无机材料学报 ›› 2022, Vol. 37 ›› Issue (4): 413-419.DOI: 10.15541/jim20210139
收稿日期:2021-03-10
修回日期:2021-04-01
出版日期:2022-04-20
网络出版日期:2021-04-30
通讯作者:
郑遗凡, 教授. E-mail: Zhengyifan@zjut.edu.cn作者简介:吴秋琴(1996-), 女, 硕士研究生. E-mail: wuqiuqin0601@foxmail.com
基金资助:
WU Qiuqin1,2( ), YAO Fenfa2, JIN Chuanhong2, ZHENG Yifan1(
), YAO Fenfa2, JIN Chuanhong2, ZHENG Yifan1( )
)
Received:2021-03-10
Revised:2021-04-01
Published:2022-04-20
Online:2021-04-30
Contact:
ZHENG Yifan, professor. E-mail: Zhengyifan@zjut.edu.cnAbout author:WU Qiuqin (1996-), female, Master candidate. E-mail: wuqiuqin0601@foxmail.com
Supported by:摘要:
超细亚化学计量比过渡金属氧化物纳米材料的可控制备具有重要应用价值, 但其可控合成仍存在挑战。本研究提出了基于碳纳米管的限域空间合成法, 首先采用溶液法将四硫代钨酸铵((NH4)2WS4)填充至碳管内, 然后真空热分解反应制得产物。通过表征发现: 碳管内限域生长的主要产物为一维亚氧化物W3O8纳米线, 其典型线宽为1.23~1.93 nm, 对应4~5列钨氧原子链, 长度可达数十微米。W3O8纳米线与碳管内壁之间主要为范德华力相互作用(间距约0.35 nm), 为分离及表征W3O8纳米线的本征特性提供了方便, 该方法也有望应用于合成其他亚化学计量比的一维过渡金属氧化物纳米线。
中图分类号:
吴秋琴, 姚奋发, 金传洪, 郑遗凡. 碳纳米管内填充生长超细一维亚化学计量比氧化钨纳米线[J]. 无机材料学报, 2022, 37(4): 413-419.
WU Qiuqin, YAO Fenfa, JIN Chuanhong, ZHENG Yifan. One-dimensional Sub-stoichiometric W3O8 Nanowires Filled Carbon Nanotubes[J]. Journal of Inorganic Materials, 2022, 37(4): 413-419.
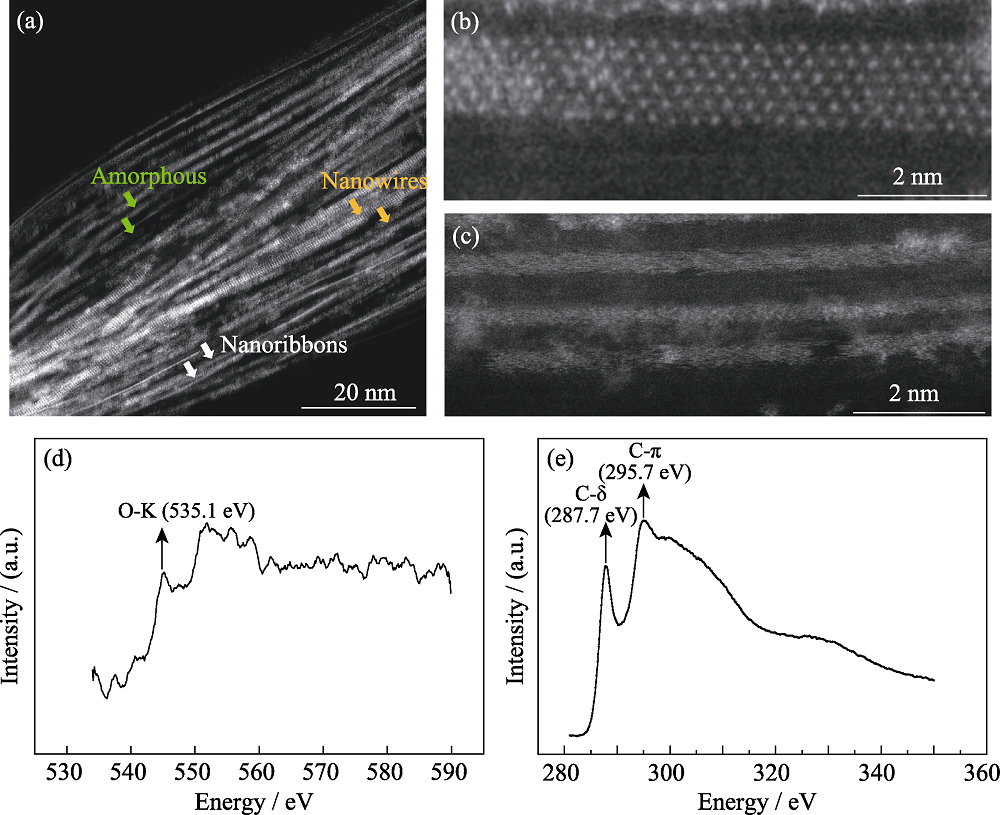
图3 充在碳纳米管内的三种结构ADF-STEM照片(a, b, c)和电子能量损失谱((EELS)(d, e)
