无机材料学报 ›› 2021, Vol. 36 ›› Issue (11): 1137-1144.DOI: 10.15541/jim20210105
收稿日期:2021-02-21
修回日期:2021-04-05
出版日期:2021-11-20
网络出版日期:2021-04-25
通讯作者:
曹余良, 教授. E-mail: ylcao@whu.edu.cn
作者简介:曾凡鑫(1995-), 男, 硕士研究生. E-mail: fxzeng1117@126.com
基金资助:
ZENG Fanxin( ), LIU Chuang, CAO Yuliang(
), LIU Chuang, CAO Yuliang( )
)
Received:2021-02-21
Revised:2021-04-05
Published:2021-11-20
Online:2021-04-25
Contact:
CAO Yuliang, professor. E-mail: ylcao@whu.edu.cn
About author:ZENG fanxin(1995-), male, Master candidate. E-mail: fxzeng1117@126.com
Supported by:摘要:
为提升Sb基负极材料的储钠循环性能, 通过简单的两步法(机械辅助化学合金化和酸溶解去合金化)制备纳米化和多孔化的去合金锑/多壁碳纳米管(De-Sb/MCNT)复合物, 采用不同方法表征材料的物理化学性质和储钠电化学性能。结果显示, De-Sb/MCNT材料的可逆比容量达到408.6 mAh·g-1 (200 mA·g-1), 首周库仑效率为69.2%; 在800 mA·g-1循环330周后, 容量保持率仍可达88%, 展现出优异的储钠循环性能。这得益于机械辅助化学合金化/酸溶解去合金化对商品化Sb的“预粉化”作用, 促进了材料的纳米化和多孔化, 缓解了充放电过程中的体积膨胀, 实现了高的循环稳定性。这种常温合金化/去合金化的方法为制备循环稳定的储钠合金负极材料提供了新的途径。
中图分类号:
曾凡鑫, 刘创, 曹余良. 去合金化制备具有高循环稳定性的纳米多孔Sb/MCNT储钠负极材料[J]. 无机材料学报, 2021, 36(11): 1137-1144.
ZENG Fanxin, LIU Chuang, CAO Yuliang. Sodium Storage Behavior of Nanoporous Sb/MCNT Anode Material with High Cycle Stability by Dealloying Route[J]. Journal of Inorganic Materials, 2021, 36(11): 1137-1144.
