无机材料学报 ›› 2022, Vol. 37 ›› Issue (1): 22-28.DOI: 10.15541/jim20210458
所属专题: 【能源环境】CO2绿色转换
• 专栏: CO 2 绿色转化(特邀编辑: 欧阳述昕, 王文中) • 上一篇 下一篇
王潇( ), 朱智杰, 吴之怡, 张城城, 陈志杰, 肖梦琦, 李超然(
), 朱智杰, 吴之怡, 张城城, 陈志杰, 肖梦琦, 李超然( ), 何乐(
), 何乐( )
)
收稿日期:2021-07-19
修回日期:2021-08-23
出版日期:2022-01-20
网络出版日期:2021-08-20
通讯作者:
何 乐, 教授. E-mail: lehe@suda.edu.cn; 李超然, 副研究员. E-mail: crli@suda.edu.cn
作者简介:王 潇(1997-), 女, 硕士研究生. E-mail: 20194214065@suda.stu.cn
基金资助:
WANG Xiao( ), ZHU Zhijie, WU Zhiyi, ZHANG Chengcheng, CHEN Zhijie, XIAO Mengqi, LI Chaoran(
), ZHU Zhijie, WU Zhiyi, ZHANG Chengcheng, CHEN Zhijie, XIAO Mengqi, LI Chaoran( ), HE Le(
), HE Le( )
)
Received:2021-07-19
Revised:2021-08-23
Published:2022-01-20
Online:2021-08-20
Contact:
HE Le, professor. E-mail: lehe@suda.edu.cn; LI Chaoran, associate professor. E-mail: crli@suda.edu.cn
About author:WANG Xiao(1997-), female, Master candidate. E-mail: 20194214065@suda.stu.cn
Supported by:摘要:
高吸光催化剂对提高光热转换效率具有重要意义, 阵列结构光热催化剂的陷光效应有助于增强光吸收并提高光热转换效率。但是, 现有阵列基光热催化剂仍存在单位面积上活性金属负载量过低的不足, 难以满足实际应用的需求。本研究发展了二氧化硅保护的MOFs热解策略, 获得了单位辐照面积上活性金属质量可调、太阳光吸收效率超过90%的粉体钴等离激元超结构光热催化剂, 通过时域有限差分法模拟计算证实其高吸光能力源于纳米颗粒的等离子杂化效应。相比阵列基等离子体超结构催化剂, 该粉体结构的催化活性和稳定性显著增强, 在相同催化条件下, 二氧化碳转化率从0.9%提高到26.2%。本研究为非贵金属光热催化剂的实际应用奠定了基础。
中图分类号:
王潇, 朱智杰, 吴之怡, 张城城, 陈志杰, 肖梦琦, 李超然, 何乐. 钴等离激元超结构粉体催化剂的制备及其光热催化应用[J]. 无机材料学报, 2022, 37(1): 22-28.
WANG Xiao, ZHU Zhijie, WU Zhiyi, ZHANG Chengcheng, CHEN Zhijie, XIAO Mengqi, LI Chaoran, HE Le. Preparation and Photothermal Catalytic Application of Powder-form Cobalt Plasmonic Superstructures[J]. Journal of Inorganic Materials, 2022, 37(1): 22-28.