Fig. 3 (a, b, c) ADF-STEM images of three types of filled structures inside the CNTs. and (d, e) EELS spectra of oxygen and carbon elements Zoom-in ADF-STEM image of WS2 nanoribbons@CNTs (b) and amorphous (c), respectively
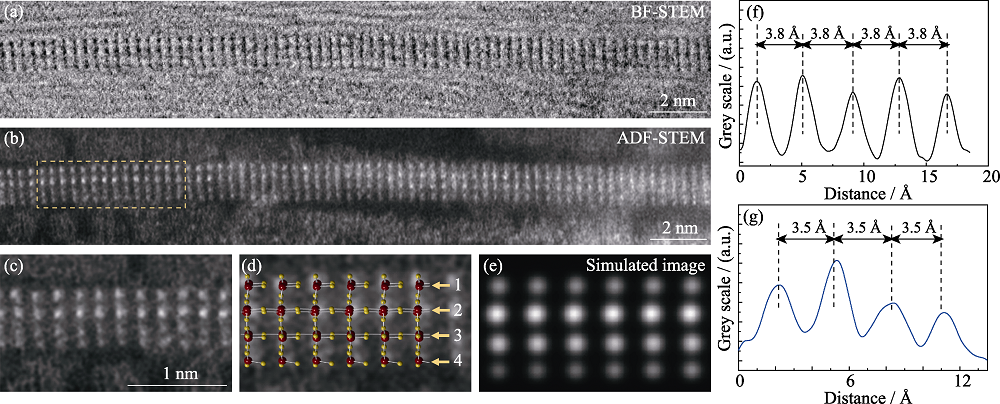
图4 填充在碳管内W3O8纳米线的原子结构图
Fig. 4 Atomic structure of an individual W3O8 nanowire filled inside CNT (a-b) BF-STEM and ADF-STEM images of an individual W3O8 nanowire filled inside CNT; (c) Zoom in ADF-STEM image of the dashed yellow rectangle in (b); (d-e) Corresponding atomic model and simulated ADF-STEM image of (c); (f-g) Intensity line profiles along the perpendicular and parallel to axial directions of the CNT (1 Å=0.1 nm)
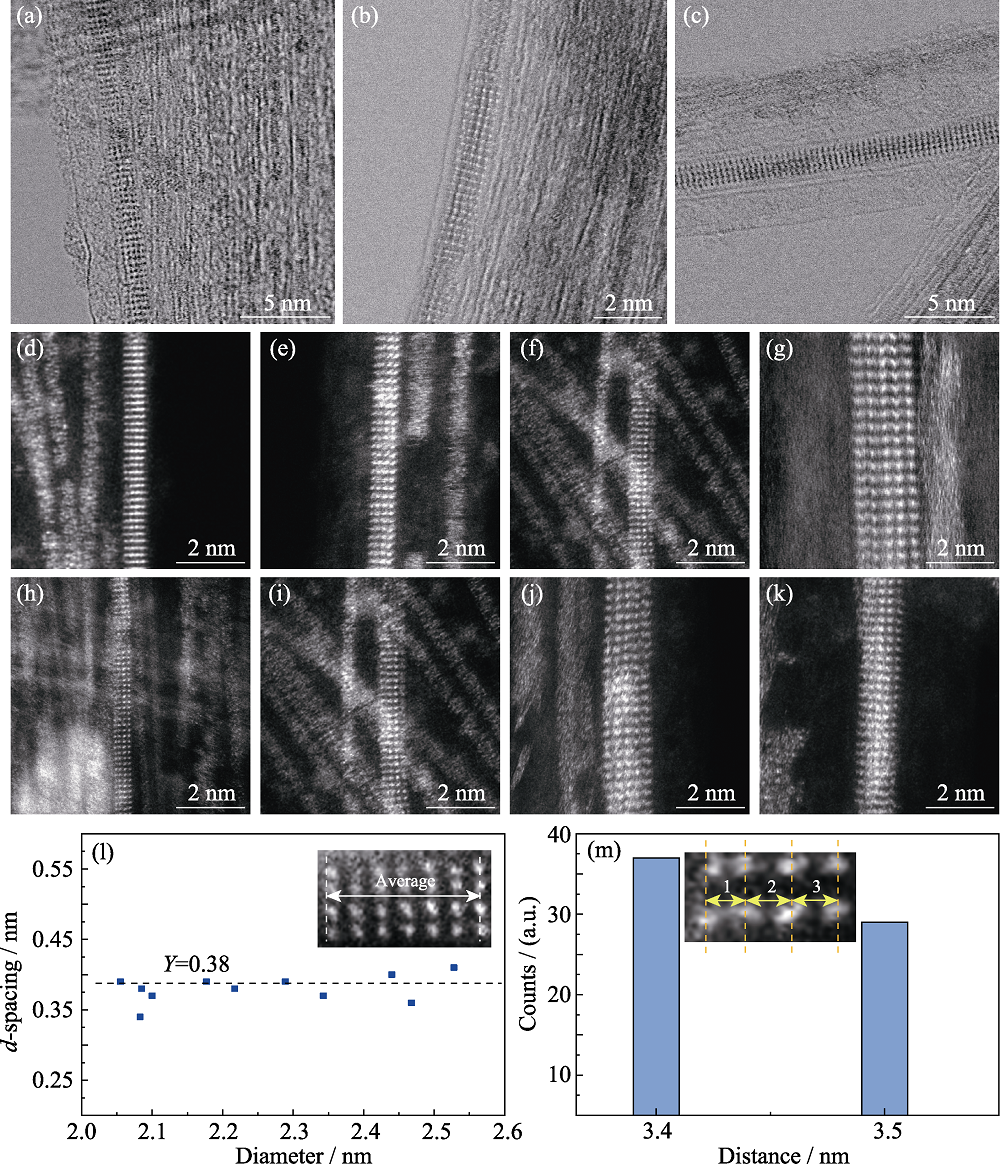
图5 两种直径W3O8链的ADF-STEM表征分析
Fig. 5 ADF-STEM analyses of W3O8 nanowires with different diameters (a-k) ADF-STEM images of W3O8 nanowires with different diameters; (l, m) Distance distributions of the W3O8 nanowire parallel and perpendicular to the axial of the CNT
| [1] |
CHOI H G, JUNG Y H, KIM D K. Solvothermal synthesis of tungsten oxide nanorod/nanowire/nanosheet. Journal of the American Ceramic Society, 2005, 88(6): 1684-1686.
DOI URL |
| [2] |
SHIM H S, KIM J W, SUNG Y E, et al. Electrochromic properties of tungsten oxide nanowires fabricated by electrospinning method. Solar Energy Materials and Solar Cells, 2009, 93(12): 2062-2068.
DOI URL |
