无机材料学报 ›› 2022, Vol. 37 ›› Issue (4): 404-412.DOI: 10.15541/jim20210261
马慧1( ), 陶疆辉1, 王艳妮1, 韩玉1, 王亚斌1,2(
), 陶疆辉1, 王艳妮1, 韩玉1, 王亚斌1,2( ), 丁秀萍3(
), 丁秀萍3( )
)
收稿日期:2021-04-20
修回日期:2021-07-11
出版日期:2022-04-20
网络出版日期:2021-07-12
通讯作者:
王亚斌, 副教授. E-mail: ybw_bingerbingo@126.com;作者简介:马慧(1996-), 女, 硕士研究生. E-mail: mh08201@163.com
基金资助:
MA Hui1( ), TAO Jianghui1, WANG Yanni1, HAN Yu1, WANG Yabin1,2(
), TAO Jianghui1, WANG Yanni1, HAN Yu1, WANG Yabin1,2( ), DING Xiuping3(
), DING Xiuping3( )
)
Received:2021-04-20
Revised:2021-07-11
Published:2022-04-20
Online:2021-07-12
Contact:
WANG Yabin, associate professor. E-mail: ybw_bingerbingo@126.com;About author:MA Hui (1996-), female, Master candidate. E-mail: mh08201@163.com
Supported by:摘要:
以能源开发(如光解水制氢)及环境保护(如有机物降解)应用为目标, 负载型贵金属催化剂在设计、制备及理论研究方面已取得了长足的发展。本工作以具有特异形貌及结构的树枝状二氧化硅纳米球载体为基础, 通过溶胶-凝胶法在其孔道引入二氧化钛纳米颗粒形成硅钛杂化结构。通过有机改性技术, 在树枝状硅钛杂化纳米球表面接枝氨基官能团。然后, 通过浸渍法和硼氢化钠还原手段, 在杂化纳米球孔道负载超细金纳米粒子。不同手段表征结果显示实验成功制备了树枝状硅钛杂化纳米球负载金纳米颗粒复合材料。在模拟太阳光下, 所得催化剂光解水产氢量及速率为69.08 μmol·g-1和13.82 μmol·g-1·h-1, 约为对比样催化剂(树枝状二氧化硅纳米球负载金纳米粒子)的7倍。在无光条件下, 其降解对硝基苯酚的表观动力学常数为6.540×10-3 s-1, 约为对比样的17倍(0.372×10-3 s-1)。由此可见, 设计合成的新型催化剂展现出优越的多功能催化活性。
中图分类号:
马慧, 陶疆辉, 王艳妮, 韩玉, 王亚斌, 丁秀萍. 硅钛杂化介孔球负载金纳米粒子及其催化性能调控[J]. 无机材料学报, 2022, 37(4): 404-412.
MA Hui, TAO Jianghui, WANG Yanni, HAN Yu, WANG Yabin, DING Xiuping. Gold Nanoparticles Supported on Silica & Titania Hybrid Mesoporous Spheres and Their Catalytic Performance Regulation[J]. Journal of Inorganic Materials, 2022, 37(4): 404-412.