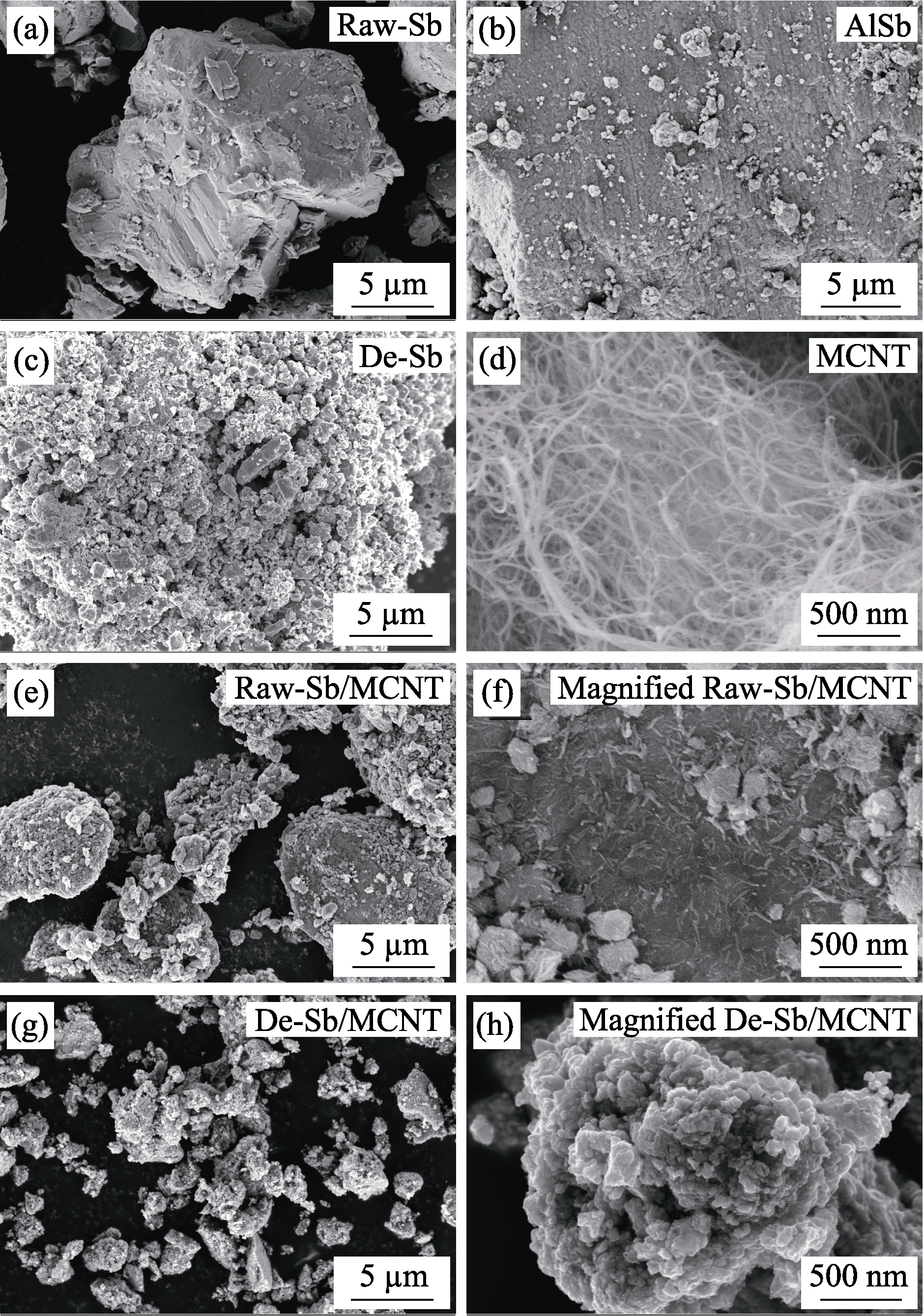
图2 (a)Raw-Sb、(b)AlSb、(c)De-Sb、(d)MCNT、(e, f)Raw-Sb/ MCNT和(g, h)De-Sb/MCNT的SEM照片
Fig. 2 SEM images of (a) Raw-Sb, (b) AlSb, (c) De-Sb, (d) MCNT, (e, f) Raw-Sb/MCNT and (g, h) De-Sb/MCNT

图3 De-Sb/MCNT的(a)TEM照片、(b)高分辨TEM照片、(c)电子衍射图、(d)SEM照片及对应的(e)Sb、(f)C和(g)O的元素分布图
Fig. 3 (a) TEM image, (b) HRTEM image, (c) SAED pattern, (d) SEM image and the corresponding (e) Sb, (f) C, and (g) O elemental mapping images of De-Sb/MCNT
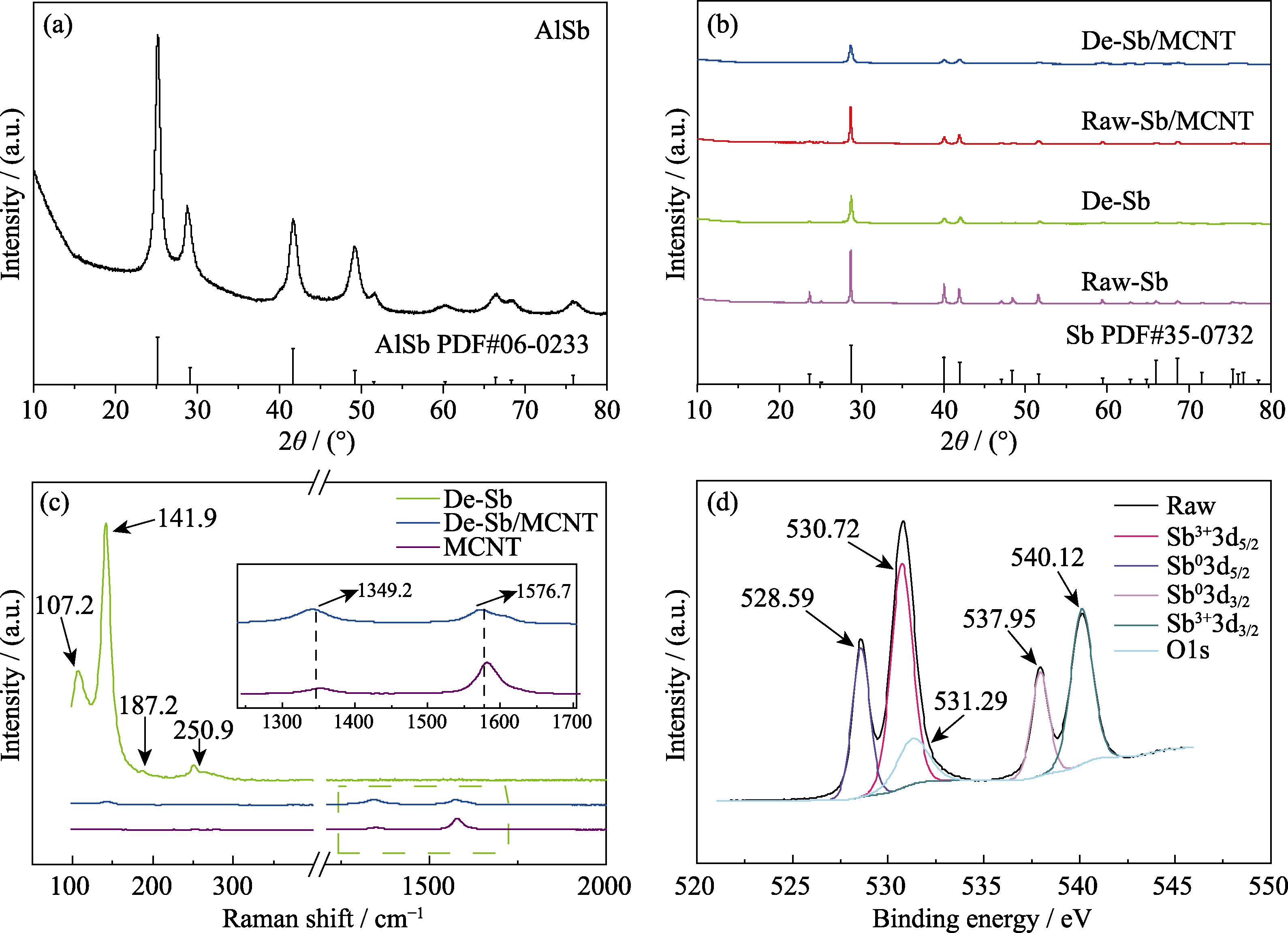
图4 (a)AlSb合金、(b)Raw-Sb、De-Sb、Raw-Sb/MCNT和De-Sb/MCNT的XRD图谱; (c)MCNT、De-Sb和De-Sb/MCNT的拉曼光谱图; (d)De-Sb/MCNT的XPS图谱
Fig. 4 XRD patterns of (a) AlSb, (b) Raw-Sb, De-Sb, Raw-Sb/MCNT and De-Sb/MCNT, (c) Raman spectra of MCNT, De-Sb and De-Sb/MCNT, and (d) XPS spectrum of De-Sb/MCNT Colorful figures are available on website
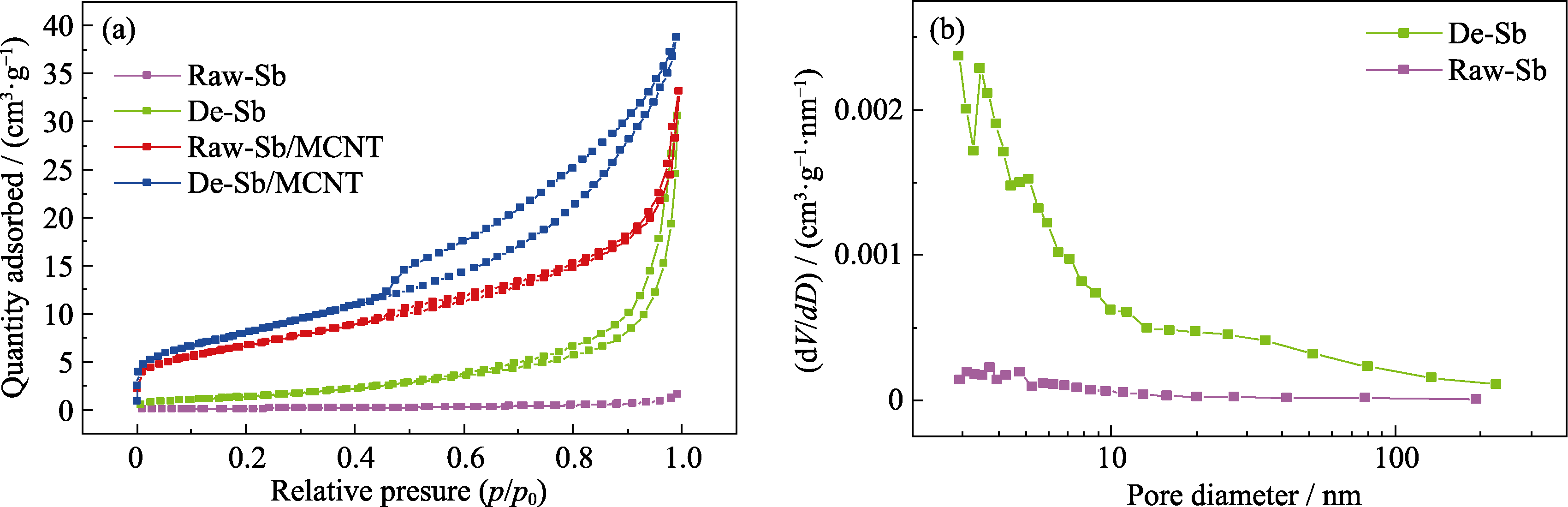
图5 (a)Raw-Sb、De-Sb、Raw-Sb/MCNT和De-Sb/MCNT的N2吸附-脱附等温线; (b)Raw-Sb和De-Sb的孔径分布
Fig. 5 (a) N2 adsorption-desorption isotherms of Raw-Sb, De-Sb, Raw-Sb/MCNT and De-Sb/MCNT, and (b) pore size distributions of Raw-Sb and De-Sb Colorful figures are available on website
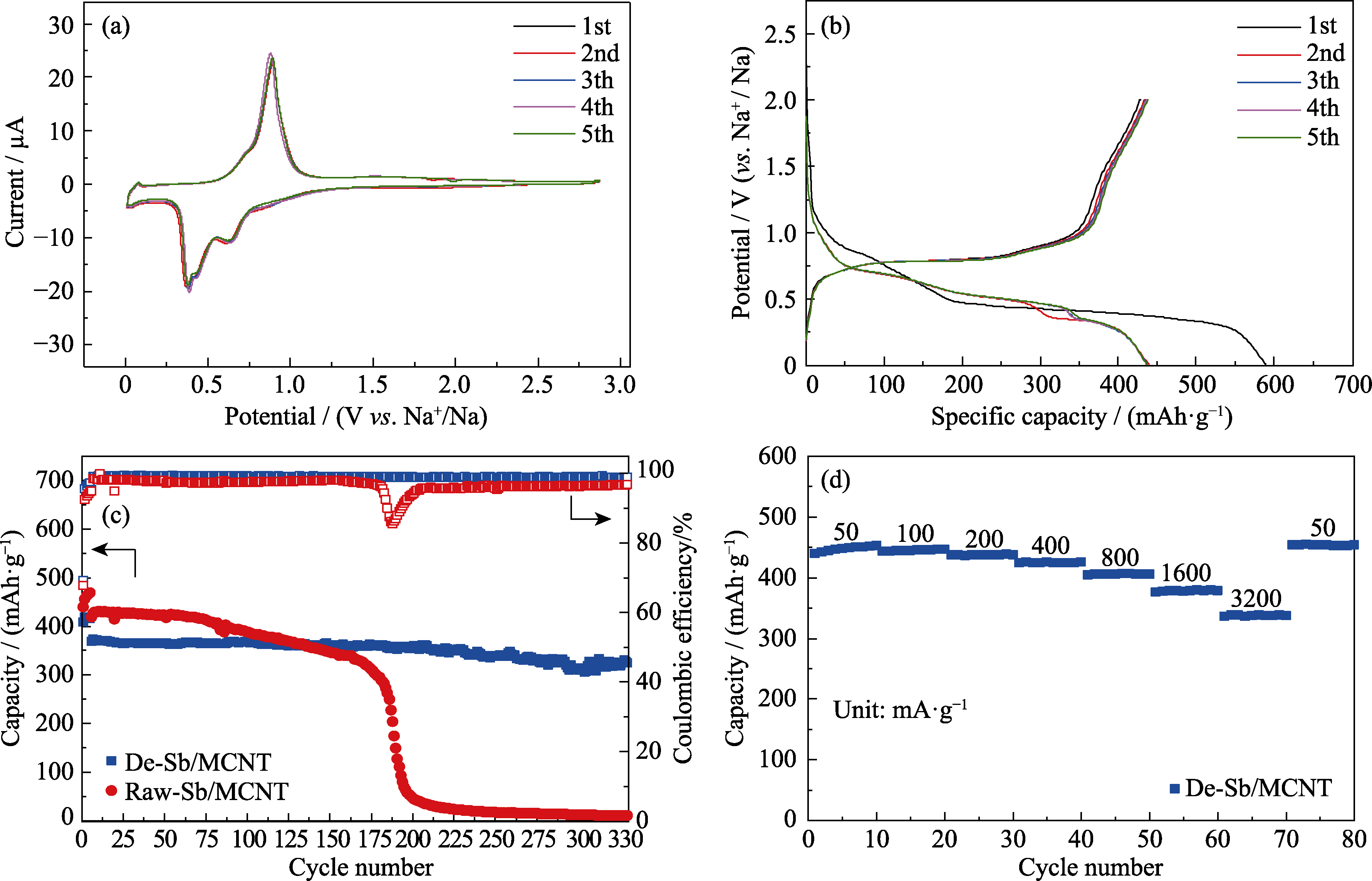