| [3] | 饶峰, 汪建勋, 韩高荣. 电子束蒸发制备氧化钨、氧化镍薄膜的电致变色性能. 材料科学与工程学报, 2004, 22(5): 683-687. |
| [4] |
WANG S F, FAN W R, LIU Z C, et al. Advances on tungsten oxide based photochromic materials: strategies to improve their photochromic properties. Journal of Materials Chemistry C, 2018, 6(2): 191-212.
DOI URL |
| [5] |
MOVLAEE K, PERIASAMY P, KRISHNAKUMAR T, et al. Microwave-assisted synthesis and characterization of WOx nanostructures for gas sensor application. Journal of Alloys and Compounds, 2018, 762: 745-753.
DOI URL |
| [6] |
SOLIS J, SAUKKO S, KISH L, et al. Semiconductor gas sensors based on nanostructured tungsten oxide. Thin Solid Films, 2001, 391(2): 255-260.
DOI URL |
| [7] |
WANG F Y, SONG L F, ZHANG H C, et al. One-dimensional metal-oxide nanostructures for solar photocatalyticwater-splitting. Journal of Electronic Materials, 2017, 46(8): 4716-4724.
DOI URL |
| [8] |
QUAN H, GAO Y, WANG W. Tungsten oxide-based visible light-driven photocatalysts: crystal and electronic structures and strategies for photocatalytic efficiency enhancement. Inorganic Chemistry Frontiers, 2020, 7(4): 817-838.
DOI URL |
| [9] |
LEE J, JO C S, PARK B. Simple fabrication of flexible electrodes with high metal-oxide content: electrospun reduced tungsten oxide/carbon nanofibers for lithium ion battery application. Nanoscale, 2014, 6: 10147-10155.