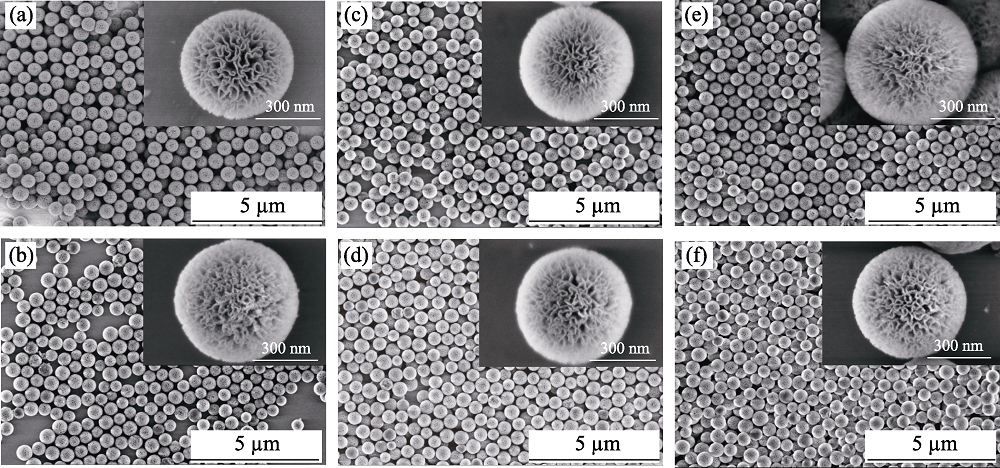
图2 DMSNs (a)、DMSTNs (b)、DMSNs-NH2 (c)、DMSTNs-NH2 (d)、DMSNs-NH2-Au (e)和DMSTNs-NH2-Au (f)的SEM照片
Fig. 2 SEM images of DMSNs (a), DMSTNs (b), DMSNs-NH2 (c), DMSTNs-NH2 (d), DMSNs-NH2-Au (e), and DMSTNs-NH2-Au (f)
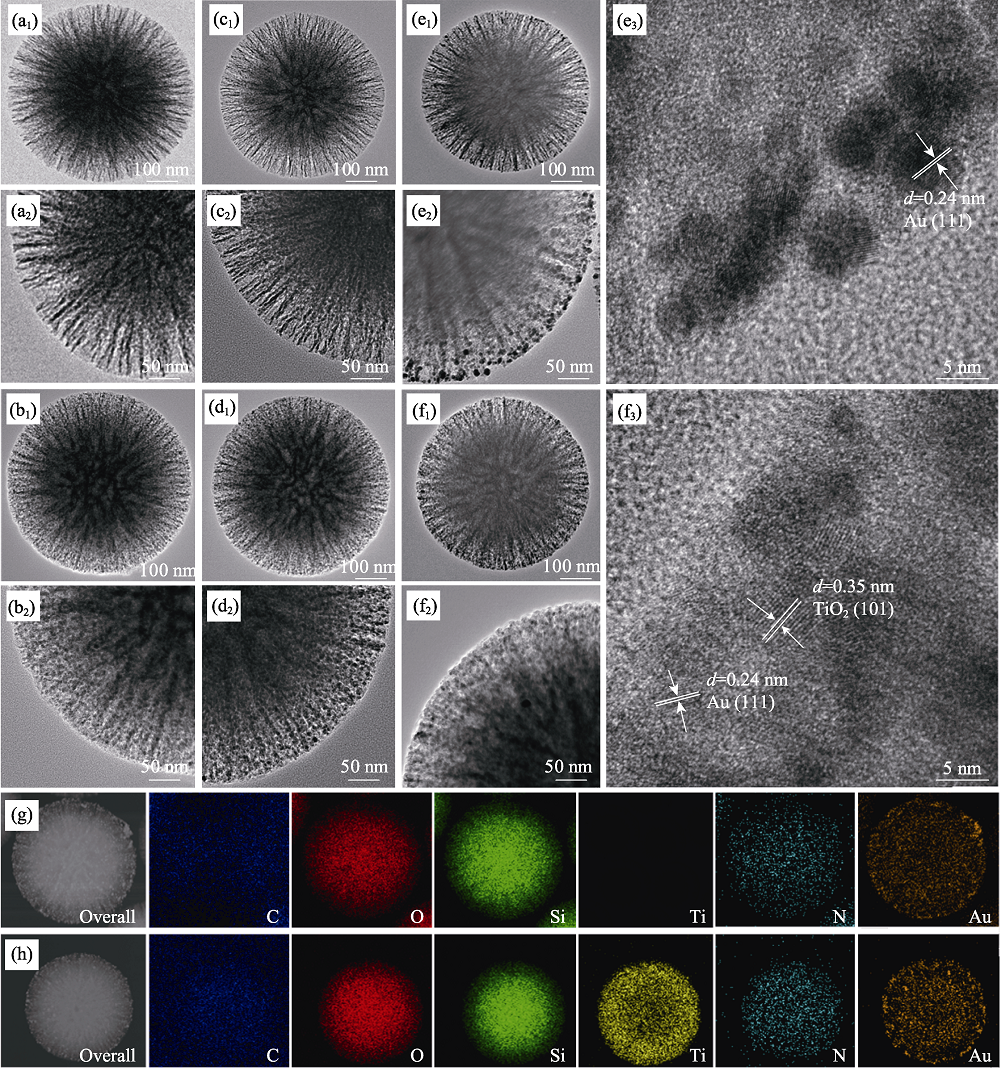
图5 DMSNs (a)、DMSTNs (b)、DMSNs-NH2 (c)、DMSTNs-NH2 (d)、DMSNs-NH2-Au (e)、DMSTNs-NH2-Au (f)的透射电镜照片, DMSNs-NH2-Au (g)和DMSTNs-NH2-Au (h)的透射电镜-元素分布图
Fig.5 TEM images of DMSNs (a), DMSTNs (b), DMSNs-NH2 (c), DMSTNs-NH2 (d), DMSNs-NH2-Au (e), and DMSTNs-NH2-Au (f), and TEM-mapping images of DMSNs-NH2-Au (g) and DMSTNs-NH2-Au (h)
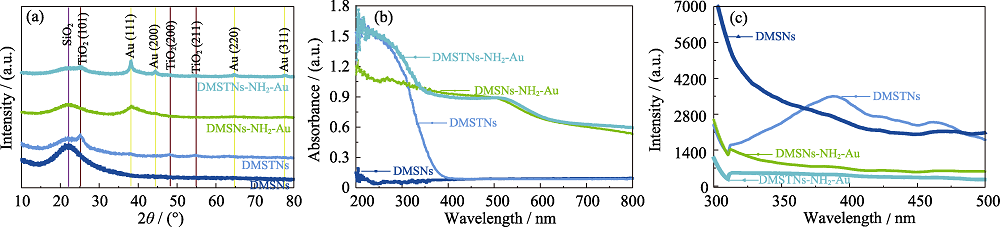
图6 DMSNs、DMSTNs、DMSNs-NH2-Au和DMSTNs-NH2-Au的XRD图谱(a), 紫外-可见漫反射光谱图(b)和光致发光谱图(c)