图1 Co-SiO2催化剂制备与表征
Fig. 1 Preparation and characterization of Co-SiO2 (a) Schematic illustration of preparation of Co and Co-SiO2 catalysts, TEM images of (b) ZIF-67-SiO2, (c) Co and (d) Co-SiO2, (e) HRTEM, (f) SAED and (g-j) elemental mappings of Co-SiO2
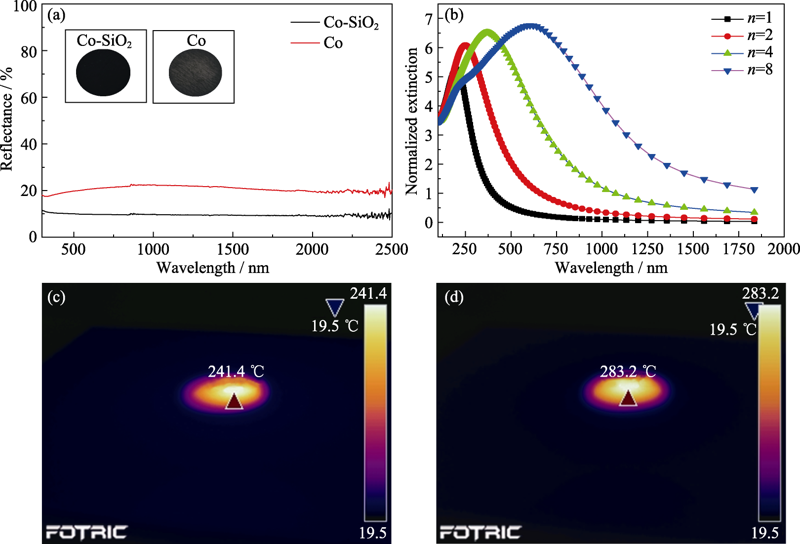
图3 (a)Co-SiO2和Co的漫反射光谱, 插图为Co-SiO2和Co催化剂的实物图; (b)不同Co颗粒堆积的归一化消光系数模拟图; 红外相机观测的 (c)Co催化剂与(d)Co-SiO2催化剂的表面平均温度
Fig. 3 (a) Diffuse reflectance spectra of Co-SiO2 and Co (insets showing photographs of two catalysts), (b) dependence of calculated normalized extinction cross-section of Co nanostructures on the number of Co nanoparticles (n) in the chain, and surface temperature of (c) Co catalyst and (d) Co-SiO2 catalyst
| Sample | Metal mass per unit area /(mg·cm-2) | Size of Co/nm | CO2 conversion /% | CO selectivity /% |
|---|---|---|---|---|
| Co-SiO2-1 | 0.41 | 27 | 11.9 | 61.0 |
| Co-SiO2-2 | 0.86 | 27 | 17.6 | 34.9 |
| Co-SiO2-3 | 1.4 | 27 | 26.2 | 29.7 |
| Co-SiO2-4 | 1.7 | 27 | 24.8 | 27.2 |
| Co-PS@SiO2 | 0.32 | 25 | 0.9 | 70.4 |
表1 不同催化剂在光热二氧化碳加氢反应中的CO2转化率
Table 1 CO2 conversion efficiencies of different catalysts in photothermal hydrogenation of carbon dioxide
| Sample | Metal mass per unit area /(mg·cm-2) | Size of Co/nm | CO2 conversion /% | CO selectivity /% |
|---|---|---|---|---|
| Co-SiO2-1 | 0.41 | 27 | 11.9 | 61.0 |
| Co-SiO2-2 | 0.86 | 27 | 17.6 | 34.9 |
| Co-SiO2-3 | 1.4 | 27 | 26.2 | 29.7 |
| Co-SiO2-4 | 1.7 | 27 | 24.8 | 27.2 |
| Co-PS@SiO2 | 0.32 | 25 | 0.9 | 70.4 |
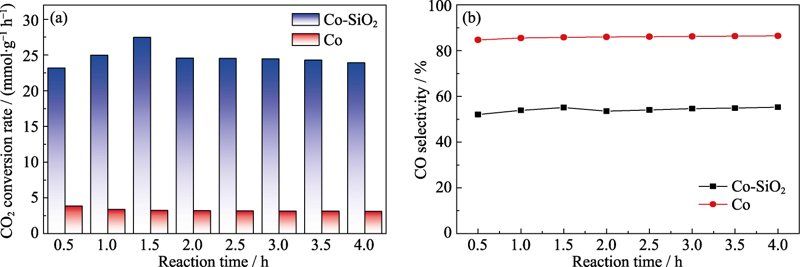
图5 Co-SiO2和Co在300 ℃连续工作4 h内的热催化二氧化碳加氢稳定性比较
Fig. 5 Stability of Co-SiO2 and Co in thermocatalytic CO2 hydrogenation at 300 ℃ (a) CO2 conversion rate; (b) CO selectivity
| [1] |
WANG W, WANG S, MA X, et al. Recent advances in catalytic hydrogenation of carbon dioxide. Chemical Society Reviews, 2011, 40(7):3703-3727.
DOI URL |
| [2] |
GAO W, LIANG S, WANG R, et al. Industrial carbon dioxide capture and utilization: state of the art and future challenges. Chemical Society Reviews, 2020, 49(23):8584-8686.
DOI URL |
| [3] |
CHEN G, WATERHOUSE GIN, SHI R, et al. From solar energy to fuels: recent advances in light-driven C1 chemistry. Angewandte Chemie International Edition, 2019, 58(49):17528-17551.
DOI URL |
| [4] |
HAN B, OU X, DENG Z, et al. Nickel metal-organic framework monolayers for photoreduction of diluted CO2: metal-node- dependent activity and selectivity. Angewandte Chemie International Edition, 2018, 57(51):16811-16815.
