无机材料学报 ›› 2022, Vol. 37 ›› Issue (4): 436-444.DOI: 10.15541/jim20210158
唐洁吟1,2( ), 王刚3,4, 刘聪1,2, 邹学农3,4, 陈晓峰1,2(
), 王刚3,4, 刘聪1,2, 邹学农3,4, 陈晓峰1,2( )
)
收稿日期:2021-03-12
修回日期:2021-04-08
出版日期:2022-04-20
网络出版日期:2021-04-30
通讯作者:
陈晓峰, 教授. E-mail: chenxf@scut.edu.cn作者简介:唐洁吟(1989-), 女, 硕士. E-mail: 1720175343@qq.com
基金资助:
TANG Jieyin1,2( ), WANG Gang3,4, LIU Cong1,2, ZOU Xuenong3,4, CHEN Xiaofeng1,2(
), WANG Gang3,4, LIU Cong1,2, ZOU Xuenong3,4, CHEN Xiaofeng1,2( )
)
Received:2021-03-12
Revised:2021-04-08
Published:2022-04-20
Online:2021-04-30
Contact:
CHEN Xiaofeng, professor. E-mail: chenxf@scut.edu.cnAbout author:TANG Jieyin (1989-), female, Master. E-mail: 1720175343@qq.com
Supported by:摘要:
封闭牙本质小管能有效减轻牙齿过敏症。本研究以不同粒径的微纳米生物活性玻璃球(MNBGs)为分散质、海藻酸钠-磷酸盐缓冲溶液为分散液, 制备了用于牙本质脱敏治疗的MNBGs糊剂(MNBGP)。在牙本质切片表面进行体外矿化并系统评价了糊剂与牙本质的结合性能, 以及糊剂体外诱导牙本质再矿化、封闭牙本质小管的能力。研究结果表明, 不同粒径MNBGs制备的糊剂均能与牙本质界面紧密结合, 粒径较小的MNBGs在脱矿牙本质切片表面分布更加均匀。MNBGP在人工唾液(AS)中能较好地诱导牙本质再矿化形成磷灰石(HA)以堵塞封闭牙本质小管, 脱矿牙本质切片表面形成的HA 层随矿化时间延长而增厚, 矿化28 d HA层的厚度可达到5~10 μm。MNBGs的尺寸影响其诱导牙本质再矿化的效果, 当颗粒大小与牙本质小管直径匹配时, MNBGs可以更好地封闭牙本质小管。因此, MNBGP具有良好的治疗牙本质过敏的应用前景。
中图分类号:
唐洁吟, 王刚, 刘聪, 邹学农, 陈晓峰. 微纳米生物活性玻璃诱导牙本质再矿化研究[J]. 无机材料学报, 2022, 37(4): 436-444.
TANG Jieyin, WANG Gang, LIU Cong, ZOU Xuenong, CHEN Xiaofeng. Dentin Remineralization Induced by Micro-nano Bioactive Glass Spheres[J]. Journal of Inorganic Materials, 2022, 37(4): 436-444.