Fig. 6 XRD patterns (a), UV-Vis-DRS spectra (b) and PL spectra (c) of DMSNs, DMSTNs, DMSNs-NH2-Au, and DMSTNs-NH2-Au
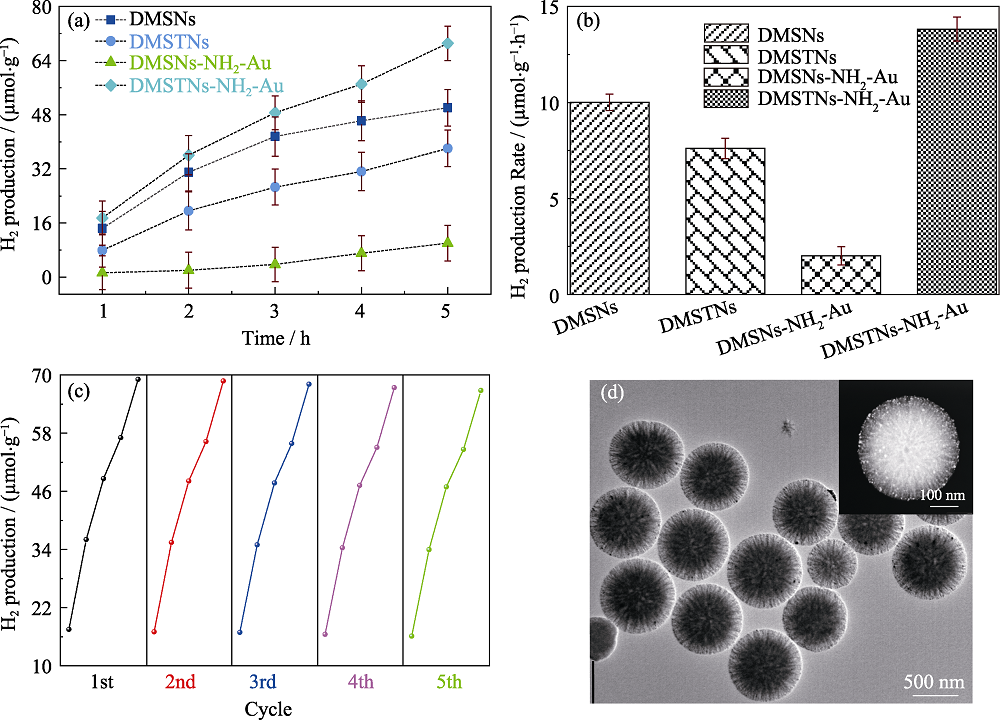
图7 DMSNs、DMSTNs、DMSNs-NH2-Au和DMSTNs-NH2-Au在模拟太阳光下的氢产量(a)及速率(b); DMSTNs-NH2-Au 的光解水产氢循环性能(c); DMSTNs-NH2-Au经过5次催化循环后的透射电镜照片(插图为高角环形暗场成像)(d)
Fig. 7 H2 production amount as a function of irradiation time (a) and the corresponding production rates (b) of DMSNs, DMSTNs, DMSNs-NH2-Au, and DMSTNs-NH2-Au, cycling tests of DMSTNs-NH2-Au for H2 production (c), and TEM image of DMSTNs-NH2-Au sample experienced five cycles, with inset showing the high-angle annular dark-field imaging (d)
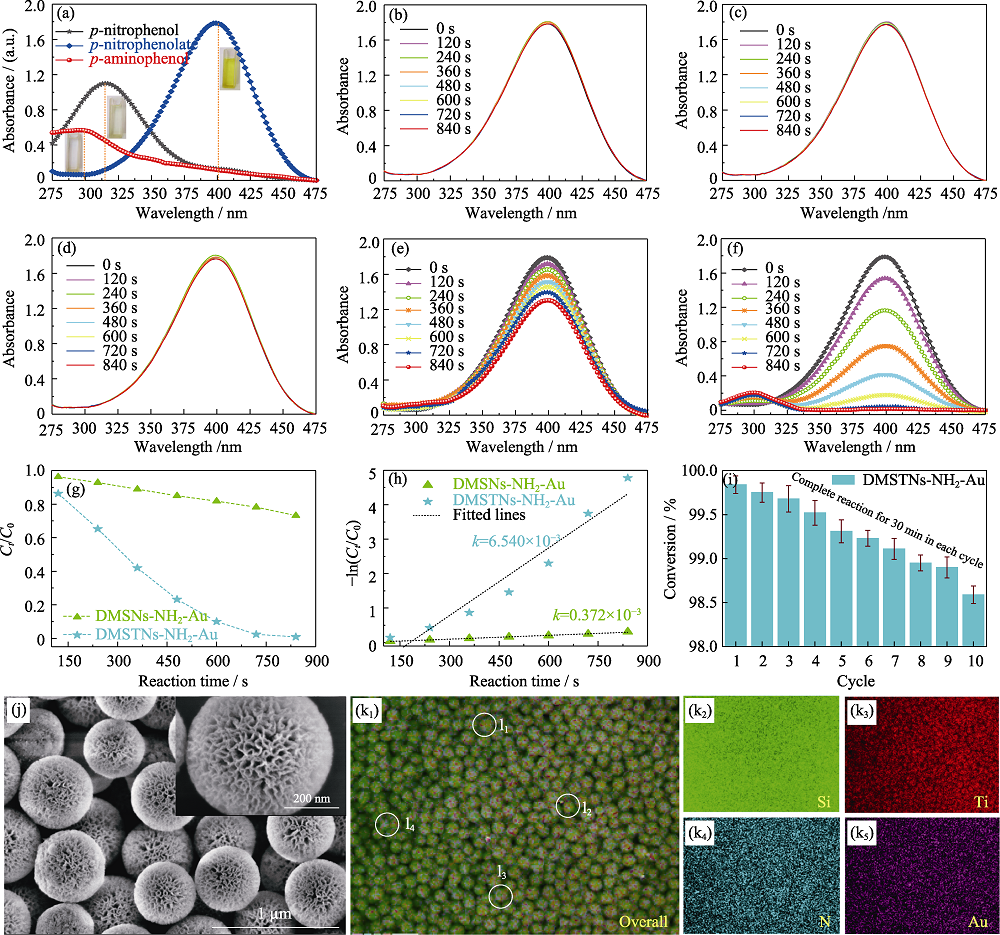