图6 De-Sb/MCNT的(a)循环伏安曲线和(b)在200 mA·g-1电流下的充放电曲线; (c) Raw-Sb/MCNT和De-Sb/MCNT在800 mA·g-1下的长循环性能; (d) De-Sb/MCNT的倍率性能图
Fig. 6 (a) Cyclic voltammogram and (b) Galvanostatic charge/discharge voltage profile at 200 mA·g-1 of De-Sb/MCNT, (c) long-term cycling performance of Raw-Sb/MCNT and De-Sb/MCNT at 800 mA·g-1, and (d) rate performance of De-Sb/MCNT Colorful figures are available on website
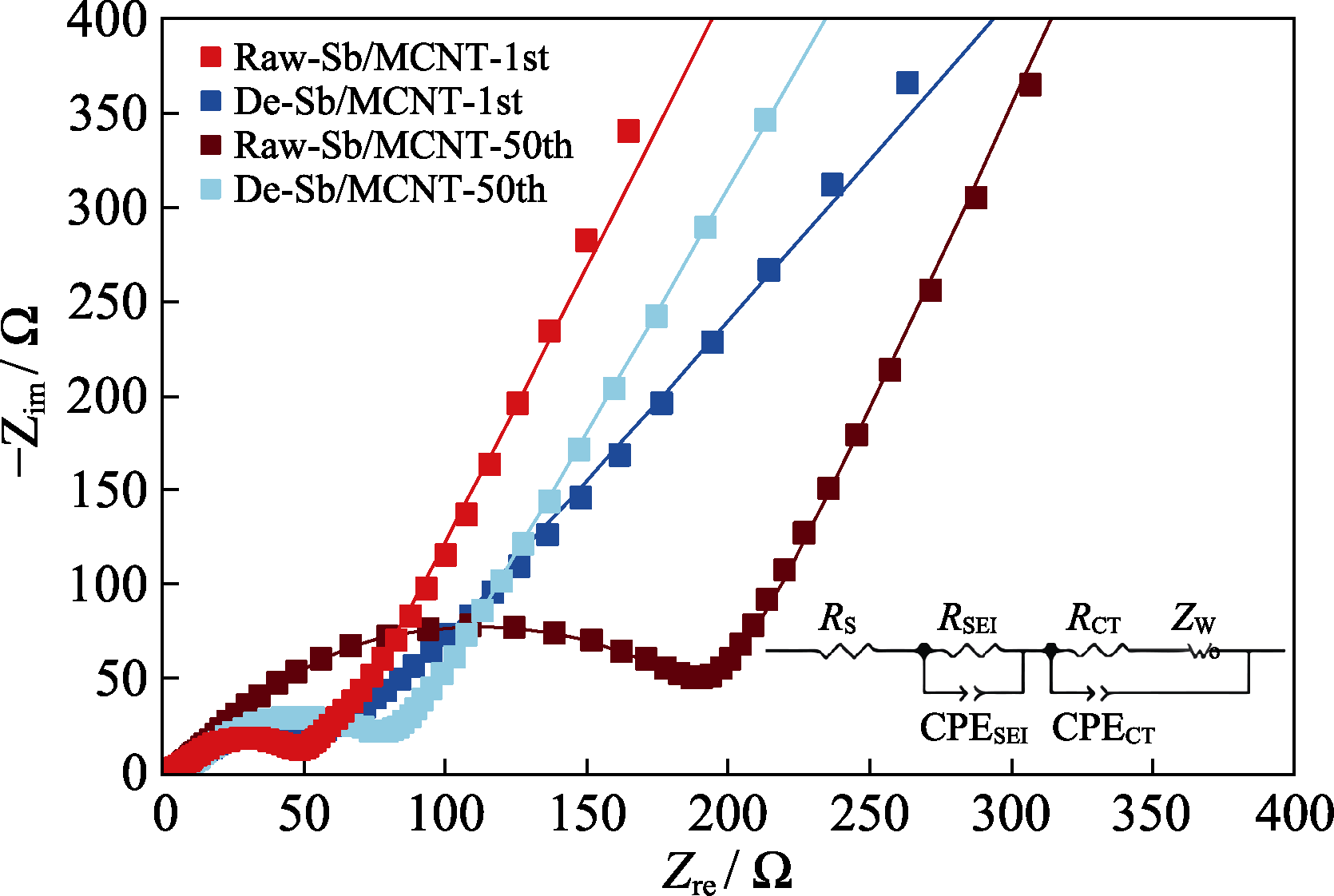
图7 Raw-Sb/MCNT和De-Sb/MCNT循环1周和50周后的EIS图谱和对应的等效电路图(插图)
Fig. 7 EIS plots of Raw-Sb/MCNT and De-Sb/MCNT after 1 and 50 cycles with inset showing the corresponding equivalent circuit Colorful figures are available on website
| Sample | Cycle number | RS/Ω | RSEI/Ω | RCT/Ω |
|---|---|---|---|---|
| Raw-Sb/MCNT | 1 | 2.89 | 37.62 | 23.70 |
| 50 | 4.38 | 43.29 | 142.90 | |
| De-Sb/MCNT | 1 | 1.32 | 21.44 | 44.84 |
| 50 | 2.68 | 24.44 | 51.25 |
表1 Raw-Sb/MCNT和De-Sb/MCNT的EIS图谱的拟合结果
Table 1 Fitting results of the EIS plots of Raw-Sb/MCNT and De-Sb/MCNT
| Sample | Cycle number | RS/Ω | RSEI/Ω | RCT/Ω |
|---|---|---|---|---|
| Raw-Sb/MCNT | 1 | 2.89 | 37.62 | 23.70 |
| 50 | 4.38 | 43.29 | 142.90 | |
| De-Sb/MCNT | 1 | 1.32 | 21.44 | 44.84 |
| 50 | 2.68 | 24.44 | 51.25 |