| Sample | SiO2 : CaO : P2O5 (molar ratio) | DDA/g | TEOS/mL | |
|---|---|---|---|---|
| Theoretical | Measured | |||
| MNBGs-1 | 80 : 16 : 4 | 87.637 : 12.363 : 0 | 3 | 8 |
| MNBGs-2 | 86.289 : 13.696 : 0.015 | 6 | 16 | |
| MNBGs-3 | 89.071 : 10.875 : 0.054 | 6 | 24 | |
表1 不同粒径MNBGs的理论、实际化学组分和试剂用量
Table 1 Theoretical and measured chemical composition, reagent dosage of MNBGs with different particle sizes
| Sample | SiO2 : CaO : P2O5 (molar ratio) | DDA/g | TEOS/mL | |
|---|---|---|---|---|
| Theoretical | Measured | |||
| MNBGs-1 | 80 : 16 : 4 | 87.637 : 12.363 : 0 | 3 | 8 |
| MNBGs-2 | 86.289 : 13.696 : 0.015 | 6 | 16 | |
| MNBGs-3 | 89.071 : 10.875 : 0.054 | 6 | 24 | |
| Samples | C/% | O/% | Ca/% | P/% | Ca/P |
|---|---|---|---|---|---|
| Without etching | 0 | 65.77 | 20.26 | 13.97 | 1.45 |
| With etching | 66.82 | 32.99 | 0.05 | 0.14 | 0.35 |
表2 牙本质切片表面EDTA酸蚀前后各元素含量(摩尔分数)及钙磷比
Table 2 Chemical components (molar percent) and Ca/P ratio on the surface of dentin before and after EDTA etching
| Samples | C/% | O/% | Ca/% | P/% | Ca/P |
|---|---|---|---|---|---|
| Without etching | 0 | 65.77 | 20.26 | 13.97 | 1.45 |
| With etching | 66.82 | 32.99 | 0.05 | 0.14 | 0.35 |
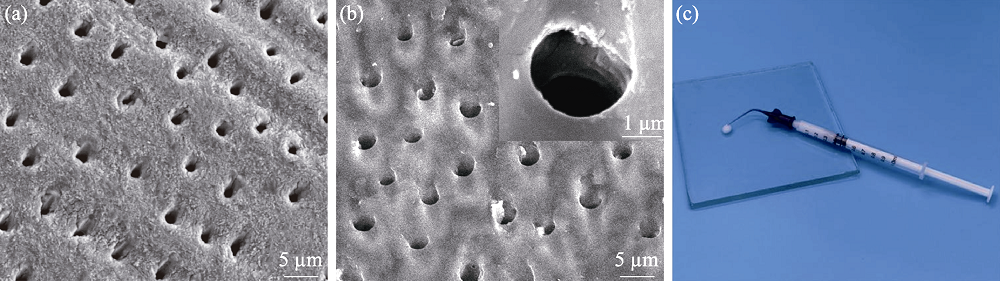
图3 牙本质切片样品酸蚀前(a)后(b)的SEM照片(插图为牙本质小管放大照片)及生物活性玻璃糊剂实物照片(c)
Fig. 3 SEM images of the dentin surface without (a) and with (b) EDTA-etching, magnified photo of dentin tubules (insert in (b)), and photo of bioactive glass paste (c)