图8 对硝基苯酚、对硝基苯酚钠和对氨基苯酚的紫外可见漫反射光谱(a), 不同样品(含有硼氢化钠的对硝基苯酚溶液作为空白试样(b)、加入DMSNs(c)、DMSTNs(d)、DMSNs-NH2-Au(e)和DMSTNs-NH2-Au(f))的紫外吸收变化, DMSNs-NH2-Au和DMSTNs-NH2-Au的转化率(g)及准一级模拟方程(h), DMSTNs-NH2-Au循环降解性能(i), DMSTNs-NH2-Au经过10次催化循环后的SEM形貌(j)及元素分布图(k), 任意4个经过10次催化循环的DMSTNs-NH2-Au单体球能谱测试标定区域(l)
Fig. 8 Characteristic ultraviolet absorption peaks of p-nitrophenol, p-nitrophenolate, and p-aminophenol (a), Ultraviolet absorption spectra of different samples, including p-nitrophenol+NaBH4 as the blank sample (b), with the addition of DMSNs (c), DMSTNs (d), DMSNs-NH2-Au (e), and DMSTNs-NH2-Au (f), the conversion (g) and pseudo first-order linear equation (h) of DMSNs-NH2-Au, and DMSTNs-NH2-Au, and cycling tests of DMSTNs-NH2-Au