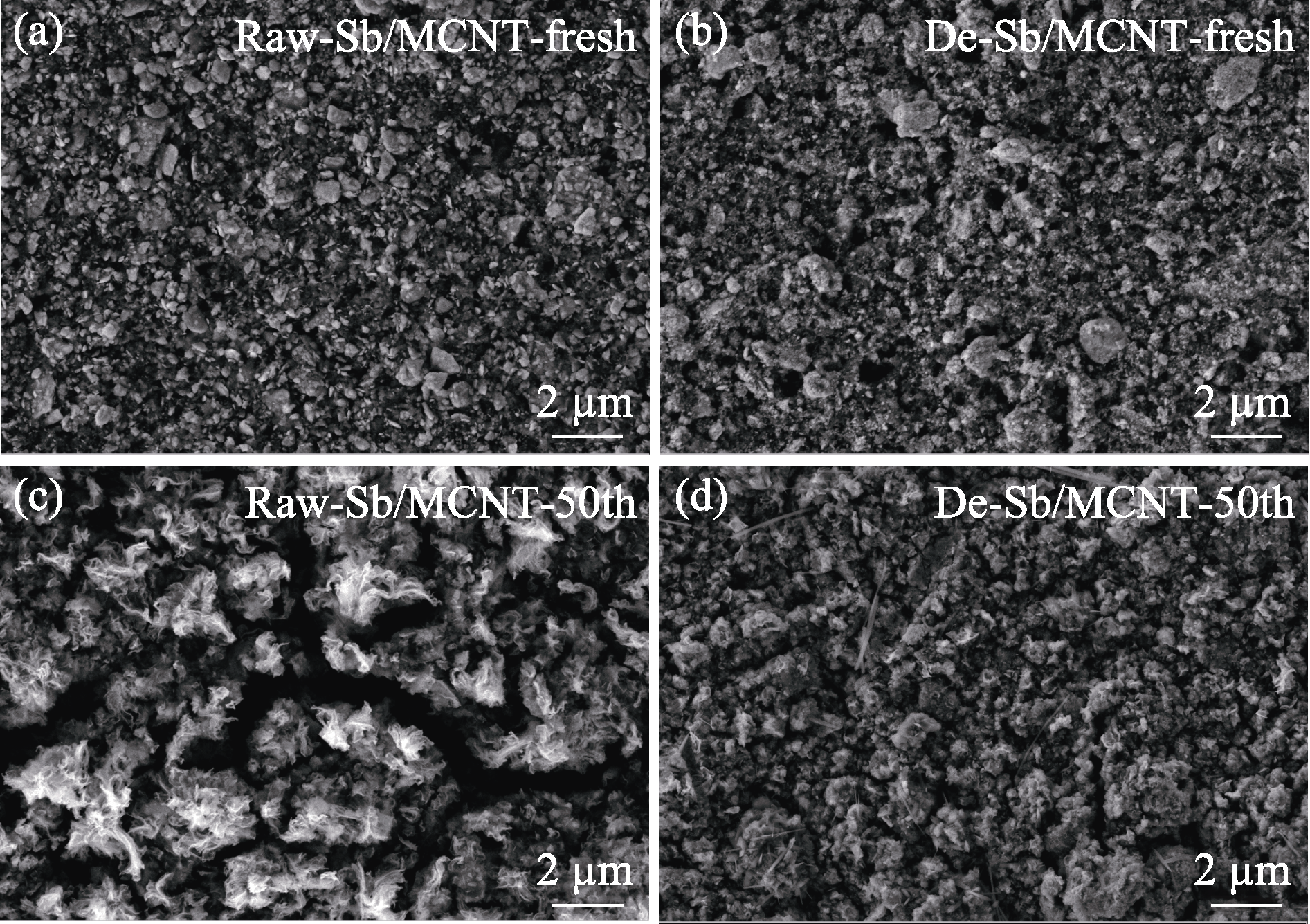
图8 (a, c)Raw-Sb/MCNT电极和(b, d)De-Sb/MCNT电极50圈循环(a, b)前(c, d)后的SEM照片
Fig. 8 SEM images of (a, c) Raw-Sb/MCNT electrode and (b, d) De-Sb/MCNT electrode (a, b) before and (c, d) after 50 cycles
| Material | Capacity/(mAh·g-1) | Cycling stability | Ref. |
|---|---|---|---|
| Sb/Super P | 610 (0.1 A·g-1) | 94% after 100 cycles | [1] |
| Sb/MCNT | 502 (0.1 A·g-1) | 76.1% after 120 cycles | [2] |
| SbOx/RGO | 427 (0.1 A·g-1) | 95% after 100 cycles | [3] |
| Sb-AlC0.75-C | 295 (0.1 A·g-1) | 83% after 100 cycles | [4] |
| Dealloyed Sb | 620 (0.1 A·g-1) | 90.2 % after 100 cycles | [5] |
| Sb nanoparticles/RGO | 476 (0.1 A·g-1) | 81% after 45 cycles | [6] |
| Sb2S3/C | 598 (0.2 A·g-1) | 93.1% after 100 cycles | [7] |
| Nanoporous Sb/C | 436 (0.05 A·g-1) | No fading after 200 cycles | [8] |
| Antimony nanocrystals/C | 520 (0.1 A·g-1) | 88% after 500 cycles | [9] |
| SiC-Sb-Cu-C | 542 (0.02 A·g-1) | No fading after 100 cycles | [10] |
| De-Sb/MCNT | 408.6 (0.2 A·g-1) | 97% after 200 cycles, 88% after 330 cycles | This work |
表S1 Sb基材料的Na离子电池电化学性能
Table S1 Electrochemical performance of different Sb-based materials as Na ion battery electrodes