DOI URL |
| [5] | WANG S, TOUNTAS A A, PAN W, et al. CO2 footprint of thermal versus photothermal CO2 catalysis. Small, 2021: 2007025. |
| [6] |
RA E C, KIM K Y, KIM E H, et al. Recycling carbon dioxide through catalytic hydrogenation: recent key developments and perspectives. ACS Catalysis, 2020, 10(19):11318-11345.
DOI URL |
| [7] |
KONG T, JIANG Y, XIONG Y. Photocatalytic CO2 conversion: What can we learn from conventional COx hydrogenation? Chemical Society Reviews, 2020, 49(18):6579-6591.
DOI URL |
| [8] |
MENG X, WANG T, LIU L, et al. Photothermal conversion of CO2 into CH4 with H2 over group VIII nanocatalysts: an alternative approach for solar fuel production. Angewandte Chemie International Edition, 2014, 53(43):11478-11482.
DOI URL |
| [9] |
NING S, XU H, QI Y, et al. Microstructure induced thermodynamic and kinetic modulation to enhance CO2 photothermal reduction: a case of atomic-scale dispersed Co-N species anchored Co@C hybrid. ACS Catalysis, 2020, 10(8):4726-4736.
DOI URL |
| [10] | YU F, WANG C H, LI Y Y, et al. Enhanced solar photothermal catalysis over solution plasma activated TiO2. Advanced Science, 2020, 7(16): 2000204. |
| [11] | LI Z, LIU J, SHI R, et al. Fe-based catalysts for the direct photohydrogenation of CO2 to value-added hydrocarbons. Advanced Energy Materials, 2021, 11(12): 2002783. |
| [12] |
WANG Y S, ZHAO Y F, LIU J J, et al. Manganese oxide modified nickel catalysts for photothermal CO hydrogenation to light olefins. Advanced Energy Materials, 2020, 10(5):1902860.
DOI URL |
| [13] |
ZHOU S Q, SHANG L, ZHAO Y X, et al. Pd single-atom catalysts on nitrogen-doped graphene for the highly selective photothermal hydrogenation of acetylene to ethylene. Advanced Materials, 2019, 31(18):1900509.
DOI URL |
| [14] |
CHEN G, GAO R, ZHAO Y, et al. Alumina-supported cofe alloy catalysts derived from layered-double-hydroxide nanosheets for efficient photothermal CO2 hydrogenation to hydrocarbons. Advanced Materials, 2018, 30(3):1704663.
DOI URL |
| [15] | WU S, LI Y, ZHANG Q, et al. High light-to-fuel efficiency and CO2 reduction rates achieved on a unique nanocomposite of Co/Co doped Al2O3 nanosheets with UV-Vis-IR irradiation. Energy & Environmental Science, 2019, 12(8):2581-2590. |
| [16] | GHOUSSOUB M, XIA M, DUCHESNE P N, et al. Principles of photothermal gas-phase heterogeneous CO2 catalysis. Energy & Environmental Science, 2019, 12(4):1122-1142. |
| [17] |
WANG Z J, SONG H, LIU H, et al. Coupling of solar energy and thermal energy for carbon dioxide reduction: status and prospects. Angewandte Chemie International Edition, 2020, 59(21):8016-8035.
DOI URL |
| [18] |
MATEO D, CERRILLO J L, DURINI S, et al. Fundamentals and applications of photo-thermal catalysis. Chemical Society Reviews, 2021, 50(3):2173-2210.
DOI URL |
| [19] |
ZHANG F, LI Y H, QI M Y, et al. Photothermal catalytic CO2 reduction over nanomaterials. Chem Catalysis, 2021, 1(2):272-297.
DOI URL |
| [20] |
LIU H, SHI L, ZHANG Q, et al. Photothermal catalysts for hydrogenation reactions. Chemical Communications, 2021, 57(11):1279-1294.
DOI URL |
| [21] |
LUO S, REN X, LIN H, et al. Plasmonic photothermal catalysis for solar-to-fuel conversion: current status and prospects. Chemical Science, 2021, 12(16):5701-5719.
DOI URL |
| [22] |
JIA J, WANG H, LU Z, et al. Photothermal catalyst engineering: hydrogenation of gaseous CO2 with high activity and tailored selectivity. Advanced Science, 2017, 4(10):1700252.
DOI URL |
| [23] |
KONG N, HAN B, LI Z, et al. Ruthenium nanoparticles supported on Mg(OH)2 microflowers as catalysts for photothermal carbon dioxide hydrogenation. ACS Applied Nano Materials, 2020, 3(3):3028-3033.
