无机材料学报 ›› 2021, Vol. 36 ›› Issue (11): 1163-1170.DOI: 10.15541/jim20210109
所属专题: 【能源环境】水体污染物去除
收稿日期:2021-02-23
修回日期:2021-04-26
出版日期:2021-11-20
网络出版日期:2021-04-30
作者简介:郭 宇(1981-), 男, 教授. E-mail: guoyu@lnut.edu.cn
基金资助:
GUO Yu( ), JIANG Xiaoqing, WU Hongmei, XIAO Yu, WU Dafu, LIU Xin
), JIANG Xiaoqing, WU Hongmei, XIAO Yu, WU Dafu, LIU Xin
Received:2021-02-23
Revised:2021-04-26
Published:2021-11-20
Online:2021-04-30
About author:GUO Yu(1981-), male, professor. E-mail: guoyu@lnut.edu.cn
Supported by:摘要:
为去除水体中Cr(III)的污染, 本研究利用席夫碱反应原理制备了2-羟基-1-萘甲醛功能化SBA-15吸附剂(Q-SBA-15)。通过不同测试手段对所制备样品的形貌、孔道结构、元素组成和表面化学状态进行了系统表征。结果表明, SBA-15经2-羟基-1-萘甲醛修饰后, 其比表面积和孔径明显减小, 但表面形貌和晶体结构没有明显变化。为研究Q-SBA-15对Cr(III)的吸附性能, 详细分析了溶液pH和离子强度的影响, 以及吸附动力学、吸附等温线、吸附热力学和再生性能。结果表明, Q-SBA-15对Cr(III)吸附过程遵循准二级吸附动力学模型和Langmuir模型。当吸附温度为 40 ℃、pH为6、吸附时间为120 min时, Q-SBA-15对Cr(III)的吸附容量最大, 达到102.3 mg/g。Q-SBA-15对Cr(III)的吸附作用主要依靠其表面官能团与Cr(III)的配位螯合作用, 且为自发吸热过程。再生实验表明Q-SBA-15具有良好的重复使用性。该Q-SBA-15吸附剂在去除Cr(III)方面具有潜在的应用价值。
中图分类号:
郭宇, 姜晓庆, 吴红梅, 肖昱, 仵大富, 刘鑫. 2-羟基-1-萘甲醛功能化SBA-15吸附剂的制备及其对Cr(III)的吸附性能[J]. 无机材料学报, 2021, 36(11): 1163-1170.
GUO Yu, JIANG Xiaoqing, WU Hongmei, XIAO Yu, WU Dafu, LIU Xin. Preparation of 2-hydroxy-1-naphthalene Functionalized SBA-15 Adsorbent for the Adsorption of Chromium(III) Ions from Aqueous Solution[J]. Journal of Inorganic Materials, 2021, 36(11): 1163-1170.
