无机材料学报 ›› 2021, Vol. 36 ›› Issue (12): 1323-1329.DOI: 10.15541/jim20210206
所属专题: 【虚拟专辑】碳中和(2020~2021); 【虚拟专辑】钙钛矿材料(2020~2021); 【能源环境】钙钛矿; 【能源环境】CO2绿色转换
王玥1,2( ), 崔常松1,2, 王士维1,2, 占忠亮1,2,3(
), 崔常松1,2, 王士维1,2, 占忠亮1,2,3( )
)
收稿日期:2021-03-26
修回日期:2021-04-27
出版日期:2021-12-20
网络出版日期:2021-05-25
通讯作者:
占忠亮, 教授. E-mail: zzhan@ustc.edu.cn
作者简介:王 玥(1994-), 女, 博士研究生. E-mail: wangyue@mail.sic.ac.cn
WANG Yue1,2( ), CUI Changsong1,2, WANG Shiwei1,2, ZHAN Zhongliang1,2,3(
), CUI Changsong1,2, WANG Shiwei1,2, ZHAN Zhongliang1,2,3( )
)
Received:2021-03-26
Revised:2021-04-27
Published:2021-12-20
Online:2021-05-25
Contact:
ZHAN Zhongliang, professor. E-mail: zzhan@ustc.edu.cn
About author:WANG Yue (1994-), female, PhD candidate. E-mail: wangyue@mail.sic.ac.cn
Supported by:摘要:
固态氧化物电解池(SOECs)因较高的能量转化效率在电化学还原CO2, 实现“碳中和”社会方面备受关注。与非对称电池结构相比, 对称SOECs的空气极和燃料极是相同或相近的材料, 可以减少界面种类, 改善电极与电解质的热膨胀匹配性, 简化电池的制备工艺。本研究合成了钙钛矿氧化物LaxSr2-xFe1.5Ni0.1Mo0.4O6-δ (LxSFNM, x=0.1、0.2、0.3、0.4), 作为固体氧化物电解池的对称电极用于评估纯CO2的电化学还原性能。掺入La3+可以有效提高反应催化活性, 其中L0.3SFNM为电极的电解池表现出最高的电化学性能, 800 ℃下, 在空气中的极化电阻为0.07 Ω∙cm2, 在50% CO-50% CO2中的极化电阻为0.62 Ω∙cm2。单电池L0.3SFNM@LSGM|LSGM|L0.3SFNM@LSGM在800 ℃和1.5 V电压下的电解电流密度为1.17 A∙cm-2, 在初始的50 h CO2短期电解测试中表现出优异的稳定性, 是一种理想的对称电极材料。
中图分类号:
王玥, 崔常松, 王士维, 占忠亮. LaxSr2-xFe1.5Ni0.1Mo0.4O6-δ对称电池电解CO2研究[J]. 无机材料学报, 2021, 36(12): 1323-1329.
WANG Yue, CUI Changsong, WANG Shiwei, ZHAN Zhongliang. Symmetrical La3+-doped Sr2Fe1.5Ni0.1Mo0.4O6-δ Electrode Solid Oxide Fuel Cells for Pure CO2 Electrolysis[J]. Journal of Inorganic Materials, 2021, 36(12): 1323-1329.