| Material | Capacity/(mAh·g-1) | Cycling stability | Ref. |
|---|---|---|---|
| Sb/Super P | 610 (0.1 A·g-1) | 94% after 100 cycles | [1] |
| Sb/MCNT | 502 (0.1 A·g-1) | 76.1% after 120 cycles | [2] |
| SbOx/RGO | 427 (0.1 A·g-1) | 95% after 100 cycles | [3] |
| Sb-AlC0.75-C | 295 (0.1 A·g-1) | 83% after 100 cycles | [4] |
| Dealloyed Sb | 620 (0.1 A·g-1) | 90.2 % after 100 cycles | [5] |
| Sb nanoparticles/RGO | 476 (0.1 A·g-1) | 81% after 45 cycles | [6] |
| Sb2S3/C | 598 (0.2 A·g-1) | 93.1% after 100 cycles | [7] |
| Nanoporous Sb/C | 436 (0.05 A·g-1) | No fading after 200 cycles | [8] |
| Antimony nanocrystals/C | 520 (0.1 A·g-1) | 88% after 500 cycles | [9] |
| SiC-Sb-Cu-C | 542 (0.02 A·g-1) | No fading after 100 cycles | [10] |
| De-Sb/MCNT | 408.6 (0.2 A·g-1) | 97% after 200 cycles, 88% after 330 cycles | This work |
| [1] | CAO Y L. The opportunities and challenges of sodium ion battery. Energy Storage Science and Technology, 2020, 9(3):757-761. |
| [2] | LU H, AI F, JIA Y, et al. Exploring sodium-ion storage mechanism in hard carbons with different microstructure prepared by ball- milling method. Small, 2018, 14(39): 1802694-1-8. |
| [3] |