for p-nitrophenol reduction (i), SEM images (j) and energy dispersive spectroscopy (EDS) mappings (k) of DMSTNs-NH2-Au sample experienced ten cycles. EDS measurement of random four DMSTNs-NH2-Au individuals (l)
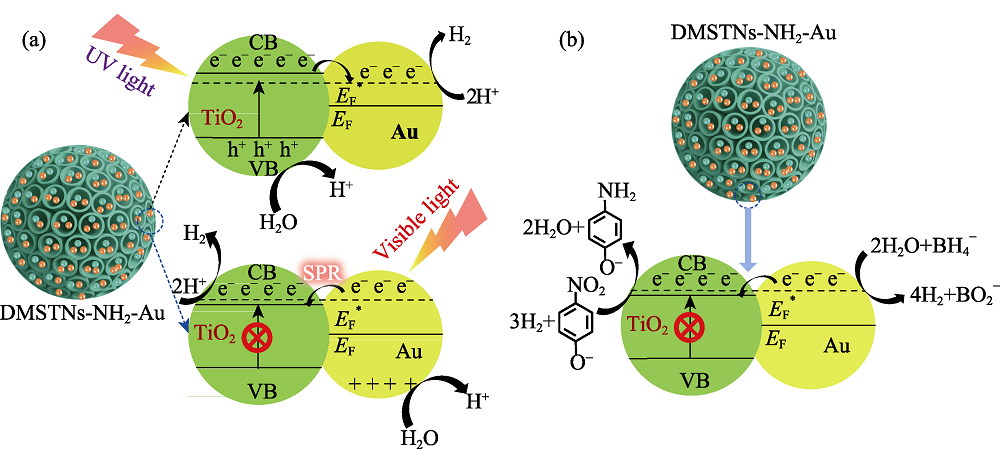
图9 DMSTNs-NH2-Au 在(a)模拟太阳光条件下光解水制氢和(b)无光照条件下还原对硝基苯酚的催化机理
Fig. 9 Schematic illustration of possible photocatalytic mechanisms for DMSTNs-NH2-Au to split water under simulated sunlight (a) and ordinary catalytic reduction of p-nitrophenol without light irritation (b)
| [1] |
MEEMKEN F, BAIKER A. Recent progress in heterogeneous asymmetric hydrogenation of C=O and C=C bonds on supported noble metal catalysts. Chemical Reviews, 2017, 117(17): 11522-11569.