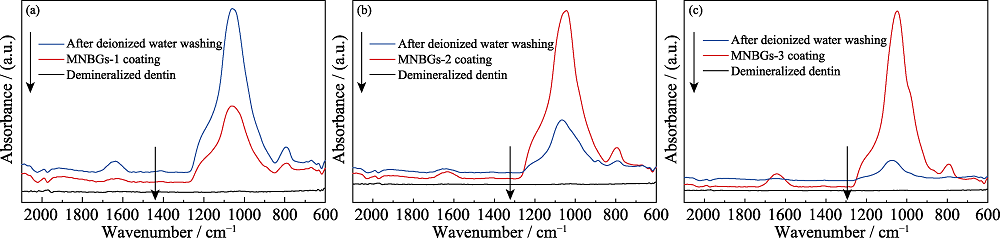
图5 脱矿牙本质切片表面涂覆不同MNBGP前后, 以及去离子水冲洗后的全反射-红外光谱
Fig. 5 ATR-FTIR spectra of demineralized dentin surface before and after coating with MNBGP, and after rinsing with water (a) MNBGP-1; (b) MNBGP-2; (c) MNBGP-3
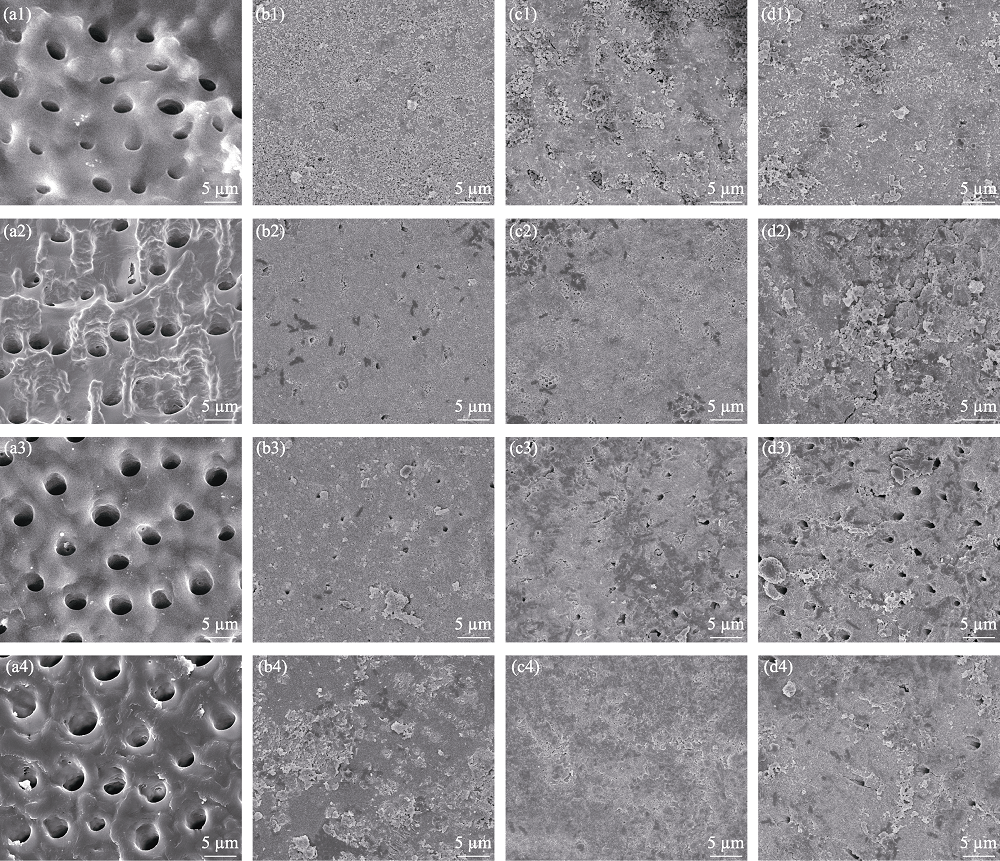
图6 未经材料处理(对照组)(a)和涂覆MNBGP-1(b)、MNBGP-2(c)、MNBGP-3(d)的脱矿牙本质切片在人工唾液中浸泡1 d (a1~d1)、7 d (a2~d2)、14 d (a3~d3)和28 d (a4~d4)后的表面SEM照片
Fig. 6 SEM images of the surfaces of demineralized dentin slices without (a) (control) and with treatment by MNBGP-1 (b), MNBGP-2 (c), and MNBGP-3 (d) after soaking in AS for 1 d (a1-d1), 7 d (a2-d2), 14 d (a3-d3) and 28 d (a4-d4)

图7 未经材料处理(a)和涂覆MNBGP-1(b)、MNBGP-2(c)、MNBGP-3(d)的脱矿牙本质切片在人工唾液中浸泡1 d (a1~d1)、7 d (a2~d2)、14 d (a3~d3)和28 d (a4~d4)后的纵截面的SEM照片
Fig. 7 SEM images of the longitudinal section of demineralized dentin samples without (a) (control) and with treatment by MNBGP-1 (b), MNBGP-2 (c), and MNBGP-3 (d) after soaking in AS for 1 d (a1-d1), 7 d (a2-d2), 14 d (a3-d3) and 28 d (a4-d4)
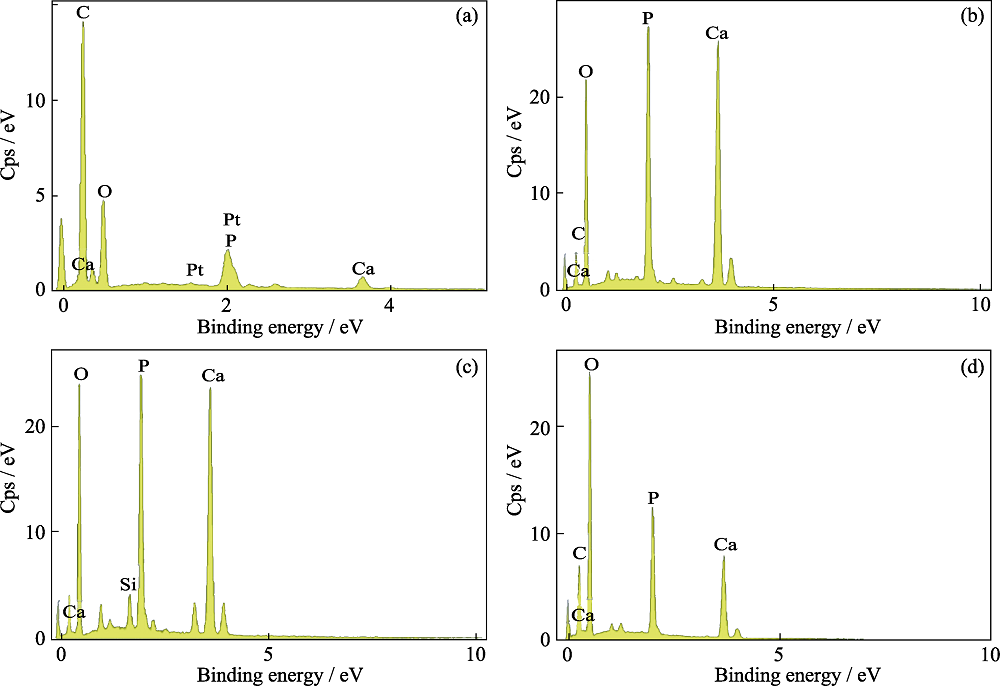
图8 对照组(a)和MNBGP-1(b)、MNBGP-2(c)、MNBGP-3(d)涂覆的脱矿牙本质切片在人工唾液中矿化28 d表面的EDS能谱分析
Fig. 8 EDS analyses of the surface of demineralized dentin slices without (a) (control) and with treatment by MNBGP-1 (b), MNBGP-2 (c), and MNBGP-3 (d) after soaking in AS for 28 d
| Sample | Ca/% | P/% | Ca/P |
|---|---|---|---|
| Control | 2.49 | 1.82 | 1.37 |
| MNBGP-1 | 16.64 | 11.69 | 1.42 |