ALLAN P K, GRIFFIN J M, DARWICHE A, et al. Tracking sodium- antimonide phase transformations in sodium-ion anodes: insights from operando pair distribution function analysis and solid-state NMR spectroscopy. Journal of the American Chemical Society, 2016, 138(7):2352-2365.
DOI URL |
| [4] |
XIE H, KALISVAART W P, OLSEN B C, et al. Sn-Bi-Sb alloys as anode materials for sodium ion batteries. Journal of Materials Chemistry A, 2017, 5(20):9661-9670.
DOI URL |
| [5] |
PAN Y, WU X J, ZHANG Z Q, et al. Binder and carbon-free SbSn-P nanocomposite thin films as anode materials for sodium-ion batteries. Journal of Alloys and Compounds, 2017, 714:348-355.
DOI URL |
| [6] |
ZHAO Y, MANTHIRAM A. High-capacity, high-rate Bi-Sb alloy anodes for lithium-ion and sodium-ion batteries. Chemistry of Materials, 2015, 27(8):3096-3101.
DOI URL |
| [7] |
WANG L, WANG C, ZHANG N, et al. High anode performance of in situ formed Cu2Sb nanoparticles integrated on Cu foil via replacement reaction for sodium-ion batteries. ACS Energy Letters, 2016, 2(1):256-262.
DOI URL |
| [8] |
LEE C W, KIM J C, PARK S, et al. Highly stable sodium storage in 3-D gradational Sb-NiSb-Ni heterostructures. Nano Energy, 2015, 15:479-489.