DOI URL |
| [2] |
DHIMAN M, CHALKE B, POLSHETTIWAR V. Organosilane oxidation with a half million turnover number using fibrous nanosilica supported ultrasmall nanoparticles and pseudo-single atoms of gold. Journal of Materials Chemistry A, 2017, 5(5): 1935-1940.
DOI URL |
| [3] |
SANTOS V P, CARABINEIRO S A C, TAVARES P B, et al. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Applied Catalysis B: Environmental, 2010, 99(1): 198-205.
DOI URL |
| [4] |
LIU M, HOF F, MORO M, et al. Carbon supported noble metal nanoparticles as efficient catalysts for electrochemical water splitting. Nanoscale, 2020, 12(39): 20165-20170.
DOI URL |
| [5] |
ZHANG Y P, ZHOU Y, ZHAO Y, et al. Recent progresses in the size and structure control of MOF supported noble metal catalysts. Catalysis Today, 2016, 263: 61-68.
DOI URL |
| [6] | KASHYAP T, BISWASI S, PAL A R, et al. Unraveling the catalytic and plasmonic roles of g-C3N4 supported Ag and Au nanoparticles under selective photoexcitation. ACS Sustainable Chemistry & Engineering, 2019, 7(23): 19295-19302. |
| [7] |
MAO S J, WANG C P, WANG Y. The chemical nature of N doping on N doped carbon supported noble metal catalysts. Journal of Catalysis, 2019, 375: 456-465.
DOI URL |
| [8] |
XU J, ZHANG J Y, PENG H G, et al. Ag supported on meso- structured SiO2 with different morphologies for CO oxidation: on the inherent factors influencing the activity of Ag catalysts. Microporous and Mesoporous Materials, 2017, 242: 90-98.
DOI URL |
| [9] |
LE X D, DONG Z P, LIU Y S, et al. Palladium nanoparticles immobilized on core-shell magnetic fibers as a highly efficient and recyclable heterogeneous catalyst for the reduction of 4-nitrophenol and Suzuki coupling reactions. Journal of Materials Chemistry A, 2014, 2(46): 19696-19706.
DOI URL |
| [10] |
SINGH B, POLSHETTIWAR V. Design of CO2 sorbents using functionalized fibrous nanosilica (KCC-1): insights into the effect of the silica morphology (KCC-1 vs. MCM-41). Journal of Materials Chemistry A, 2016, 4(18): 7005-7019.
DOI URL |
| [11] |
SINGH R, BAPAT R, QIN L, et al. Atomic layer deposited (ALD) TiO2 on fibrous nano-silica (KCC-1) for photocatalysis: nanoparticle formation and size quantization effect. ACS Catalysis, 2016, 6(5): 2770-2784.
DOI URL |
| [12] |
MAITY A, BELGAMWAR R, POLSHETTIWAR V. Facile synthesis to tune size, textural properties and fiber density of dendritic fibrous nanosilica for applications in catalysis and CO2 capture. Nature Protocols, 2019, 14: 2177-2204.
DOI URL |
| [13] | POLSHETTIWAR V, CHA D, ZHANG X, et al. High-surface-area silica nanospheres (KCC-1) with a fibrous morphology. Angewandte Chemie International Edition, 2010, 49(50): 9652-9656. |
| [14] |
WANG Y, TANG J, YANG Y N, et al. Functional nanoparticles with a reducible tetrasulfide motif to upregulate mRNA translation and enhance transfection in hard-to-transfect cells. Angewandte Chemie International Edition, 2020, 59(7): 2695-2699.
DOI URL |
| [15] | DONG Z P, LE X D, LI X L, et al. Silver nanoparticles immobilized on fibrous nano-silica as highly efficient and recyclable heterogeneous catalyst for reduction of 4-nitrophenol and 2-nitroaniline. Applied Catalysis B Environmental, 2014, 158: 129-135. |
| [16] |
DONG Z P, LE X D, DONG C X, et al. Ni@Pd core-shell nanoparticles modified fibrous silica nanospheres as highly efficient and recoverable catalyst for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. Applied Catalysis B Environmental, 2015, 162: 372-380.