| MNBGP-2 | 18.37 | 12.97 | 1.42 |
| MNBGP-3 | 6.16 | 6.12 | 1.01 |
表3 脱矿牙本质切片表面钙、磷元素含量(摩尔分数)及钙磷比
Table 3 Chemical components ( molar percent) and Ca/P ratio in molar on the surface of remineralized dentin
| Sample | Ca/% | P/% | Ca/P |
|---|---|---|---|
| Control | 2.49 | 1.82 | 1.37 |
| MNBGP-1 | 16.64 | 11.69 | 1.42 |
| MNBGP-2 | 18.37 | 12.97 | 1.42 |
| MNBGP-3 | 6.16 | 6.12 | 1.01 |
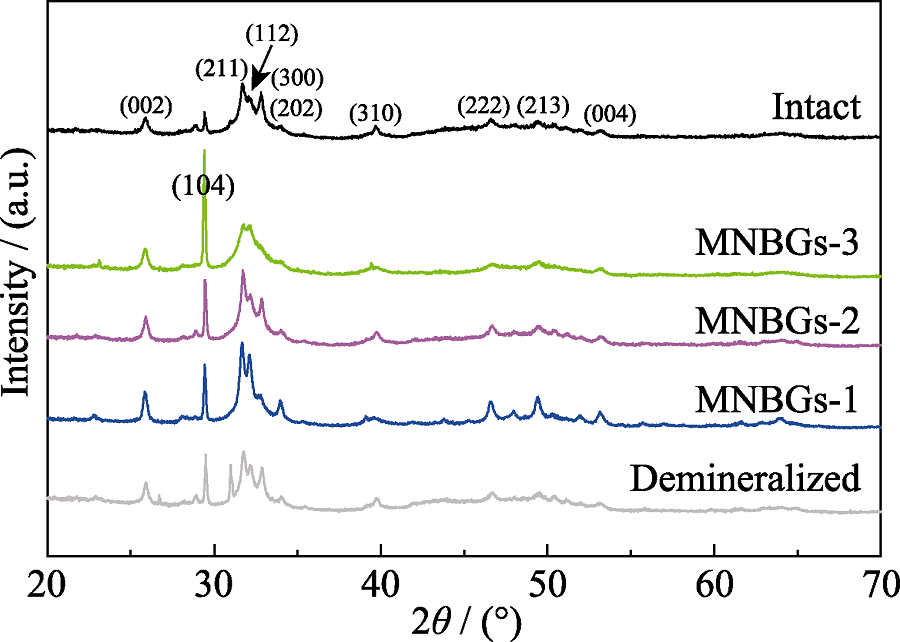
图9 天然牙本质、脱矿牙本质和在人工唾液中浸泡28 d的脱矿牙本质切片样品表面的XRD 图谱
Fig. 9 XRD patterns of the surface of intact dentin, demineralized dentin and slices without treatment (control) and being treated with MNBGP after being soaked in AS for 28 d
| [1] |
HOLLAND G R, NARHI M N, ADDY M, et al. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. Journal of Clinical Periodontology, 1997, 24(11): 808-813.
DOI URL |
| [2] | ADDY M, WEST N X. The role of toothpaste in the aetiology and treatment of dentine hypersensitivity. Monogr. Oral. Sci., 2013, 23: 75-87. |
| [3] |
ARNOLD W H, PRANGE M, NAUMOVA E A. Effectiveness of various toothpastes on dentine tubule occlusion. Journal of Dentistry, 2015, 43(4): 440-449.
DOI URL |
| [4] | BERKATHULLAH M, FAROOK M S, MAHMOUD O. The effectiveness of remineralizing agents on dentinal permeability. BioMed Research International, 2018, 2018: 4072815. |
| [5] |
BAINO F, HAMZEHLOU S, KARGOZAR S. Bioactive glasses: where are we and where are we going? Journal of Functional Biomaterials, 2018, 9(1):25.