DOI URL |
| [10] | MIGAS D B, SHAPOSHNIKOV V L, BORISISENKO V E. Tungsten oxides. II. The metallic nature of Magnéli phases. Journal of Applied Physics, 2010, 108(9): 093714-1-6. |
| [11] |
HUANG X, ZHAI H J, LI J, et al. On the structure and chemical bonding of tri-tungsten oxide cluster W3On- and W3On (n=7-10): W3O8 as a potential model for O-deficient defect sites in tungsten oxides. Journal of Physics and Chemistry A, 2006, 110: 85-92.
DOI URL |
| [12] |
QIN Y X, XIE W W, LIU Y, et al. Thermal-oxidative growth of aligned W18O49 nanowire arrays for high performance gas sensor. Sensors and Actuators B: Chemical, 2016, 223: 487-495.
DOI URL |
| [13] |
CHATTEN R, CHADWICK A V, ROUGER A, et al. The oxygen vacany in crystal phases of WO3. Journal of Physics and Chemistry B, 2005, 109: 3146-3156.
DOI URL |
| [14] |
ZHENG H D, OUJ Z, STRANO M S, et al. Nanostructured tungsten oxide-properties, synthesis, and applications. Advanced Functional Materials, 2011, 21(12): 2175-2196.
DOI URL |
| [15] | WANG F G, VALENTIN D C, PACCHIONI G. Semiconductor- to-metal transition in WO3-x: nature of the oxygen vacancy. Physical Review B, 2011, 84(7): 073103-1-3. |
| [16] |
WANG S F, FAN W E, LIU Z C, et al. Advances on tungsten oxide based photochromic materials: strategies to improve their photochromic properties. Journal of Materials Chemistry C, 2018, 6: 191-212.
DOI URL |
| [17] |
HE T, YAO J N. Photochromic materials based on tungsten oxide. Journal of Materials Chemistry, 2007, 17: 4547-4557.
DOI URL |
| [18] |
VEMURIER S, BHARATHI K K, GULLAPLLI S K, et al. Effect of structure and size on the electrical of nanocrystalline WO3 films. ACS Applied Materials Interfaces, 2006, 2(9): 2623-2628.
DOI URL |
| [19] |
POLACZEK A, PEKATA M, OBUSZKO Z, et al. Magnetic susceptibility and thermoelectric power of tungsten intermediary oxides. Journal of Physics: Condensed Matter, 1994, 6: 7909-7919.
DOI URL |
| [20] |
HU R, WU H S, HONG K Q. Growth of uniform tungsten oxide nanowires with small diameter via a two-step heating process. Journal of Crystal Growth, 2007, 306(2): 395-399.
DOI URL |
| [21] |
BAEK Y, YONG K. Controlled growth and characterization of tungsten oxide nanowires using thermal evaporation of WO3 powder. The Journal of Physical Chemistry C, 2007, 111(3): 1213-1218.
DOI URL |
| [22] |
VADDIRAJU S, CHANDRASEKARANh H, SUNKARA M K. Vapor phase synthesis of tungsten nanowires. Journal of the American Chemical Society, 2003, 125(36): 10792-10793.
DOI URL |
| [23] |
MIAO B, ZENG W, HUSSAIN S, et al. Large scale hydrothermal synthesis of monodisperse hexagonal WO3nanowire and the growth mechanism. Materials Letters, 2015, 147: 12-15.
DOI URL |
| [24] |
ZHOU J, YAN Y, ZHANG C Y. A low-temperature solid-phase method to synthesize highly fluorecent carbon nitride dots with tunable emission. Chemical Communication, 2013, 49: 8605-8607.
DOI URL |
| [25] |
GUO C, YIN S, YAN M, et al. Morphology-controlled synthesis of W18O49 nanostructures and their near-infrared absorption properties. Inorganic Chemistry, 2012, 51(8): 4763-4771.
DOI URL |
| [26] |
CHAKRAPANI V. Modulation of stoichiometry, morphology and composition of transition metal oxide nanostructure through hot wire chemical vapor deposition. Journal of Materials Research, 2006, 31(1): 17-25.
DOI URL |
| [27] | WU C M, NASEEM S, CHOU M H, et al. Recent advance in tungsten oxide based materials and their applications. Frontier in Materials, 2019, 6: 49-1-17. |
| [28] | DAI T T, DENG Z H, MENG G, et al. Controllable synthesis and gas sensing properties of bridged tungsten oxide nanowires. Acta Physico-Chimica Sinica, 2021, 37(10): 1911036-1-11. |
| [29] |
LI Y, BANDO Y, GOLBERG D. Quasi-aligned single-crystalline W18O49 nanotubes and nanowires. Advanced Materials, 2003, 15(15): 1294-1296.