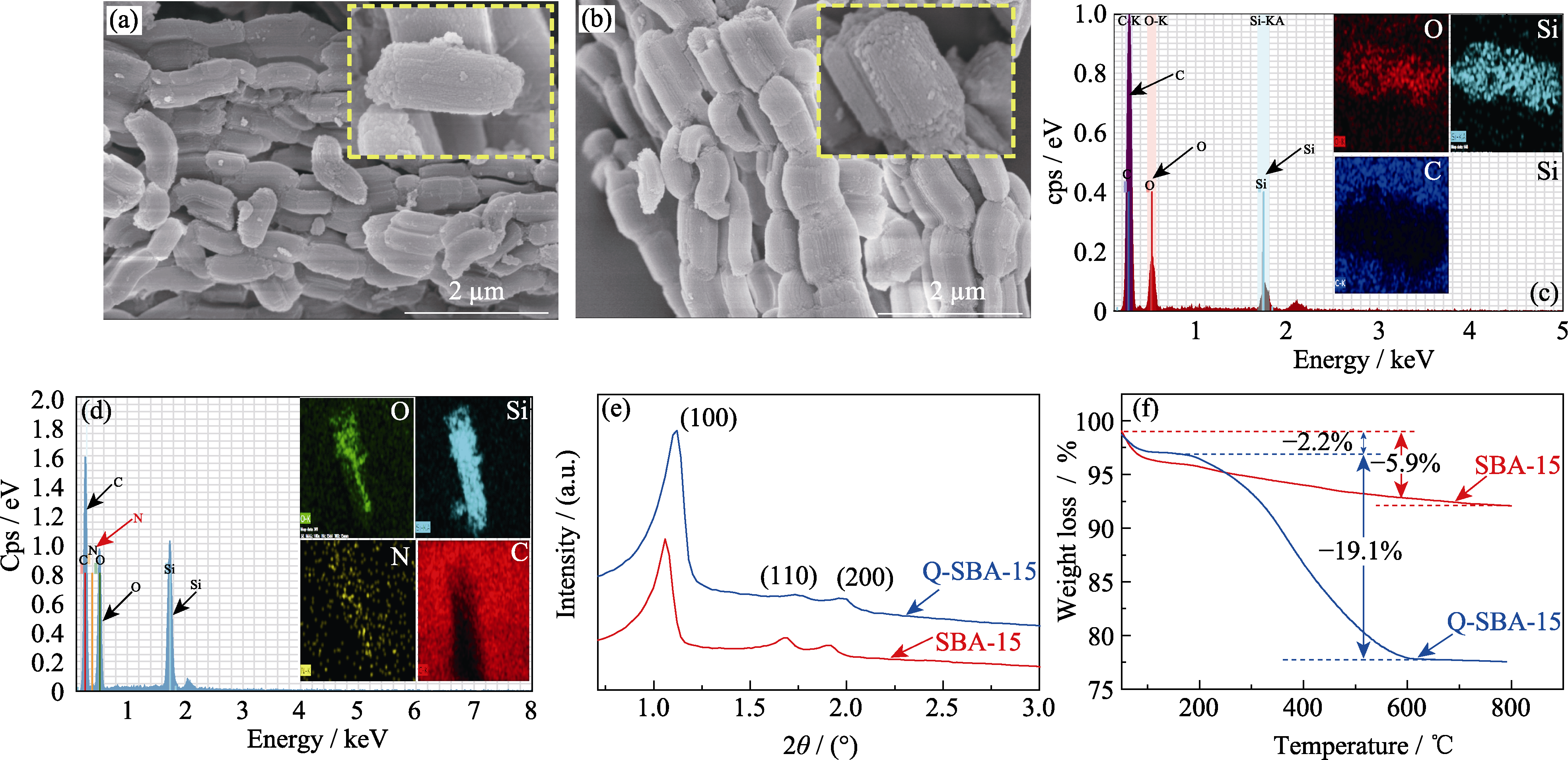
图2 (a, c)SBA-15和(b, d)Q-SBA-15的(a, b)SEM照片, (c, d)EDX分析, (e)XRD图谱和(f)TG曲线
Fig. 2 (a, b) SEM images, (c, d) EDX elemental analyses, (e) XRD patterns and (f) TG curves of (a, c) SBA-15 and (b, d) Q-SBA-15 samples
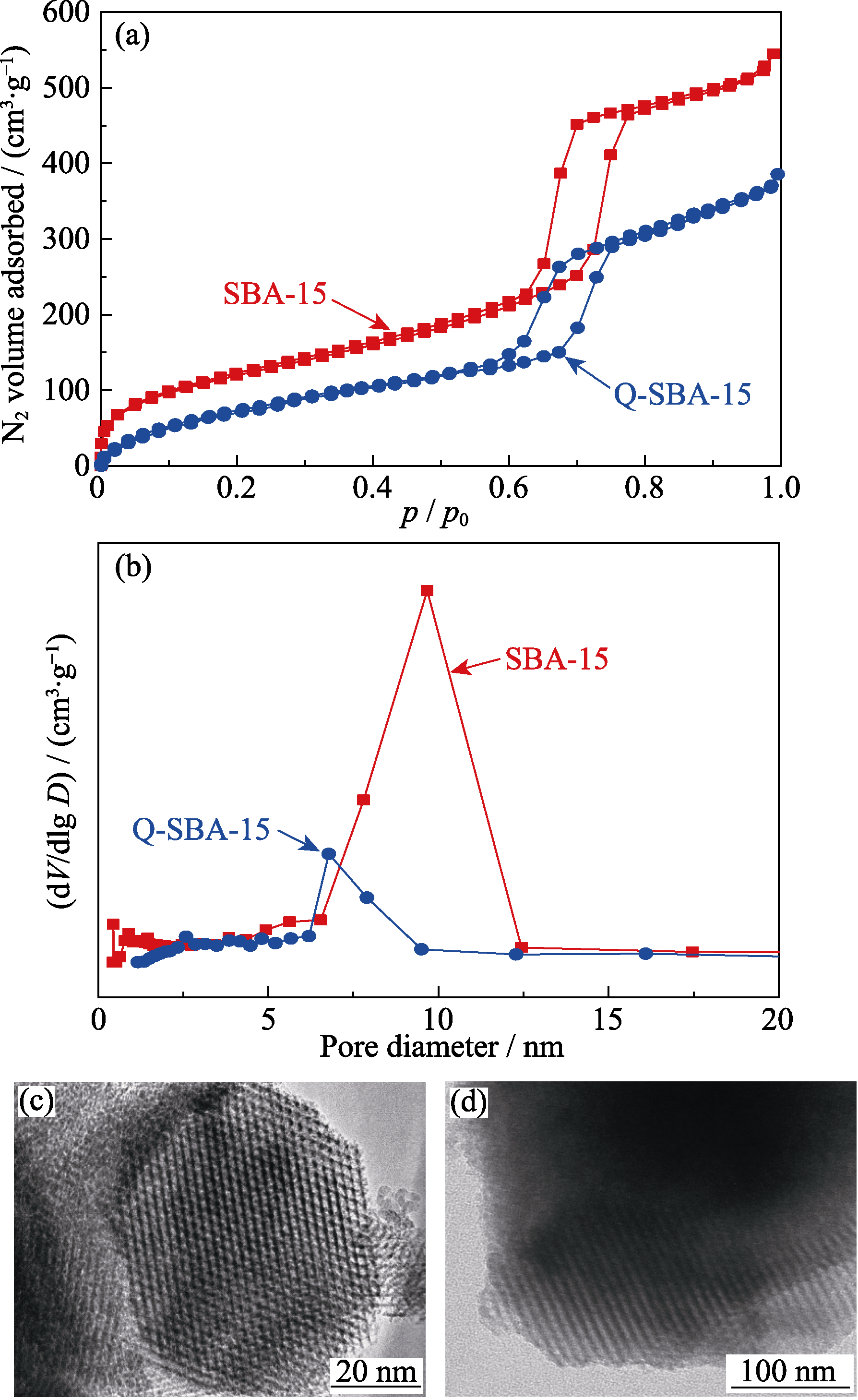
图3 SBA-15和Q-SBA-15的(a)N2吸附-解吸等温线和(b)孔径分布; (c, d)Q-SBA-15的TEM照片
Fig. 3 (a) N2 adsorption-desorption isotherms, (b) pore size distributions of SBA-15 and Q-SBA-15, (c, d) TEM images of Q-SBA-15
| Sample | SBET/(cm2·g-1) | Pore size/nm |
|---|---|---|
| SBA-15 | 757 | 9.6 |
| Q-SBA-15 | 283 | 6.7 |
表1 样品的孔结构分析
Table 1 Textural properties of the synthesized samples
| Sample | SBET/(cm2·g-1) | Pore size/nm |
|---|---|---|
| SBA-15 | 757 | 9.6 |
| Q-SBA-15 | 283 | 6.7 |
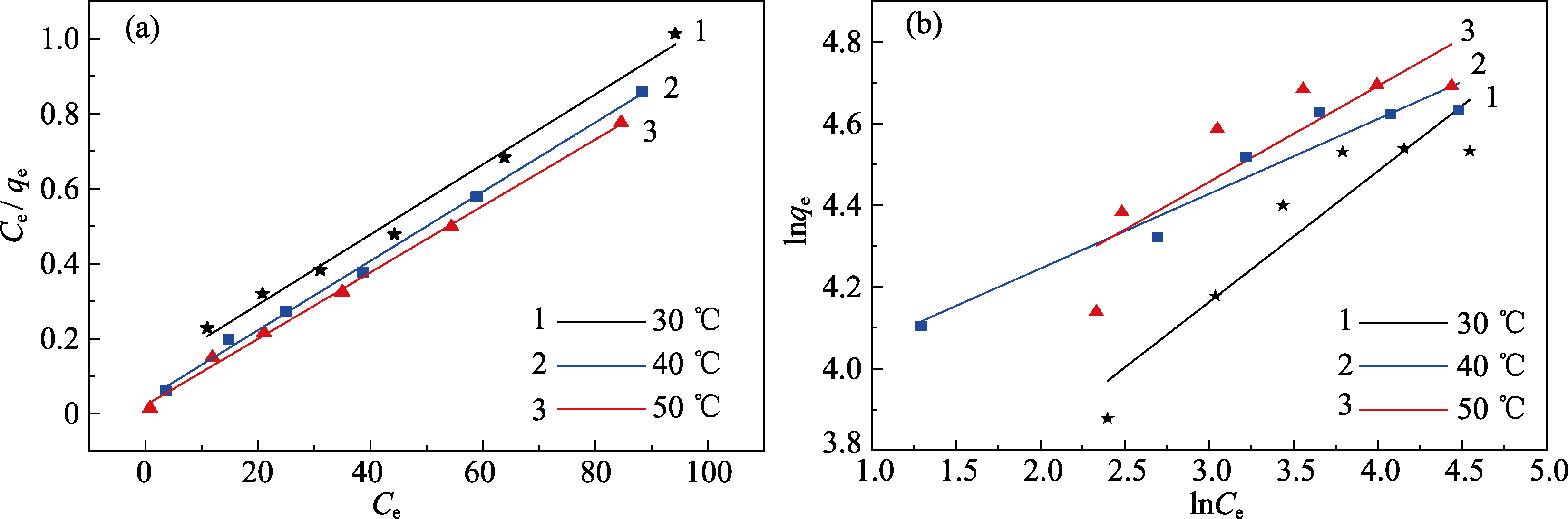
图7 Q-SBA-15吸附Cr(III)的(a)Langmuir方程和(b)Freundlich方程拟合曲线
Fig. 7 Linearized fitting curves of (a) Langmuir model and (b) Freundlich model for adsorption of Cr(III) on Q-SBA-15
| Temperature /℃ | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm/(g·mg-1) | KL | R2 | KF | n | R2 | ||
| 30 | 106.84 | 0.09 | 0.988 | 24.59 | 3.12 | 0.836 | |
| 40 | 108.23 | 0.25 | 0.997 | 48.39 | 5.47 | 0.913 | |
| 50 | 112.74 | 0.41 | 0.997 | 42.72 | 4.27 | 0.697 | |
表2 不同温度下Q-SBA-15吸附Cr(III)的吸附等温线拟合常数
Table 2 Different model parameters for adsorption of Cr(III) by Q-SBA-15 at different temperatures