DOI URL |
| [17] |
LE X D, DONG Z P, ZHANG W, et al. Fibrous nano-silica containing immobilized Ni@Au core-shell nanoparticles: a highly active and reusable catalyst for the reduction of 4-nitrophenol and 2-nitroaniline. Journal of Molecular Catalysis A: Chemical, 2014, 395: 58-65.
DOI URL |
| [18] |
WANG Y B, HE J, SHI Y M, et al. Structure- dependent adsorptive or photocatalytic performances of solid and hollow dendritic mesoporous silica&titania nanospheres. Microporous and Mesoporous Materials, 2020, 305 110326.
DOI URL |
| [19] |
WANG Y B, HE J, LI X L, et al. Dendritic mesoporous silica&titania nanospheres (DMSTNs) coupled with amorphous carbon nitride (ACN) for improved visible-light-driven hydrogen production. Applied Surface Science, 2021, 538 148147.
DOI URL |
| [20] |
WANG Y B, HU K K, HE J, et al. Improving the size uniformity of dendritic fibrous nano-silica by a facile one-pot rotating hydrothermal approach. RSC Advances, 2019, 9(43): 24783-24790.
DOI URL |
| [21] |
FIHRI A, CHA D, BOUHRARA M, et al. Fibrous nano-silica (KCC-1)-supported palladium catalyst: Suzuki coupling reactions under sustainable conditions. ChemSusChem, 2012, 5(1): 85-89.
DOI URL |
| [22] |
SADEGHZADEH S M. A heteropolyacid-based ionic liquid immobilized onto fibrous nano-silica as an efficient catalyst for the synthesis of cyclic carbonate from carbon dioxide and epoxides. Green Chemistry, 2015, 17(5): 3059-3066.
DOI URL |
| [23] |
REN J, LI Z, LIU S S, et al. Silica-titania mixed oxides: Si-O-Ti connectivity, coordination of titanium, and surface acidic properties. Catalysis Letters, 2008, 124(34): 185-194.
DOI URL |
| [24] |
VAIANO V, MATARANGOLO M, MURCIA J J, et al. Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag. Applied Catalysis B: Environmental, 2018, 225: 197-206.
DOI URL |
| [25] |
JING L Q, QU Y C, WANG B Q, et al. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Solar Energy Materials and Solar Cells, 2006, 90(12): 1773-1787.
DOI URL |
| [26] |
HAMID M Y S, FIRMANSYAH M L, TRIWAHYONO S, et al. Oxygen vacancy-rich mesoporous silica KCC-1 for CO2 methanation. Applied Catalysis A General, 2017, 532: 86-94.
DOI URL |
| [27] |
JAKOB M, LEVANON H, KAMAT P V. Charge distribution between UV-irradiated TiO2 and gold nanoparticles: determination of shift in the fermi level. Nano Letters, 2003, 3(3): 353-358.
DOI URL |
| [28] |
SUBRAMANIAN V, WOLF E E, KAMAT P V. Catalysis with TiO2/gold nanocomposites. effect of metal particle size on the fermi level equilibration. Journal of the American Chemical Society, 2004, 126(15): 4943-4950.
DOI URL |
| [29] |
CYBULA A, PRIEBE J B, POHL M M, et al. The effect of calcination temperature on structure and photocatalytic properties of Au/Pd nanoparticles supported on TiO2. Applied Catalysis B: Environmental, 2014, 152-153: 202-211.
DOI URL |
| [30] | LIU B, JIANG Y, WANG Y, et al. Influence of dimensionality and crystallization on visible-light hydrogen production of Au@TiO2 core-shell photocatalysts based on localized surface plasmon resonance. Catalysis Science&Technology, 2018, 8(4): 1094-1103. |
| [31] |
MISRA M, CHOWDHURY S R, LEE T I. Sunlight driven decomposition of toxic organic compound, coumarin, p-nitrophenol, and photo reduction of Cr(VI) ions, using a bridge structure of Au@CNT@TiO2 nanocomposite. Applied Catalysis B: Environmental, 2020, 272: 118991.
