URL |
| [21] |
YU Q, CHANG J, WU C. Silicate bioceramics: from soft tissue regeneration to tumor therapy. Journal of Materials Chemistry B, 2019, 7(36):5449-5460.
DOI URL |
| [22] |
LI H, CHANG J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic. Acta Biomaterialia, 2013, 9(2):5379-5389.
DOI URL |
| [23] |
LIU Z, JU X J, HUANG Y H, et al. Diffusional permeability characteristics of positively K+-responsive membranes caused by spontaneously changing membrane pore size and surface wettability. Journal of Membrane Science, 2016, 497:328-338.
DOI URL |
| [24] | SUN H, CHEN J, HAN X, et al. Multi-responsive hydrogels with UCST- and LCST-induced shrinking and controlled release behaviors of rhodamine B. Materials Science & Engineering C-Materials for Biological Applications, 2018, 82:284-290. |
| [25] | ZHU W, WAN L, ZHANG C, et al. Exploitation of 3D face- centered cubic mesoporous silica as a carrier for a poorly water soluble drug: influence of pore size on release rate. Materials Science & Engineering C-Materials for Biological Applications, 2014, 34:78-85. |
| [26] |
DANTAS M J L, SANTOS B F F D, TAVARES A A, et al. The impact of the ionic cross-linking mode on the physical and in vitro dexamethasone release properties of chitosan/hydroxyapatite beads. Molecules, 2019, 24(24):4510.
DOI URL |
| [27] |
LIN K L, CHANG J, CHEN G F, et al. A simple method to synthesize single-crystalline β-wollastonite nanowires. Journal of Crystal Growth, 2006, 300(2):267-271.
DOI URL |
| [28] |
NISHA U, MERLINE C, LAKSHMINARAYANAN R, et al. Tunable release of combined contraceptive steroids from core-shell gelatin/PCL fibers. Fibers and Polymers, 2020, 21(9):1906-1916.
DOI URL |
| [29] |
YOHE S T, HERRERA V L M, COLSON Y L, et al. 3D super hydrophobic electrospun meshes as reinforcement materials for sustained local drug delivery against colorectal cancer cells. Journal of Control Release, 2012, 162(1):92-101.
DOI URL |
| [30] |
EMAMI B, TAFRESHI H V, GAD-EL-HAK M, et al. Effect of fiber orientation on shape and stability of air-water interface on submerged super hydrophobic electrospun thin coatings. Journal of Applied Physics, 2012, 111(6):064325.
DOI URL |
| [31] |
MUNJ H R, LANNUTTI J J, TOMASKO D L. Understanding drug release from PCL/gelatin electrospun blends. Journal of Biomaterials Applications, 2017, 31(6):933-949.
DOI URL |
| [32] | IMMICH A P S, ARIAS M L, CARRERAS N, et al. Drug delivery systems using sandwich configurations of electrospun poly(lactic acid) nanofiber membranes and ibuprofen. Materials Science & Engineering C-Materials for Biological Applications, 2013, 33(7):4002-4008. |
| [33] | BHARADWAZ A, JAYASURIYA A. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Materials Science & Engineering C-Materials for Biological Applications, 2020, 110:110698. |
| [34] |
RNJAK K J, WISE G S, LI Z, et al. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials, 2011, 32(28):6729-6736.
DOI URL |
| [35] |
DOU Y D, WU C T, CHANG J. Preparation, mechanical property and cytocompatibility of poly(l-lactic acid)/calcium silicate nanocomposites with controllable distribution of calcium silicate nanowires. Acta Biomaterialia, 2012, 8(11):4139-4150.
DOI URL |
| [1] | 王磊, 李建军, 宁军, 胡天玉, 王洪阳, 张占群, 武琳馨. CoFe2O4@Zeolite催化剂活化过一硫酸盐对甲基橙的强化降解: 性能与机理[J]. 无机材料学报, 2023, 38(4): 469-476. |
| [2] | 盛丽丽, 常江. 光/磁热Fe2SiO4/Fe3O4双相生物陶瓷及其复合电纺丝膜制备及抗菌性能研究[J]. 无机材料学报, 2022, 37(9): 983-990. |
| [3] | 魏子钦, 夏翔, 李勤, 李国荣, 常江. 钛酸钡/硅酸钙复合生物活性压电陶瓷的制备及性能研究[J]. 无机材料学报, 2022, 37(6): 617-622. |
| [4] | 陈士昆, 王楚楚, 陈晔, 李莉, 潘路, 文桂林. 磁性Ag2S/Ag/CoFe1.95Sm0.05O4 Z型异质结的制备及光催化降解性能[J]. 无机材料学报, 2022, 37(12): 1329-1336. |
| [5] | 吴爱军, 朱敏, 朱钰方. 含铜硅酸钙纳米棒复合水凝胶用于肿瘤治疗和皮肤伤口愈合性能研究[J]. 无机材料学报, 2022, 37(11): 1203-1216. |
| [6] | 刘城, 赵倩, 牟志伟, 雷洁红, 段涛. 新型铋基SiOCNF复合膜对放射性气态碘的吸附性能[J]. 无机材料学报, 2022, 37(10): 1043-1050. |
| [7] | 张晓山, 王兵, 吴楠, 韩成, 刘海燕, 王应德. 高红外遮蔽SiZrOC纳米纤维膜的制备及其性能研究[J]. 无机材料学报, 2022, 37(1): 93-100. |
| [8] | 马玲玲, 常江. Nd掺杂硅酸钙及其复合电纺丝膜的制备及性能研究[J]. 无机材料学报, 2021, 36(9): 974-980. |
| [9] | 王袁杰, 裴学良, 李好义, 徐鑫, 何流, 黄政仁, 黄庆. 自由基引发活性聚碳硅烷交联及其在制备SiC纤维中的应用[J]. 无机材料学报, 2021, 36(9): 967-973. |
| [10] | 李铁, 李玥, 王颖异, 张珽. 石墨烯-铁酸铋纳米晶复合材料的制备及其催化性能研究[J]. 无机材料学报, 2021, 36(7): 725-732. |
| [11] | 李婷婷, 张志明, 韩正波. 基于静电纺丝技术的聚合物基MOFs纳米纤维膜的研究进展[J]. 无机材料学报, 2021, 36(6): 592-600. |
| [12] | 蔡苗, 陈子航, 曾实, 杜江慧, 熊娟. CuS纳米片修饰Bi5O7I复合材料用于光催化还原Cr(VI)水溶液[J]. 无机材料学报, 2021, 36(6): 665-672. |
| [13] | 安伟佳, 李静, 王淑瑶, 胡金山, 蔺在元, 崔文权, 刘利, 解珺, 梁英华. Fe(III)/rGO/Bi2MoO6复合光催化剂制备及光催化芬顿协同降解苯酚[J]. 无机材料学报, 2021, 36(6): 615-622. |
| [14] | 熊金艳, 罗强, 赵凯, 张梦梦, 韩朝, 程刚. 界面电荷快速转移提升铜修饰氧化钨光催化性能[J]. 无机材料学报, 2021, 36(3): 325-331. |
| [15] | 兰青, 孙盛睿, 吴萍, 杨庆峰, 刘阳桥. 钴掺杂氧化铜/可见光协同活化PMS降解罗丹明B及其机理研究[J]. 无机材料学报, 2021, 36(11): 1171-1177. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||