| Temperature /℃ | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm/(g·mg-1) | KL | R2 | KF | n | R2 | ||
| 30 | 106.84 | 0.09 | 0.988 | 24.59 | 3.12 | 0.836 | |
| 40 | 108.23 | 0.25 | 0.997 | 48.39 | 5.47 | 0.913 | |
| 50 | 112.74 | 0.41 | 0.997 | 42.72 | 4.27 | 0.697 | |
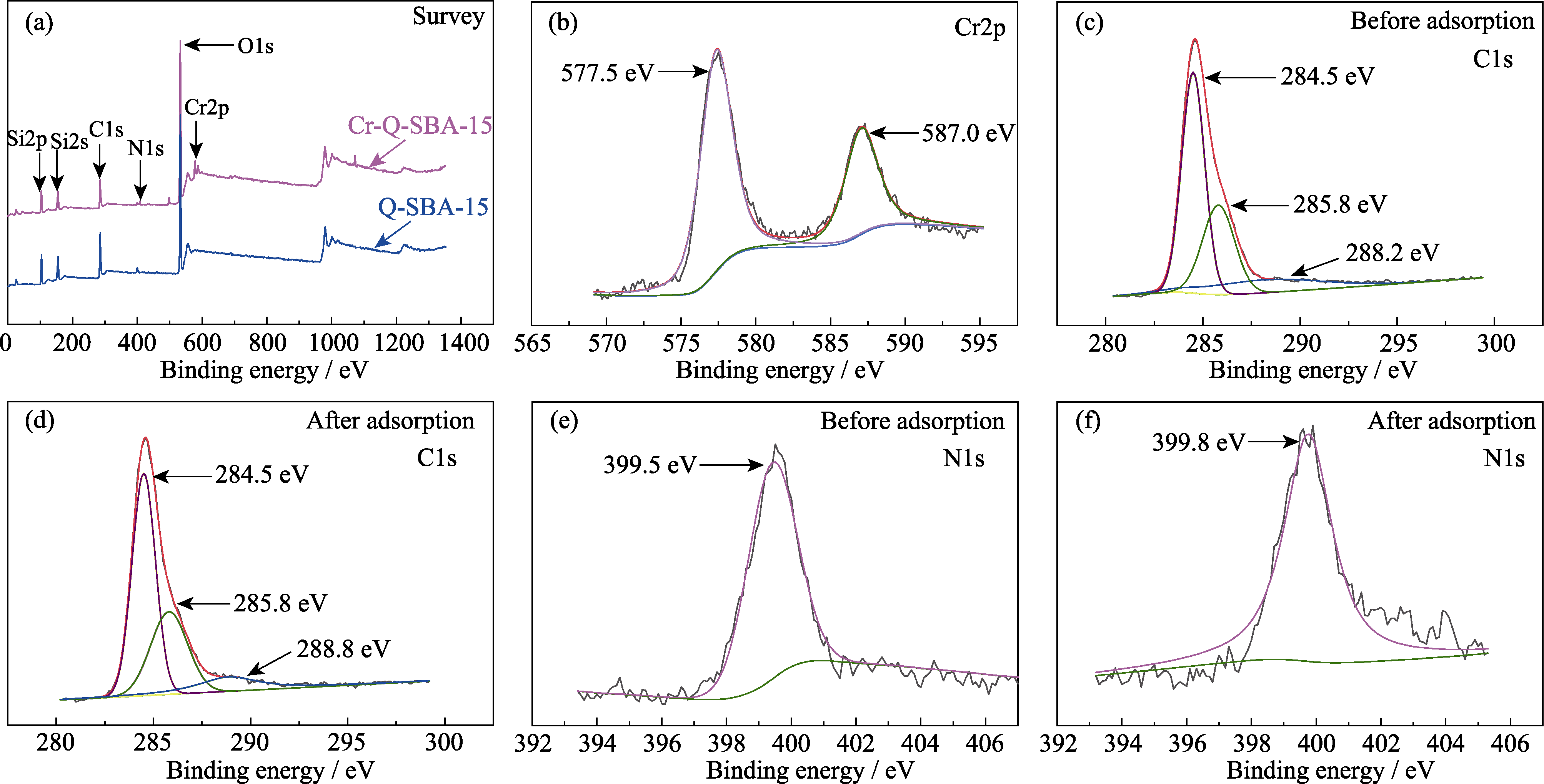
图8 Q-SBA-15吸附Cr(III)前/后的XPS谱图
Fig. 8 XPS spectra of Q-SBA-15 (a) Overview spectra of Q-SBA-15 before and after Cr(III) adsorption; (b) Cr2p spectrum of Q-SBA-15 after Cr(III) adsorption; C1s spectra of (c) Q-SBA-15 (before adsorption) and (d) Q-SBA-15 (after adsorption); N1s spectra of (e) Q-SBA-15 (before adsorption) and (f) Q-SBA-15 (after adsorption)
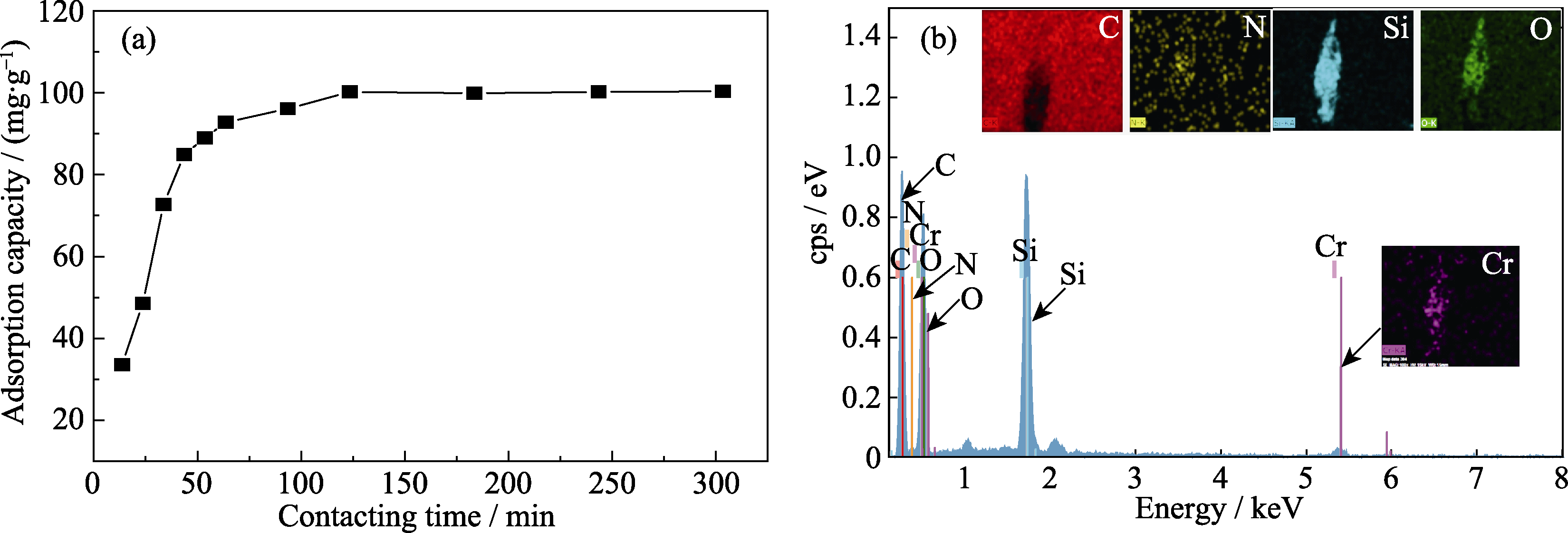
图S1 (a)Q-SBA-15随着时间变化对Cr(III)的吸附性能和(b)Q-SBA-15吸附Cr(III)后的EDX元素分析
Fig. S1 (a) Effect of contacting time on the adsorption capacity of Cr(III) by Q-SBA-15 and (b) EDX analyses of Q-SBA-15 after adsorption of Cr(III)
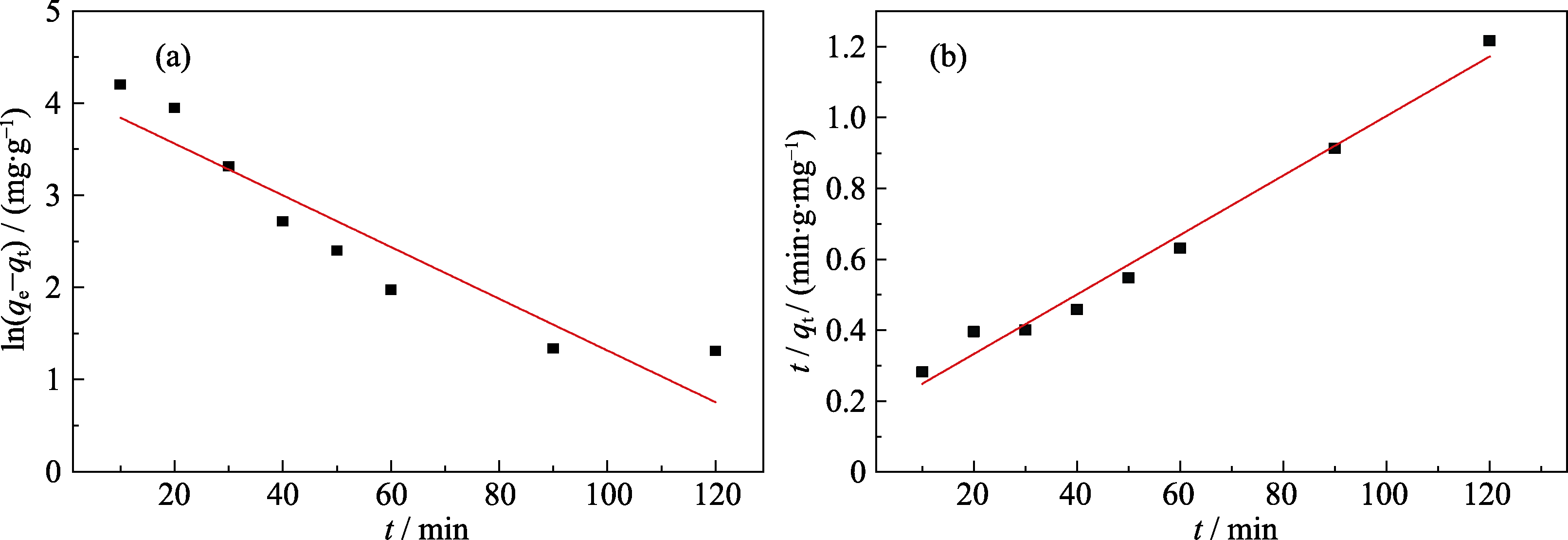
图S2 Q-SBA-15吸附Cr(III)的(a)准一级和(b)准二级动力学模型拟合曲线
Fig. S2 Fitting curves for adsorption of Cr(III) by (a) pseudo-first-order model and (b) pseudo-first-order model
| Sample | qe,exp/(mg·g-1) | Pseudo first-order model | Pseudo second-order model | ||||
|---|---|---|---|---|---|---|---|
| K1/ min-1 | qe,cal/(mg·g-1) | R2 | K2/(g·mg-1·min-1) | qe,cal/(mg·g-1) | R2 | ||