DOI URL |
| [24] |
DONG C, LIAN C, HU S, et al. Size-dependent activity and selectivity of carbon dioxide photocatalytic reduction over platinum nanoparticles. Nature Communications, 2018, 9(1):1252.
DOI URL |
| [25] |
WU Z, LI C, LI Z, et al. Niobium and titanium carbides (MXenes) as superior photothermal supports for CO2 photocatalysis. ACS Nano, 2021, 15(3):5696-5705.
DOI URL |
| [26] |
SHEN J, WU Z, LI C, et al. Emerging applications of MXene materials in CO2 photocatalysis. FlatChem, 2021, 28:100252.
DOI URL |
| [27] |
DENG B, SONG H, PENG K, et al. Metal-organic framework- derived Ga-Cu/CeO2 catalyst for highly efficient photothermal catalytic CO2 reduction. Applied Catalysis B: Environmental, 2021, 298:120519.
DOI URL |
| [28] |
NGUYEN N T, YAN T, WANG L, et al. Plasmonic titanium nitride facilitates indium oxide CO2 photocatalysis. Small, 2020, 16(49):2005754.
DOI URL |
| [29] |
XU Y F, DUCHESNE P N, WANG L, et al. High-performance light-driven heterogeneous CO2 catalysis with near-unity selectivity on metal phosphides. Nature Communications, 2020, 11:5149.
DOI URL |
| [30] |
XIE B, WONG R J, TAN T H, et al. Synergistic ultraviolet and visible light photo-activation enables intensified low-temperature methanol synthesis over copper/zinc oxide/alumina. Nature Communications, 2020, 11:1615.
DOI URL |
| [31] |
O’BRIEN P G, SANDHEL A, WOOD T E, et al. Photomethanation of gaseous CO2 over Ru/Silicon nanowire catalysts with visible and near-infrared photons. Advanced Science, 2014, 1(1):1400001.
DOI URL |
| [32] |
FANG Y, LV K, LI Z, et al. Solution-liquid-solid growth and catalytic applications of silica nanorod arrays. Advanced Science, 2020, 7(13):2000310.
DOI URL |
| [33] |
HOCH L B, O'BRIEN P G, JELLE A, et al. Nanostructured indium oxide coated silicon nanowire arrays: A hybrid photothermal/ photochemical approach to solar fuels. ACS Nano, 2016, 10(9):9017-9025.
DOI URL |
| [34] | LOU D, XU A B, FANG Y, et al. Cobalt-sputtered anodic aluminum oxide membrane for efficient photothermal CO2 hydrogenation. ChemNanoMat, 2021, DOI: 10.1002/cnma.202100162. |
| [35] |
ZHANG D, LV K, LI C, et al. All-earth-abundant photothermal silicon platform for CO2 catalysis with nearly 100% sunlight harvesting ability. Solar RRL, 2020, 5(2):2000387.
DOI URL |
| [36] |
JELLE A A, GHUMAN K K, O'BRIEN P G, et al. Highly efficient ambient temperature CO2 photomethanation catalyzed by nanostructured RuO2 on silicon photonic crystal support. Advanced Energy Materials, 2018, 8(9):1702277.
DOI URL |
| [37] | O’BRIEN P G, GHUMAN K K, JELLE A A, et al. Enhanced photothermal reduction of gaseous CO2 over silicon photonic crystal supported ruthenium at ambient temperature. Energy & Environmental Science, 2018, 11(12):3443-3451. |
| [38] |
FENG K, WANG S, ZHANG D, et al. Cobalt plasmonic superstructures enable almost 100% broadband photon efficient CO2 photocatalysis. Advanced Materials, 2020, 32(24):2000014.
DOI URL |
| [39] |
WANG M, LIU J, GUO C, et al. Metal-organic frameworks (ZIF-67) as efficient cocatalysts for photocatalytic reduction of CO2: the role of the morphology effect. Journal of Materials Chemistry A, 2018, 6(11):4768-4775.
DOI URL |
| [40] |
WANG L, GUAN E, WANG Y, et al. Silica accelerates the selective hydrogenation of CO2 to methanol on cobalt catalysts. Nature Communications, 2020, 11(1):1033.
DOI URL |
| [1] | 唐小华, 李辉, 杨爱梅, 查飞, 常玥. 咪唑/镍(II)改性SAPO-34的制备及其催化CO2加氢制备乙烯[J]. 无机材料学报, 2017, 32(11): 1209-1214. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||