DOI URL |
| [9] | KIM D, HWANG C, JEONG J, et al. Bipolymer-cross-linked binder to improve the reversibility and kinetics of sodiation and desodiation of antimony for sodium-ion batteries. ACS Applied Materials & Interfaces, 2019, 11(46):43039-43045. |
| [10] | GAO H, ZHOU W, JANG J H, et al. Cross-linked chitosan as a polymer network binder for an antimony anode in sodium-ion batteries. Advanced Energy Materials, 2016, 6(6): 1502130-1-7. |
| [11] |
FENG J, WANG L, LI D, et al. Enhanced electrochemical stability of carbon-coated antimony nanoparticles with sodium alginate binder for sodium-ion batteries. Progress in Natural Science: Materials International, 2018, 28(2):205-211.
DOI URL |
| [12] |
DIMITRIJEVIC B J, AIFANTIS K E, HACKL K. The influence of particle size and spacing on the fragmentation of nanocomposite anodes for Li batteries. Journal of Power Sources, 2012, 206:343-348.
DOI URL |
| [13] |
LIU Y, ZHOU B, LIU S, et al. Galvanic replacement synthesis of highly uniform Sb nanotubes: reaction mechanism and enhanced sodium storage performance. ACS Nano, 2019, 13(5):5885-5892.
DOI URL |
| [14] |
HOU H S, JING M J, YANG Y C, et al. Sb porous hollow microspheres as advanced anode materials for sodium-ion batteries. Journal of Materials Chemistry A, 2015, 3(6):2971-2977.
DOI URL |
| [15] | LIU S, FENG J, BIAN X, et al. The morphology-controlled synthesis of a nanoporous-antimony anode for high-performance sodium-ion batteries. Energy & Environmental Science, 2016, 9(4):1229-1236. |
| [16] |
LI H, WANG K, ZHOU M, et al. Facile tailoring of multidimensional nanostructured Sb for sodium storage applications. ACS Nano, 2019, 13(8):9533-9540.
DOI URL |
| [17] |
WOLFENSTINE J, FOSTER D, READ J, et al. Experimental confirmation of the model for microcracking during lithium charging in single-phase alloys. Journal of Power Sources, 2000, 87(1/2):1-3.
DOI URL |
| [18] |
QIAN J, CHEN Y, WU L, et al. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chemical Communications, 2012, 48(56):7070-7072.
DOI URL |
| [19] |
ZHOU X, DAI Z, BAO J, et al. Wet milled synthesis of an Sb/ MWCNT nanocomposite for improved sodium storage. Journal of Materials Chemistry A, 2013, 1(44):13727-13731.
DOI URL |
| [20] |
ZHOU X, LIU X, XU Y, et al. An SbOX/reduced graphene oxide composite as a high-rate anode material for sodium-ion batteries. The Journal of Physical Chemistry C, 2014, 118(41):23527-23534.
DOI URL |
| [21] |
JUNG G J, LEE Y, MUN Y S, et al. Sb-AlC0.75-C composite anodes for high-performance sodium-ion batteries. Journal of Power Sources, 2017, 340:393-400.
DOI URL |
| [22] | GU J, DU Z, ZHANG C, et al. Liquid-phase exfoliated metallic antimony nanosheets toward high volumetric sodium storage. Advanced Energy Materials Liquid-phase exfoliated metallic antimony nanosheets toward high volumetric sodium storage. Advanced Energy Materials, 2017, 7(17): 1700447-1-8 |
| [23] |
LIN Z, WANG G, XIONG X, et al. Ni-polymer gels-derived hollow NiSb alloy confined in 3D interconnected carbon as superior sodium- ion battery anode. Electrochimica Acta, 2018, 269:225-231.
DOI URL |
| [24] |
WU P, ZHANG A, PENG L, et al. Cyanogel-enabled homogeneous Sb-Ni-C ternary framework electrodes for enhanced sodium storage. ACS Nano, 2018, 12(1):759-767.
DOI URL |
| [25] | GUO M, CHEN J, LIU X, et al. Three-dimensional polypyrrole nano-network with Sb nanocrystals as electrode material for sodium- ion Three-dimensional polypyrrole nano-network with Sb nanocrystals as electrode material for sodium- ion and lithium-ion batteries. Journal of The Electrochemical Society, 2020, 167(2): 020527-1-8. |
| [26] |
LI N, LIAO S, SUN Y, et al. Uniformly dispersed self-assembled growth of Sb2O3/Sb@graphene nanocomposites on a 3D carbon sheet network for high Na-storage capacity and excellent stability. Journal of Materials Chemistry A, 2015, 3(11):5820-5828.
DOI URL |
| [27] | WANG H, FAN S, CAO Y, et al. Building a cycle-stable Fe-Si alloy/carbon nanocomposite anode for Li-ion batteries through a covalent-bonding method. ACS Applied Materials & Interfaces, 2020, 12(27):30503-30509. |
| [28] | FERRARI A C, ROBERTSON J. Resonant Raman spectroscopy of disordered, amorphous, diamond like carbon. Physical Review B, 2001, 64(7): 075414-1-13. |
| [29] |
ZHANG M, OUYANG L, ZHU M, et al. A phosphorus and carbon composite containing nanocrystalline Sb as a stable and high-capacity anode for sodium ion batteries. Journal of Materials Chemistry A, 2020, 8(1):443-452.
DOI URL |
| [30] |
DREWETT N E, ALDOUS I M, ZOU J, et al. In situ Raman spectroscopic analysis of the lithiation and sodiation of antimony microparticles. Electrochimica Acta, 2017, 247:296-305.