DOI URL |
| [32] |
ISMAIL A A, HAKKI A, BAHNEMANN D W. Mesostructure Au/TiO2 nanocomposites for highly efficient catalytic reduction of p-nitrophenol. Journal of Molecular Catalysis A: Chemical, 2012, 358: 145-151.
DOI URL |
| [33] |
HUANG M Q, ZHANG Y W, ZHOU Y M, et al. Synthesis and characterization of hollow ZrO2-TiO2/Au spheres as a highly thermal stability nanocatalyst. Journal of Colloid and Interface Science, 2017, 497: 23-32.
DOI URL |
| [1] | 汤嘉伟, 王永邦, 马成, 杨海潇, 王际童, 乔文明, 凌立成. 甲基萘沥青基有序中孔炭的制备及电化学性能[J]. 无机材料学报, 2021, 36(10): 1031-1038. |
| [2] | 刘芳芳, 传秀云, 杨扬, 李爱军. 氮/硫共掺杂对纤水镁石模板碳纳米管电化学性能的影响[J]. 无机材料学报, 2021, 36(7): 711-717. |
| [3] | 李子宜, 章佳佳, 邹小勤, 左家玉, 李俊, 刘应书, 裴有康. 菱沸石分子筛膜的合成调控与气体分离研究进展[J]. 无机材料学报, 2021, 36(6): 579-591. |
| [4] | 陈仁德, 郭鹏, 左潇, 许世鹏, 柯培玲, 汪爱英. Ag掺杂非晶碳膜结构、力学与电学行为研究[J]. 无机材料学报, 2019, 34(4): 387-393. |
| [5] | 马志军, 莽昌烨, 赵海涛, 关智浩, 程亮. 石墨烯装载不同含量钴锌铁氧体及其电磁行为对比[J]. 无机材料学报, 2019, 34(4): 407-416. |
| [6] | 王佳玮, 杨艳青, 高泽宇, 梁颖, 邓钏, 张卫珂. 水热法合成Bi2WO6/CNOs纳米材料及其电化学性能[J]. 无机材料学报, 2018, 33(11): 1208-1212. |
| [7] | 魏菁, 李汉超, 柯培玲, 汪爱英. 不同厚度四面体非晶碳薄膜的高通量制备及表征[J]. 无机材料学报, 2018, 33(11): 1173-1178. |
| [8] | 李汉超, 刘盼盼, 孙丽丽, 柯培玲, 崔平, 汪爱英. 金属催化非晶碳转化制备石墨烯方法的研究进展[J]. 无机材料学报, 2018, 33(6): 587-595. |
| [9] | 王浩, 李琳, 王春雷, 王倩, 梁长海, 王同华. 聚酰亚胺基高比表面活性炭及其电化学性能研究[J]. 无机材料学报, 2017, 32(11): 1181-1187. |
| [10] | 魏 婕, 李雪冬, 王宏志, 张青红, 李耀刚. NCDs/TiO2复合材料的制备及其在太阳光下催化制氢的应用[J]. 无机材料学报, 2015, 30(9): 925-930. |
| [11] | 郑艳彬, 姜志刚, 朱品文. 洋葱碳的制备与应用研究进展[J]. 无机材料学报, 2015, 30(8): 793-801. |
| [12] | 张珍容, 姚异渊, 罗 凯, 万 隆, 刘宣勇. 纯钛表面立方介孔SiO2薄膜诱导沉积碳磷灰石层[J]. 无机材料学报, 2015, 30(7): 725-731. |
| [13] | 孙志娟, 陈雪莲, 蒋春跃. 自组装法制备中空二氧化硅纳米粒子减反射薄膜[J]. 无机材料学报, 2014, 29(9): 947-955. |
| [14] | 于庆春, 万 红. 片状石墨增强树脂基复合材料的耐激光烧蚀性能研究[J]. 无机材料学报, 2012, 27(2): 157-161. |
| [15] | 鲍 英, 王春晓, 詹 亮, 王艳莉, 杨光智, 杨俊和, 凌立成. 纳米碳管对泡沫炭的超临界发泡行为及其力学性能的影响机制[J]. 无机材料学报, 2011, 26(10): 1020-1024. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||