| Q-SBA-15 | 102.3 | 0.0647 | 61.75 | 0.857 | 4.26×10-4 | 119.19 | 0.979 |
表S1 Q-SBA-15吸附Cr(III)的动力学方程拟合参数
Table S1 Kinetic parameters for Cr(III) adsorption onto Q-SBA-15
| Sample | qe,exp/(mg·g-1) | Pseudo first-order model | Pseudo second-order model | ||||
|---|---|---|---|---|---|---|---|
| K1/ min-1 | qe,cal/(mg·g-1) | R2 | K2/(g·mg-1·min-1) | qe,cal/(mg·g-1) | R2 | ||
| Q-SBA-15 | 102.3 | 0.0647 | 61.75 | 0.857 | 4.26×10-4 | 119.19 | 0.979 |
| Adsorbent | qm/(mg·g-1) | References |
|---|---|---|
| Biomass-based hydrogel | 41.7 | [1] |
| m-MCM-41-NH2 | 36.92 | [2] |
| Graphene oxide | 13.3 | [3] |
| Thiol-functionalized MCM-41 | 15.34 | [4] |
| TS-SBA-15 | 95.68 | [5] |
| NH2-SBA-15 | 24.88 | [6] |
| Q-SBA-15 | 102.3 | This work |
表S2 不同吸附剂对Cr(III)的吸附性能对比
Table S2 Comparison of Cr(III) adsorption performance with different materials selected from literature
| Adsorbent | qm/(mg·g-1) | References |
|---|---|---|
| Biomass-based hydrogel | 41.7 | [1] |
| m-MCM-41-NH2 | 36.92 | [2] |
| Graphene oxide | 13.3 | [3] |
| Thiol-functionalized MCM-41 | 15.34 | [4] |
| TS-SBA-15 | 95.68 | [5] |
| NH2-SBA-15 | 24.88 | [6] |
| Q-SBA-15 | 102.3 | This work |
| Sample | Tempera- ture/K | Kc | ΔG/ (kJ·mol-1) | ΔS/ (kJ·mol·K-1) | ΔH/ (kJ·mol-1) |
|---|---|---|---|---|---|
| Q-SBA-15 | 293 | 1.846 | -4.386 | 0.128 | 33.118 |
| 303 | 2.095 | -5.666 | |||
| 313 | 2.650 | -6.946 | |||