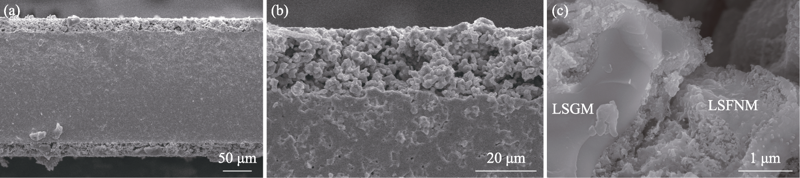
Fig. 4 (a) Cross-sectional SEM image of the tri-layer symmetrical structure of “porous|dense|porous” LSGM and (b) high magnification view of symmetrical cell, and (c) high-magnification view of impregnated L0.3SFNM catalyst

Fig. 5 Nyquist plots of impedance data measured with LxSFNM at 800 ℃ in (a) air and (c) 50% CO-50% CO2; (b, d) DRT curves of impedance data shown in (a, c)
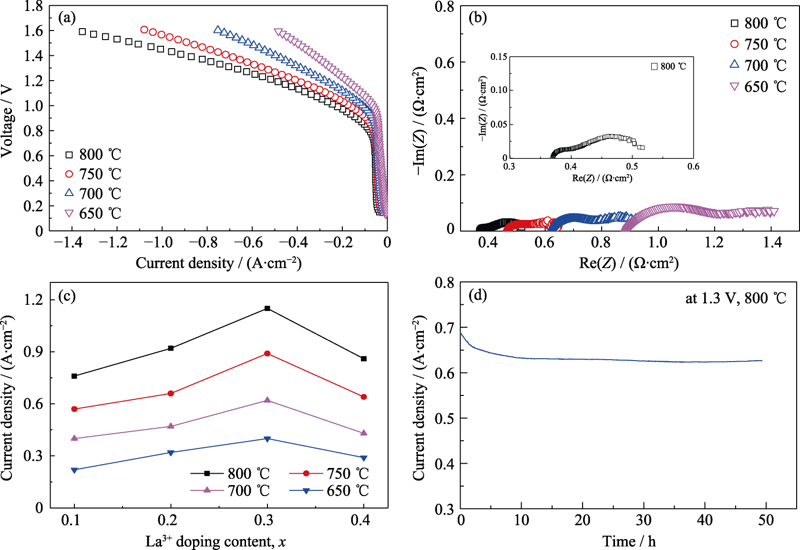
Fig. 7 (a) I-V curves and (b) impedance spectra measured at the 1.5 V of a single electrolyte-supported electrolysis cell with L0.3SFNM at different temperatures with inset in (b) showing corresponding enlarged spectrum of the cell with L0.3SFNM at 800 ℃, (c) current densities with varied LaxSFNM anodes at 1.5 V under different temperatures, and (d) short-term stability test of the symmetrical SOECs with L0.3SFNM electrode, operating under 800 ℃ at an applied voltage of 1.3 V
| Electrode | Electrolyte | Performance/(A∙cm-2) | Ref. |
|---|---|---|---|
| La0.3Sr0.7Fe0.7Ti0.3O3 | YSZ | 0.52 (2V) | [ |
| La0.6Sr0.4Fe0.9Mn0.1O3-δ-GDC | YSZ | 1.107 (2V) | [ |
| La0.6Sr0.4Fe0.8Ni0.2O3-δ-GDC | YSZ | 1.03 | [ |
| La0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δ | YSZ | 0.442 | [ |
| La0.6Ca0.4Fe0.8Ni0.2O3-δ-GDC | YSZ | 0.78 | [ |
| La0.75Sr0.25Cr0.5Mn0.5O3-δ | YSZ | 0.09 | [ |
| La0.3Sr0.7Cr0.3Fe0.7O3-δ | YSZ | 0.32 | [ |
| (PrBa)0.95(Fe0.9Mo0.1)2O5+δ | LSGM | 0.51 (1.3V) | [ |
| La0.3Sr1.7Fe1.5Ni0.1Mo0.4O6-δ | LSGM | 1.17 | This work |
Table 1 Comparison of literature values of current densities achieved for pure CO2 electrolysis in electrolyte-supported cells at 1.5 V under 800 ℃
| Electrode | Electrolyte | Performance/(A∙cm-2) | Ref. |
|---|---|---|---|
| La0.3Sr0.7Fe0.7Ti0.3O3 | YSZ | 0.52 (2V) | [ |
| La0.6Sr0.4Fe0.9Mn0.1O3-δ-GDC | YSZ | 1.107 (2V) | [ |
| La0.6Sr0.4Fe0.8Ni0.2O3-δ-GDC | YSZ | 1.03 | [ |
| La0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δ | YSZ | 0.442 | [ |
| La0.6Ca0.4Fe0.8Ni0.2O3-δ-GDC | YSZ | 0.78 | [ |
| La0.75Sr0.25Cr0.5Mn0.5O3-δ | YSZ | 0.09 | [ |
| La0.3Sr0.7Cr0.3Fe0.7O3-δ | YSZ | 0.32 | [ |
| (PrBa)0.95(Fe0.9Mo0.1)2O5+δ | LSGM | 0.51 (1.3V) | [ |
| La0.3Sr1.7Fe1.5Ni0.1Mo0.4O6-δ | LSGM | 1.17 | This work |
| [1] |
ALBO J, ALVAREZ-GUERRA M, CASTAÑO P, et al. Towards the electrochemical conversion of carbon dioxide into methanol. Green Chemistry, 2015, 17(4): 2304-2324.