DOI URL |
| [31] | HONG K S, NAM D H, LIM S J, et al. Electrochemically synthesized Sb/Sb2O3 composites as high-capacity anode materials utilizing a reversible conversion reaction for Na-ion batteries. ACS Applied Materials & Interfaces, 2015, 7(31):17264-17271. |
| [32] |
BODENES L, DARWICHE A, MONCONDUIT L, et al. The solid electrolyte interphase a key parameter of the high performance of Sb in sodium-ion batteries: comparative X-ray photoelectron spectroscopy study of Sb/Na-ion and Sb/Li-ion batteries. Journal of Power Sources, 2015, 273:14-24.
DOI URL |
| [33] |
ERLEBACHER J, AZIZ M J, KARMA A, et al. Evolution of nanoporosity in dealloying. Nature, 2001, 410(6827):450-453.
DOI URL |
| [34] |
LU X, WANG Z, LIU K, et al. Hierarchical Sb2MOO6 microspheres for high-performance sodium-ion battery anode. Energy Storage Materials, 2019, 17:101-110.
DOI URL |
| [35] |
DARWICHE A, MARINO C, SOUGRATI M T, et al. Better cycling performances of bulk Sb in Na-ion batteries compared to Li-ion systems: an unexpected electrochemical mechanism. Journal of the American Chemical Society, 2012, 134(51):20805-20811.
DOI URL |
| [36] | LEE Y, LEE K Y, CHOI W. One-pot synthesis of antimony-embedded silicon oxycarbide materials for high-performance sodium-ion batteries. Advanced Functional Materials, 2017, 27(43): 1702607-1-13. |
| [1] | 孔国强, 冷明哲, 周战荣, 夏池, 沈晓芳. Sb掺杂O3型Na0.9Ni0.5Mn0.3Ti0.2O2钠离子电池正极材料[J]. 无机材料学报, 2023, 38(6): 656-662. |
| [2] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [3] | 赵伟, 徐阳, 万颖杰, 蔡天逊, 穆金潇, 黄富强. 金属氰胺化合物的结构、合成及电化学储能应用[J]. 无机材料学报, 2022, 37(2): 140-151. |
| [4] | 刘丹, 赵亚欣, 郭锐, 刘艳涛, 张志东, 张增星, 薛晨阳. 退火条件对磁控溅射MgO-Ag3Sb-Sb2O4柔性薄膜热电性能的影响[J]. 无机材料学报, 2022, 37(12): 1302-1310. |
| [5] | 王晶, 徐守冬, 卢中华, 赵壮壮, 陈良, 张鼎, 郭春丽. 钠离子电池中空结构CoSe2/C负极材料的制备及储钠性能研究[J]. 无机材料学报, 2022, 37(12): 1344-1350. |
| [6] | 逯旭, 侯绩翀, 张强, 樊建锋, 陈少平, 王晓敏. Mg含量对Mg3(1+z)Sb2化合物热电传输性能的影响[J]. 无机材料学报, 2021, 36(8): 835-840. |
| [7] | 张晓君, 李佳乐, 邱吴劼, 杨淼森, 刘建军. 钠离子电池正极材料P2-Nax[Mg0.33Mn0.67]O2的电化学活性研究[J]. 无机材料学报, 2021, 36(6): 623-628. |
| [8] | 郭宇, 姜晓庆, 吴红梅, 肖昱, 仵大富, 刘鑫. 2-羟基-1-萘甲醛功能化SBA-15吸附剂的制备及其对Cr(III)的吸附性能[J]. 无机材料学报, 2021, 36(11): 1163-1170. |
| [9] | 肖民,邢如月,姚寿广,程杰,申亚举,杨裕生. Mn掺杂Ni(OH)2的合成及电化学性能研究[J]. 无机材料学报, 2019, 34(7): 703-708. |
| [10] | 李鹏, 聂晓蕾, 田烨, 方文兵, 魏平, 朱婉婷, 孙志刚, 张清杰, 赵文俞. Bi0.5Sb1.5Te3/环氧树脂柔性复合热电厚膜的制备及其面内制冷性能[J]. 无机材料学报, 2019, 34(6): 679-684. |
| [11] | 罗世强, 郑春满, 孙巍巍, 谢威, 柯剑煌, 刘双科, 洪晓斌, 李宇杰, 许静. ZIF-67衍生Co-NC多孔碳材料的可控制备及其在锂-硫二次电池中的应用研究[J]. 无机材料学报, 2019, 34(5): 502-508. |
| [12] | 李勇, 何玮鑫, 郑芯月, 于胜兰, 李海同, 黎弘毅, 张蓉, 王雨. 水系钠离子电池普鲁士蓝正极材料的制备与电化学性能研究[J]. 无机材料学报, 2019, 34(4): 365-372. |
| [13] | 余冠廷, 忻佳展, 朱铁军, 赵新兵. Zn-Sb双掺杂Mg2(Si,Sn)合金的热电性能[J]. 无机材料学报, 2019, 34(3): 310-314. |
| [14] | 王武练, 张军, 王秋实, 陈亮, 刘兆平. 高质量水系钠离子电池正极Fe4[Fe(CN)6]3的合成及其电化学性能[J]. 无机材料学报, 2019, 34(12): 1301-1308. |
| [15] | 王家虎, 王文馨, 杜鹏, 胡芳东, 姜晓蕾, 杨剑. Na3V2(PO4)2F3@V2O5-x复合材料的制备及储钠性能研究[J]. 无机材料学报, 2019, 34(10): 1097-1102. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||