| 318 | 2.780 | -7.586 | |||
| 323 | 3.088 | -8.226 |
表S3 Q-SBA-15吸附Cr(III)的热力学参数
Table S3 Thermodynamic parameters of Cr(III) adsorption on Q-SBA-15
| Sample | Tempera- ture/K | Kc | ΔG/ (kJ·mol-1) | ΔS/ (kJ·mol·K-1) | ΔH/ (kJ·mol-1) |
|---|---|---|---|---|---|
| Q-SBA-15 | 293 | 1.846 | -4.386 | 0.128 | 33.118 |
| 303 | 2.095 | -5.666 | |||
| 313 | 2.650 | -6.946 | |||
| 318 | 2.780 | -7.586 | |||
| 323 | 3.088 | -8.226 |
| [1] |
ALAGU K, VENU H, JAYARAMAN J, et al. Novel water hyacinth biodiesel as a potential alternative fuel for existing unmodified diesel engine: performance, combustion and emission characteristics. Energy, 2004, 179:295-305.
DOI URL |
| [2] | EBO D A, NANNE D V, BIRITWUM N K. Assessment of heavy metal pollution in the main Pra River and its tributaries in the Pra Basin of Ghana. Environmental Nanotechnology Monitoring & Management, 2018, 10:264-271. |
| [3] |
HASHEM M A, ISLAM A, MOHSIN S, et al. Green environment suffers by discharging of high-chromium-containing wastewater from the tanneries at Hazaribagh. Bangladesh. Sustainable Water Resources Management, 2015, 1(4):343-347.
DOI URL |
| [4] |
EL-SHAHAWI M S, HASSAN S M, OTHMAN A M, et al. Retention profile and subsequent chemical speciation of chromium(III) and (VI) in industrial wastewater samples employing some onium cations loaded polyurethane foams. Microchemical Journal, 2008, 89(1):13-19.