DOI URL |
| [2] |
FREUND H J, ROBERTS M W. Surface chemistry of carbon dioxide. Surface Science Reports, 1996, 25(8): 225-273.
DOI URL |
| [3] |
ZHENG Y, WANG J, YU B, et al. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): advanced materials and technology. Chem. Soc. Rev., 2017, 46(5): 1427-1463.
DOI URL |
| [4] |
LIU S, LIU Q, LUO J L. CO2-to-CO conversion on layered perovskite with in situ exsolved Co-Fe alloy nanoparticles: an active and stable cathode for solid oxide electrolysis cells. Journal of Materials Chemistry A, 2016, 4(44): 17521-17528.
DOI URL |
| [5] |
SINGH V, MUROYAMA H, MATSUI T, et al. Feasibility of alternative electrode materials for high temperature CO2 reduction on solid oxide electrolysis cell. Journal of Power Sources, 2015, 293: 642-648.
DOI URL |
| [6] |
YUE X L, IRVINE J T S. Alternative cathode material for CO2 reduction by high temperature solid oxide electrolysis cells. Journal of the Electrochemical Society, 2012, 159(8): F442-F448.
DOI URL |
| [7] | LI Y, CHEN X, YANG Y, et al. Mixed-conductor Sr2Fe1.5Mo0.5O6-δ as robust fuel electrode for pure CO2 reduction in solid oxide electrolysis cell. ACS Sustainable Chemistry & Engineering, 2017, 5(12): 11403-11412. |
| [8] |
LÜ H, LIN L, ZHANG X, et al. In situ investigation of reversible exsolution/dissolution of CoFe alloy nanoparticles in a Co-doped Sr2Fe1.5Mo0.5O6-δ cathode for CO2 electrolysis. Advanced Materials, 2020, 32(6): 1906193.
DOI URL |
| [9] |
YUE X, IRVINE J T S. Modification of LSCM-GDC cathodes to enhance performance for high temperature CO2 electrolysis using solid oxide electrolysis cells (SOECs). Journal of Materials Chemistry A, 2017, 5(15): 7081-7090.
DOI URL |
| [10] |
LU L, NI C, CASSIDY M, et al. Demonstration of high performance in a perovskite oxide supported solid oxide fuel cell based on La and Ca co-doped SrTiO3-δ. Journal of Materials Chemistry A, 2016, 4(30): 11708-11718.
DOI URL |
| [11] |
QI W, GAN Y, YIN D, et al. Remarkable chemical adsorption of manganese-doped titanate for direct carbon dioxide electrolysis. Journal of Materials Chemistry A, 2014, 2(19): 6904-6915.
DOI URL |
| [12] |
LIU S, LIU Q, LUO J L. Highly stable and efficient catalyst with in situ exsolved Fe-Ni alloy nanospheres socketed on an oxygen deficient perovskite for direct CO2 electrolysis. ACS Catalysis, 2016, 6(9): 6219-6228.
DOI URL |
| [13] |
TIAN Y, ZHANG L, LIU Y, et al. A self-recovering robust electrode for highly efficient CO2 electrolysis in symmetrical solid oxide electrolysis cells. Journal of Materials Chemistry A, 2019, 7(11): 6395-6400.
DOI URL |
| [14] | LI Y, ZHAN Z, XIA C. Highly efficient electrolysis of pure CO2 with symmetrical nanostructured perovskite electrodes. Catalysis Science & Technology, 2018, 8(4): 980-984. |
| [15] |
ZHANG Y Q, LI J H, SUN Y F, et al. Highly active and redox- stable Ce-doped LaSrCrFeO based cathode catalyst for CO2 SOECs. ACS Applied Materials Interfaces, 2016, 8(10): 6457-6463.
DOI URL |
| [16] | SÃNCHEZ D, ALONSO J A, GARCÍA-HERNÁNDEZ M, et al. Microscopic nature of the electron doping effects in the double perovskite Sr2-xLaxFeMoO6(0≤x≤1) series. Journal of Materials Chemistry A, 2003, 13(7): 1771-1777. |
| [17] |
SUGAHARA T, OHTAKI M, SOUMA T. Thermoelectric properties of double-perovskite oxide Sr2-xMxFeMoO6 (M=Ba, La). Journal of Ceramic Society Japan, 2008, 116(1360): 1278-1282.