DOI URL |
| [30] |
GUANG C X, OUYANG S X, LI P, et al. Ultrathin W18O49nanowires with diameter below 1 nm: synthesis, near-infrared absorption, photoluminescence, and photochemical reduction of carbon dioxide. Angewandte Chemie International Edition, 2012, 51(10): 2395-2399.
DOI URL |
| [31] |
GU H X, GUO C S, ZHANG S H, et al. Highly efficient, near-infrared and visible light modulated electrochromic devices based on polyoxometalates and W18O49 nanowires. ACS Nano, 2018, 12(1): 559-567.
DOI URL |
| [32] |
IIJIMA S. Helical microtubes of graphitic carbon. Nature, 1991, 354(7): 56-58.
DOI URL |
| [33] |
NIE C, GALIBERT A M, SOULA B, et al. A new insight on the mechanisms of filling closed carbon nanotubes with molten metal iodides. Carbon, 2016, 110: 48-50.
DOI URL |
| [34] |
ANOSHKIN I V, TALYZIN A V, NASIBULIN A G, et al. Coronene Encapsulation in single-walled carbon nanotubes: stacked columns, peapods, and nanoribbons. ChemPhysChem, 2014, 15(8): 1660-1665.
DOI URL |
| [35] |
CHAMBERLAIN T W, BISKUPEK J, RANCE G A, et al. Size, structure, and helical twist of graphenenanoribbons controlled by confinement in carbon nanotubes. ACS Nano, 2012, 6(5): 3943-3953.
DOI URL |
| [36] |
NAGATA M, SHUKLA S, NAKANISHI Y, et al. Isolation of single-wired transition-metal monochalcogenidesby carbon nanotubes. Nano Letters, 2019, 19(8): 4845-4851.
DOI URL |
| [37] |
HIRAHARA K, SUENAGA K, BANDOW S, et al. One- dimensional metallofullerene crystal generated inside single-walled carbon nanotubes. Physical Review Letters, 2000, 85(25): 5384-5387.
DOI URL |
| [38] |
GAO H J, KONG Y, CUI D X, et al. Spontaneous insertion of DNA oligonucleotides into carbon nanotubes. Nano Letters, 2003, 3(4): 471-473.
DOI URL |
| [39] | 王治宇, 赵宗彬, 邱介山. 碳纳米管填充技术研究. 化学进展, 2006, 5(18): 564-571. |
| [40] |
GAUTAM U K, COSTA P M, BANDO Y, et al. Recent developments in inorganically filled carbon nanotubes: successes and challenges. Science and Technology of Advanced Materials, 2010, 11(5):054501.
DOI URL |
| [41] |
ANDREI A E, NIKOLAY S F, NIKOLAY I V, et al. Size- dependent structure relation between nanotubes and encapsulated nanocrystal. Nano Letters, 2005, 17: 805-810.
DOI URL |
| [42] |
SCHNIZLER M C, OLIVEIRA M M, UGARTE D, et al. One-step route to iron oxide-filled carbon nanotubes and bucky-onions based on the pyrolysis of organmetallic precursors. Chemical Physics Letters, 2003, 381: 541-548.
DOI URL |
| [43] |
HOU P X, LIU C, CHENG H M. Purification of carbon nanotubes. Carbon, 2008, 46(15): 2003-2025.
DOI URL |
| [44] |
AJAYAN P, EBBESEN T, ICHIHASHI T, et al. Opening carbon nanotubes with oxygen and implications for filling. Nature, 1993, 362(6420): 522-525.
DOI URL |
| [45] |
张莹莹, 张锦. 共振增强拉曼光谱技术在单壁碳纳米管表征中的应用. 化学学报, 2012, 70(22): 2293-2305.
DOI |
| [46] | 卢东昱, 陈建, 周军, 等. 氧化钨纳米线结构相变的拉曼光谱研究. 光散射学报, 2006, 18(2): 121-123. |
| [47] |
LI L J, LIN T W, DOIG J, et al. Crystal-encapsulation-induced band-structure change in single-walled carbon nanotubes: photoluminescence and Raman spectra. Physical Review B, 2006, 74(24):245414.
DOI URL |
| [48] |
WANG Z Y, ZHAO K K, LI H, et al. Ultra-narrow WS2nanoribbons encapsulated in carbon nanotubes. Journal of Materials Chemistry, 2011, 21(1): 171-180.
DOI URL |
| [49] |
PENNYCOOK S. Z-contrast STEM for materials science. Ultramicroscopy, 1989, 30(1/2): 58-69.
DOI URL |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||