DOI URL |
| [5] |
BULUT V N, OZDES D, BEKIRCAN O, et al. Carrier element- free coprecipitation (CEFC) method for the separation, preconcentration and speciation of chromium using an isatin derivative. Analytica Chimica Acta, 2009, 632(1):35-41.
DOI URL |
| [6] |
MAO C P, SONG Y X, CHEN L X, et al. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena, 2019, 175:339-348.
DOI URL |
| [7] |
LIU X L, PANG H W, LIU X W, et al. Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions. The Innovation, 2021, 2(1):100076.
DOI URL |
| [8] | WANG X X, LI X, WANG J Q, et al. Recent advances in carbon nitride-based nanomaterials for the removal of heavy metal ions from aqueous solution. Journal of Inorganic Materials, 2020, 35:260-270. |
| [9] |
GAO X P, GUO C, ZHAO Z, et al. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. International Journal of Biological Macromolecules, 2020, 164(1):4423-4434.
DOI URL |
| [10] |
CHIRANGANO M, TONNI A K, AHMAD B A. Comparative biosorption of chromium(VI) using chemically modified date pits (CM-DP) and olive stone (CM-OS): kinetics, isotherms and influence of co-existing ions. Chemical Engineering Research and Design, 2020, 156:251-262.
DOI URL |
| [11] |
GODIYA C B, CHENG X, LI D, et al. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. Journal of Hazardous Materials, 2018, 364(1):28-38.