DOI URL |
| [18] |
YANG X, CHEN J, PANTHI D, et al. Electron doping of Sr2FeMoO6-δ as high performance anode materials for solid oxide fuel cells. Journal of Materials Chemistry A, 2019, 7(2): 733-743.
DOI URL |
| [19] |
AZIZI F, KAHOUL A, AZIZI A. Effect of La doping on the electrochemical activity of double perovskite oxide Sr2FeMoO6 in alkaline medium. Journal of Alloys and Compounds, 2009, 484(1/2): 555-560.
DOI URL |
| [20] |
YANG X, PANTHI D, HEDAYAT N, et al. Molybdenum dioxide as an alternative catalyst for direct utilization of methane in tubular solid oxide fuel cells. Electrochemistry Communications, 2018, 86: 126-129.
DOI URL |
| [21] |
SARMA D D, MAHADEVAN P, DASGUPTA T S, et al. Electronic structure of Sr2FeMoO6-δ. Physical Review Letter, 2000, 85(12): 2549-2552.
DOI URL |
| [22] |
LIU Q, DONG X, XIAO G, et al. A novel electrode material for symmetrical SOFCs. Advanced Materials, 2010, 22(48): 5478-5482.
DOI URL |
| [23] |
DAI N, FENG J, WANG Z, et al. Synthesis and characterization of B-site Ni-doped perovskites Sr2Fe1.5-xNixMo0.5O6-δ (x = 0, 0.05, 0.1, 0.2, 0.4) as cathodes for SOFCs. Journal of Materials Chemistry A, 2013, 1(45): 14147-14153.
DOI URL |
| [24] |
LU X, YANG Y, DING Y, et al. Mo-doped Pr0.6Sr0.4Fe0.8Ni0.2O3-δ as potential electrodes for intermediate-temperature symmetrical solid oxide fuel cells. Electrochimica Acta, 2017, 227: 33-40.
DOI URL |
| [25] | PENG X, TIAN Y, LIU Y, et al. An efficient symmetrical solid oxide electrolysis cell with LSFM-based electrodes for direct electrolysis of pure CO2. Journal of CO2 Utilization, 2020, 36: 18-24. |
| [26] |
WANG R, DOGDIBEGOVIC E, LAU G Y, et al. Metal-supported solid oxide electrolysis cell (MS-SOEC) with significantly enhanced catalysis. Energy Technology, 2019, 7(5): 1801154-1801166.
DOI URL |
| [27] |
CAO Z, WEI B, MIAO J, et al. Efficient electrolysis of CO2 in symmetrical solid oxide electrolysis cell with highly active La0.3Sr0.7Fe0.7Ti0.3O3 electrode material. Electrochemistry Communications, 2016, 69: 80-83.
DOI URL |
| [28] |
TIAN Y, ZHENG H, ZHANG L, et al. Direct electrolysis of CO2 in symmetrical solid oxide electrolysis cell based on La0.6Sr0.4Fe0.8Ni0.2O3-δ electrode. Journal of The Electrochemical Society, 2018, 165(2): F17-F23.
DOI URL |
| [29] | YANG Z, MA C, WANG N, et al. Electrochemical reduction of CO2 in a symmetrical solid oxide electrolysis cell with La0.4Sr0.6Co0.2Fe0.7Nb0.1O3-δ electrode. Journal of CO2 Utilization, 2019, 33: 445-451. |
| [30] |
XU S, LI S, YAO W, et al. Direct electrolysis of CO2 using an oxygen-ion conducting solid oxide electrolyzer based on La0.75Sr0.25Cr0.5Mn0.5O3-δ electrode. Journal of Power Sources, 2013, 230: 115-121.
DOI URL |
| [31] |
ADDO P K, MOLERO-SANCHEZ B, CHEN M, et al. CO/CO2 study of high performance La0.3Sr0.7Fe0.7Cr0.3O3-δ reversible SOFC electrodes. Fuel Cells, 2015, 15(5): 689-696.