DOI URL |
| [6] |
JONES J R. Review of bioactive glass: from Hench to hybrids. Acta Biomaterialia, 2013, 9(1): 4457-4486.
DOI URL |
| [7] |
SKALLEVOLD H E, ROKAYA D, KHURSHID Z, et al. Bioactive glass applications in dentistry. International Journal of Molecular Sciences, 2019, 20(23):5960.
DOI URL |
| [8] | LITKOWSKI L, GREENSPAN D C. A clinical study of the effect of calcium sodium phosphosilicate on dentin hypersensitivity-proof of principle. J. Clin. Dent., 2010, 21(3): 77-81. |
| [9] | GENDREAU L, BARLOW A P, MASON S C. Overview of the clinical evidence for the use of NovaMin in providing relief from the pain of dentin hypersensitivity. J. Clin. Dent., 2011, 22(3): 90-95. |
| [10] |
HOFFMAN D A, CLARK A E, RODY W J, et al. A prospective randomized clinical trial into the capacity of a toothpaste containing NovaMin to prevent white spot lesions and gingivitis during orthodontic treatment. Progress in Orthodontics, 2015, 16(1):25.
DOI URL |
| [11] | FAROOQ I, MAJEED A, ALSHWAIMI E, et al. Efficacy of a novel fluoride containing bioactive glass based dentifrice in remineralizing artificially induced demineralization in human enamel. Fluoride, 2019, 52(3): 447-455. |
| [12] |
WANG Z, SA Y, SAURO S, et al. Effect of desensitising toothpastes on dentinal tubule occlusion: a dentine permeability measurement and SEM in vitro study. Journal of Dentistry, 2010, 38(5): 400-410.