DOI URL |
| [12] |
GIL C, MARIA L, FERRI A, et al. Distribution of chromium species in a Cr-polluted soil: presence of Cr(III) in glomalin related protein fraction. Science of the Total Environment, 2014, 493:828-833.
DOI URL |
| [13] |
ATIKAH M N, PEI S G, MOHD S A, et al. Adsorptive nanocomposite membranes for heavy metal remediation: recent progresses and challenges. Chemosphere, 2019, 232:96-112.
DOI URL |
| [14] |
RUIHUA M, BIN L, XI C, et al. Adsorption of Cu(II)and Co(II) from aqueous solution using lignosulfonate/chitosan adsorbent. International Journal of Biological Macromolecules, 2020, 163(1):120-127.
DOI URL |
| [15] |
BERA A, TRIVEDI J S, KUMAR S B, et al. Anti-organic fouling and anti-biofouling poly(piperazineamide) thin film nanocomposite membranes for low pressure removal of heavy metal ions. Journal of Hazardous Materials, 2018, 343:86-97.
DOI URL |
| [16] |
WU H M, XIAO Y, GUO Y, et al. Functionalization of SBA-15 mesoporous materials with 2-acetylthiophene for adsorption of Cr(III) ion. Microporous and Mesoporous Materials, 2020, 292:109754.
DOI URL |
| [17] |
CAROLIN C F, KUMAR P S, SARAVANAN A, et al. Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. Journal of Environmental Chemical Engineering, 2017, 5(3):2782-2799.
DOI URL |
| [18] |
SURENDRAN P, ANEESH M, ANANDHU M, et al. Chelation dependent selective adsorption of metal ions by Schiff base modified SBA-15 from aqueous solutions. Journal of Environmental Chemical Engineering, 2020, 8(5):104248.
DOI URL |
| [19] | BETIHA M A, MOUSTAFA Y M, EL-SHAHAT M F, et al. Polyvinylpyrrolidone-aminopropyl-SBA-15 Schiff-base hybrid for efficient removal of divalent heavy metal cations from wastewater. Journal of Hazardous Materials, 2020, 397:112675. |
| [20] | XIAO Y, GUO Y, WU H M, et al. Adsorption of chromium(III) ions with amino functionalized mesoporous silica adsorbent. Chemical Industry and Engineering Progress, 2020, 30(9):263-272. |
| [21] |
CE L, WANG B D, HAN Y F, et al. Adsorption of lead ion on amino-functionalized fly-ash-based SBA-15 mesoporous molecular sieves prepared via two-step hydrothermal method. Microporous and Mesoporous Materials, 2017, 252:105-115.
DOI URL |
| [22] |
MOHAMMAD M S, ALI S R, MEHDI A, et al. Functionalization of SBA-15 by dithiooxamide towards removal of Co(II) ions from real samples: isotherm, thermodynamic and kinetic studies. Advanced Powder Technology, 2019, 30(9):1823-1834.
DOI URL |
| [23] |
ANBARASU G, MALATHY M, KARTHIKEYAN P, et al. Silica functionalized Cu(II) acetylacetonate Schiff base complex: an efficient catalyst for the oxidative condensation reaction of benzyl alcohol with amines. Journal of Solid State Chemistry, 2017, 253:305-312.
DOI URL |
| [24] |
HUANG S J, MA C Z, LIAO Y Z, et al. Superb adsorption capacity and mechanism of poly(1-amino-5-chloroanthraquinone) nanofibrils for lead and trivalent chromium ions. Reactive and Functional Polymers, 2016, 106:76-85.
DOI URL |
| [25] |
NICALAS F, FRANCISCO J, PEREZ A, et al. Chromium(VI) removal from water by means of adsorption-reduction at the surface of amino-functionalized MCM-41 sorbents. Microporous and Mesoporous Materials, 2017, 239:138-146.
DOI URL |
| [26] |
KRISHNA S K, YADAV D, SHARMA S K, et al. Cu(II) Schiff base complex grafted guar gum: catalyst for benzophenone derivatives synthesis. Applied Catalysis A: General, 2020, 601:117529.
DOI URL |
| [27] |
HEMANDEZ-MORALES V, NAVA R, ACOSTA-SILVA Y J, et al. Adsorption of lead(II) on SBA-15 mesoporous molecular sieve functionalized with -NH2 groups. Microporous and Mesoporous Materials, 2012, 160:133-142.
DOI URL |
| [28] |
FELLENZ N, PEREZ-ALONSO F J, MARTIN P P, et al. Chromium(VI) removal from water by means of adsorption- reduction at the surface of amino-functionalized MCM-41 sorbents. Microporous and Mesoporous Materials, 2017, 239:138-146.
DOI URL |
| [29] |
CHEN F Y, HONG M Z, YOU W J, et al. Simultaneous efficient adsorption of Pb2+ and MnO4- ions by MCM-41 functionalized with amine and nitrilotriacetic acid anhydride. Applied Surface Science, 2015, 357:856-865.
DOI URL |
| [30] |
LIU S, CUI H Z, LI Y L, et al. Bis-pyrazolyl functionalized mesoporous SBA-15 for the extraction of Cr(III) and detection of Cr(VI) in artificial jewelry samples. Microchemical Journal, 2017, 131:130-136.
DOI URL |
| [31] |
KUMAR P A, RAY M, CHAKRABORTY S. Adsorption behaviour of trivalent chromium on amine-based polymer aniline formaldehyde condensate. Chemical Engineering Journal, 2009, 149(1/2/3):340-347.