DOI URL |
| [32] |
LU C, NIU B, YI W, et al. Efficient symmetrical electrodes of PrBaFe2-xCoxO5+δ (x=0, 0.2, 0.4) for solid oxide fuel cells and solid oxide electrolysis cells. Electrochimica Acta, 2020, 358: 136916-136927.
DOI URL |
| [1] | 李泽晖,谭美娟,郑元昊,骆雨阳,经求是,蒋靖坤,李明杰. 导电金属有机骨架材料在超级电容器中的应用[J]. 无机材料学报, 2020, 35(7): 769-780. |
| [2] | 夏天, 孟燮, 骆婷, 占忠亮. Ca掺杂Sr2Fe1.5Mo0.5O6-δ材料的合成与作为对称固体氧化物燃料电池电极催化剂的性能研究[J]. 无机材料学报, 2019, 34(10): 1109-1114. |
| [3] | 王浩, 罗永春, 邓安强, 赵磊, 姜婉婷. 退火温度对无镁La-Y-Ni系A2B7型合金相结构和电化学性能的影响[J]. 无机材料学报, 2018, 33(4): 434-440. |
| [4] | 王玲玲, 黄 昊, Ramon Alberto Paredes Camacho, 吴爱民, 曹国忠. (Mn7C3, Ni)@C核壳型纳米粒子制备及超级电容器电化学特性[J]. 无机材料学报, 2017, 32(2): 135-140. |
| [5] | 赵晓虹, 王勇, 刘立敏, 李斌. 固体氧化物燃料电池新型钙钛矿La0.9Ca0.1Fe0.9Nb0.1O3-δ阳极的制备及其性能研究[J]. 无机材料学报, 2017, 32(11): 1188-1194. |
| [6] | 常希望, 陈 宁, 王丽君, 李福燊, 卞刘振, 周国治. 固体氧化物燃料电池Sr系钙钛矿电极B位元素成分优化规律[J]. 无机材料学报, 2017, 32(10): 1055-1062. |
| [7] | 李金宏, 周岐雄, 米红宇, 李 显, 李惠萍. 基于煤萃取物的类石墨状多孔炭的制备及其电容性能研究[J]. 无机材料学报, 2016, 31(1): 39-46. |
| [8] | 陈 耿, 刘桃香, 唐新峰, 苏贤礼, 鄢永高. Mg-Si-Sn基热电器件电极材料的优化选择与连接工艺[J]. 无机材料学报, 2015, 30(6): 639-646. |
| [9] | 李 磊, 梁笠智, 吴 恒, 梁 爽, 朱瑛莺, 朱信华. 钙钛矿锰氧化物低维纳米结构研究进展[J]. 无机材料学报, 2015, 30(4): 337-344. |
| [10] | 毛 磊, 索红莉, 刘 敏, 张子立, 叶 帅, 马 麟. Nb5+掺杂对低氟MOD法制备的YBCO薄膜性能的影响[J]. 无机材料学报, 2013, 28(9): 956-960. |
| [11] | 马 文, 韩鹏献, 孔庆山, 张克军, 毕彩丰, 崔光磊. MoN/氮化石墨烯复合物用作锂离子电容器电极材料的研究[J]. 无机材料学报, 2013, 28(7): 733-738. |
| [12] | 李兆龙, 孙华君, 徐 杰, 张晓艳, 黄胜男, 朱泉峣, 陈 文, Galina S. Zakharova. Mn2+掺杂对V2O5纳米材料结构与电化学性能的影响[J]. 无机材料学报, 2013, 28(11): 1200-1206. |
| [13] | 刘 林, 方有维, 邓新峰, 庄文东, 唐 斌, 张树人. (Ba1-xSrx)La4Ti4O15(x=0.8~0.95)陶瓷的微结构及微波介电性能研究[J]. 无机材料学报, 2012, 27(3): 281-284. |
| [14] | 姚春发, 李财富, 刘志权, 尚建库. (1-x)Pb(Fe2/3W1/3)O3-xPb(Mg1/2W1/2)O3多铁性材料的有序结构及磁性能[J]. 无机材料学报, 2011, 26(6): 649-654. |
| [15] | 侯育冬,崔 磊,王 赛,王 超,朱满康,严 辉. BiAlO3基高温无铅压电陶瓷的研究进展[J]. 无机材料学报, 2010, 25(3): 225-229. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||