DOI URL |
| [13] |
VOLLENWEIDER M, BRUNNER T J, KNECHT S, et al. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater., 2007, 3(6): 936-943.
DOI URL |
| [14] |
CURTIS A R, WEST N X, SU B. Synthesis of nanobioglass and formation of apatite rods to occlude exposed dentine tubules and eliminate hypersensitivity. Acta Biomater., 2010, 6(9): 3740-3746.
DOI URL |
| [15] |
SHENG X Y, GONG W Y, HU Q, et al. Mineral formation on dentin induced by nano-bioactive glass. Chinese Chemical Letters, 2016, 27(9): 1509-1514.
DOI URL |
| [16] |
FERNANDES H R, GADDAM A, REBELO A, et al. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials, 2018, 11(12):2530.
DOI URL |
| [17] |
ZHENG K, BOCCACCINI A R. Sol-Gel processing of bioactive glass nanoparticles: a review. Advances in Colloid and Interface Science, 2017, 249: 363-373.
DOI URL |
| [18] |
HU Q, CHEN X, ZHAO N, et al. Fabrication and characterization of dodecylamine derived monodispersed mesoporous bioactive glass sub-micron spheres. Journal of Sol-Gel Science and Technology, 2014, 69(1): 9-16.
DOI URL |
| [19] |
LIANG K, GAO Y, LI J, et al. Effective dentinal tubule occlusion induced by polyhydroxy-terminated PAMAM dendrimer in vitro. RSC Advances, 2014, 4(82): 43496-43503.
DOI URL |
| [20] |
GAL J Y, FOVET Y, ADIB-YADZI M. About a synthetic saliva for in vitro studies. Talanta, 2001, 53(6): 1103-1115.
DOI URL |
| [21] |
SCHUMACHER M, HABIBOVIC P, van RIJT S. Mesoporous bioactive glass composition effects on degradation and bioactivity. Bioactive Materials, 2021, 6(7): 1921-1931.
DOI URL |
| [22] |
ZHANG X, ZENG D, LI N, et al. Large-pore mesoporous Ca-Si-based bioceramics with high in vitro bioactivity and protein adsorption capability for bone tissue regeneration. Journal of Materials Chemistry B, 2016, 4(22): 3916-3924.
DOI URL |
| [23] |
XU Z, NEOH K G, KISHEN A. A biomimetic strategy to form calcium phosphate crystals on type I collagen substrate. Materials Science and Engineering: C, 2010, 30(6): 822-826.
DOI URL |
| [24] |
ZHAO F, ZHANG W, FU X, et al. Fabrication and characterization of bioactive glass/alginate composite scaffolds by a self-crosslinking processing for bone regeneration. RSC Advances, 2016, 6(94): 91201-91208.
DOI URL |
| [25] |
XU C, WANG X, ZHOU J, et al. Bioactive tricalcium silicate/alginate composite bone cements with enhanced physicochemical properties. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2018, 106(1): 237-244.
DOI URL |
| [26] |
MARELLI B, GHEZZI C E, BARRALET J E, et al. Three-dimensional mineralization of dense nanofibrillar collagen- bioglass hybrid scaffolds. Biomacromolecules, 2010, 11(6): 1470-1479.
DOI URL |
| [27] |
FAN Y, SUN Z, MORADIAN-OLDAK J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials, 2009, 30(4): 478-483.
DOI URL |
| [28] |
DAI L L, MEI M L, CHU C H, et al. Remineralizing effect of a new strontium-doped bioactive glass and fluoride on demineralized enamel and dentine. Journal of Dentistry, 2021, 108: 103633.
DOI URL |
| [29] |
WU Y, TANG L, ZHANG Q, et al. A novel synthesis of monodispersed bioactive glass nanoparticles via ultrasonic-assisted surfactant-free microemulsion approach. Materials Letters, 2021, 285: 129053.