DOI URL |
| [32] | WAN L, TONG S T. Adsorption of Cr3+ from aqueous solution on mesoporous activated carbon. Environmental Protection of Chemical industry, 2012, 32(1):75-80. |
| [33] | ZHANG X P. Modification of UIO-66-NH2 by 3, 4-dihydroxy benzaldehyde and its adsorption properties for U(VI). Rubber and Plastic Technology and Equipment, 2021, 47(2):43-50. |
| [34] | YI C L, YE X, LI T L, et al. Study the adsorption characteristics of biomanganese oxide to four heavy metals. Industrial Safety and Environmental Protection, 2021, 47(3):94-98. |
| [35] | CHENG FU-QIANG, JI TIAN-TIAN, XUE MIN, et al. Preparation of thiohydroxy-functionalized mesoporous materials and its adsorption to Cr6+. Journal of Inorganic Materials, 2020, 35(2):194-198. |
| [36] | ZHU MING-YU, FAN DE-ZE, LIU BEI, et al. C@K2Ti6O13 hierarchical nano materials: effective adsorption removel of Cr(VI). Journal of Inorganic Materials, 2020, 35(3):310-314. |
| [37] | PONCE-LIRA B, OTAZO-SÁNCHEZ E M, REGUERA E, et al. Lead removal from aqueous solution by basaltic scoria: adsorption equilibrium and kinetics. International Journal of Environmental Science and Technology, 2017, 14:1181-1196. |
| [38] | SONG X T, NIU Y Z, ZHANG P P, et al. Removal of Co(II) from fuel ethanol by silica-gel supported PAMAM dendrimers: combined experimental and theoretical study. Fuel, 2017, 199(1):91-101. |
| [39] | BHAUMIK M, MAITY A, SRINIVASU V V, et al. Enhanced removal of Cr(VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. Journal of Hazardous Materials, 2011, 190(1/2/3):381-390. |
| [1] | 马晓森, 张丽晨, 刘砚超, 汪全华, 郑家军, 李瑞丰. 13X@SiO2合成及其甲苯吸附性能[J]. 无机材料学报, 2023, 38(5): 537-543. |
| [2] | 郭春霞, 陈伟东, 闫淑芳, 赵学平, 杨傲, 马文. 埃洛石纳米管负载锆氧化物吸附水中砷的研究[J]. 无机材料学报, 2023, 38(5): 529-536. |
| [3] | 王世怡, 冯爱虎, 李晓燕, 于云. Fe3O4负载Ti3C2Tx对Pb(II)的吸附性能研究[J]. 无机材料学报, 2023, 38(5): 521-528. |
| [4] | 于业帆, 徐玲, 倪忠斌, 施冬健, 陈明清. 普鲁士蓝/生物炭材料的制备及其氨氮吸附机理[J]. 无机材料学报, 2023, 38(2): 205-212. |
| [5] | 王红宁, 黄丽, 清江, 马腾洲, 黄维秋, 陈若愚. 有机-无机氧化硅空心球的合成及VOCs吸附应用[J]. 无机材料学报, 2022, 37(9): 991-1000. |
| [6] | 王虹力, 王男, 王丽莹, 宋二红, 赵占奎. 功能化石墨烯担载型AuPd纳米催化剂增强甲酸制氢反应[J]. 无机材料学报, 2022, 37(5): 547-553. |
| [7] | 刘城, 赵倩, 牟志伟, 雷洁红, 段涛. 新型铋基SiOCNF复合膜对放射性气态碘的吸附性能[J]. 无机材料学报, 2022, 37(10): 1043-1050. |
| [8] | 周帆, 毕辉, 黄富强. 用稻壳制备亚甲基蓝高吸附容量的超高比表面积活性炭[J]. 无机材料学报, 2021, 36(8): 893-903. |
| [9] | 余祥坤, 刘坤, 李志鹏, 赵雨露, 沈锦优, 茆平, 孙爱武, 蒋金龙. 铜/凹凸棒石复合材料高效吸附放射性碘离子性能[J]. 无机材料学报, 2021, 36(8): 856-864. |
| [10] | 苏莉, 杨建平, 兰悦, 王连军, 江莞. 纳米铁颗粒及其复合材料的界面设计及环境修复应用[J]. 无机材料学报, 2021, 36(6): 561-569. |
| [11] | 席文, 李海波. TiO2/Ti3C2Tx复合材料的制备及其杂化电容脱盐特性的研究[J]. 无机材料学报, 2021, 36(3): 283-291. |
| [12] | 王婷婷, 史书梅, 柳晨媛, 朱万诚, 张恒. 多级多孔硅酸镍微球的合成及其对碱性品红的高效吸附[J]. 无机材料学报, 2021, 36(12): 1330-1336. |
| [13] | 张瑞鸿, 魏鑫, 卢占会, 艾玥洁. 基于机器学习训练金属离子吸附能预测模型的研究[J]. 无机材料学报, 2021, 36(11): 1178-1184. |
| [14] | 何俊龙, 宋二红, 王连军, 江莞. DFT方法研究一氧化氮在铬掺杂石墨烯上的吸附行为[J]. 无机材料学报, 2021, 36(10): 1047-1052. |
| [15] | 李敬,刘晓月,邱千峰,李苓,曹晓燕. 不同结构铝氧化物对磷的吸附特征[J]. 无机材料学报, 2020, 35(9): 1005-1010. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||