DOI URL |
| [30] |
NIRMALA DEVI M, SANJIV RAJ K, SUBRAMANIAN V K. Synergistic effects of magnesium and EDTA on polymorphism and morphology of CaCO3 and its influence on scale. Journal of Crystal Growth, 2021, 564: 126108.
DOI URL |
| [1] | 马磊, 黄毅, 邓浩, 银航, 田强, 晏敏皓. 氟磷灰石对酸性水溶液中铀(VI)的去除研究[J]. 无机材料学报, 2022, 37(4): 395-403. |
| [2] | 陈亚玲, 舒松, 王劭鑫, 李建军. Mn-HAP基低温SCR催化剂的制备及抗硫中毒性能[J]. 无机材料学报, 2022, 37(10): 1065-1072. |
| [3] | 朱雨桐, 谭佩洁, 林海, 朱向东, 张兴栋. 可注射透明质酸/羟基磷灰石复合材料: 制备、理化性能和细胞相容性[J]. 无机材料学报, 2021, 36(9): 981-990. |
| [4] | 林子扬, 常宇辰, 吴章凡, 包荣, 林文庆, 王德平. 不同模拟体液对硼硅酸盐生物活性玻璃基骨水泥矿化性能的影响[J]. 无机材料学报, 2021, 36(7): 745-752. |
| [5] | 吴永豪, 李向锋, 朱向东, 张兴栋. 高强度羟基磷灰石纳米陶瓷的构建及其促成骨细胞活性研究[J]. 无机材料学报, 2021, 36(5): 552-560. |
| [6] | 宋可可, 黄浩, 鲁梦婕, 杨安春, 翁杰, 段可. 水热制备锌、硅、镁、铁等元素掺杂羟基磷灰石及其表征[J]. 无机材料学报, 2021, 36(10): 1091-1096. |
| [7] | 邵悦婷, 朱英杰, 董丽颖, 蔡安勇. 羟基磷灰石超长纳米线/植物纤维纳米复合“宣纸”及其防霉性能[J]. 无机材料学报, 2021, 36(1): 107-112. |
| [8] | 孙团伟,朱英杰. 一步溶剂热法合成锶掺杂羟基磷灰石超长纳米线[J]. 无机材料学报, 2020, 35(6): 724-728. |
| [9] | 刘子阳, 耿振, 李朝阳. 牡蛎壳为原料制备医用CaCO3/HA复合生物材料[J]. 无机材料学报, 2020, 35(5): 601-607. |
| [10] | 代钊,王铭,王双,李静,陈翔,汪大林,祝迎春. 氧化锆基微量元素共掺杂羟基磷灰石增韧涂层研究[J]. 无机材料学报, 2020, 35(2): 179-186. |
| [11] | 付亚康,翁杰,刘耀文,张科宏. 钛网表面含hBMP-2的复合涂层制备及hBMP-2的释放研究[J]. 无机材料学报, 2020, 35(2): 173-178. |
| [12] | 胡亚萍, 田正芳, 朱敏, 朱钰方. 喷雾干燥可控制备生物玻璃微球及其体外生物活性研究[J]. 无机材料学报, 2020, 35(11): 1268-1276. |
| [13] | 周子航, 王群, 葛翔, 李朝阳. 掺锶羟基磷灰石纳米颗粒的合成、表征及模拟研究[J]. 无机材料学报, 2020, 35(11): 1283-1289. |
| [14] | 肖文谦,张静,李克江,邹新宇,蔡昱东,李波,刘雪,廖晓玲. 荔枝状CaCO3@HA/Fe3O4磁性介孔多级微球的制备[J]. 无机材料学报, 2019, 34(9): 925-932. |
| [15] | 吴金结, 李艳, 魏仁初, 汪建新, 屈树新, 翁杰, 智伟. 微振动应力环境影响羟基磷灰石陶瓷生物活性及力学稳定性的体外评价[J]. 无机材料学报, 2019, 34(4): 417-424. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||