无机材料学报 ›› 2021, Vol. 36 ›› Issue (5): 527-534.DOI: 10.15541/jim20200396
郑燕宁1,2( ), 季军荣3, 梁雪玲1, 赖正杰1, 陈启帆1, 廖丹葵1,2
), 季军荣3, 梁雪玲1, 赖正杰1, 陈启帆1, 廖丹葵1,2
收稿日期:2020-07-04
修回日期:2020-08-20
出版日期:2021-05-20
网络出版日期:2021-04-19
通讯作者:
廖丹葵, 教授. E-mail: liaodk@gxu.edu.cn
作者简介:郑燕宁(1995-), 女, 硕士研究生. E-mail:ningningzy@sina.cn
基金资助:
ZHENG Yanning1,2( ), JI Junrong3, LIANG Xueling1, LAI Zhengjie1, CHENG Qifan1, LIAO Dankui1,2
), JI Junrong3, LIANG Xueling1, LAI Zhengjie1, CHENG Qifan1, LIAO Dankui1,2
Received:2020-07-04
Revised:2020-08-20
Published:2021-05-20
Online:2021-04-19
Contact:
LIAO Dankui, professor.E-mail: liaodk@gxu.edu.cn
About author:ZHENG Yanning(1995-), female, Master candidate. E-mail:ningningzy@sina.cn
Supported by:摘要:
纳米酶由于其独特、高效、稳定的催化性质而在生化反应中备受关注。本研究以碳酸钙微球为绿色模板剂, 多巴胺为氮源与碳源, 合成了氮掺杂中空碳球(N-HCSs)。以3,3°,5,5°-四甲基联苯胺(TMB)为底物, 采用紫外分光光度法探究了N-HCSs的类氧化物酶的催化活性, 并研究其催化机理。结果表明, N-HCSs具有氧化物模拟酶催化活性, KOH活化后氮掺杂中空碳球的催化活性提高了3倍; N-HCSs氧化物模拟酶催化反应符合Michaelis-Menten方程, 活化前后的米氏常数Km分别为0.105和0.083, 对底物具有良好的亲和能力; N-HCSs氧化物模拟酶催化反应中起主要作用的活性氧基团是超氧阴离子(O2 ?-)。本研究为高活性无机非金属类氧化物模拟酶的设计和制备提供了理论依据。
中图分类号:
郑燕宁, 季军荣, 梁雪玲, 赖正杰, 陈启帆, 廖丹葵. 氮掺杂中空碳球氧化物模拟酶性能研究[J]. 无机材料学报, 2021, 36(5): 527-534.
ZHENG Yanning, JI Junrong, LIANG Xueling, LAI Zhengjie, CHENG Qifan, LIAO Dankui. Performance of Nitrogen-doped Hollow Carbon Spheres as Oxidase Mimic[J]. Journal of Inorganic Materials, 2021, 36(5): 527-534.
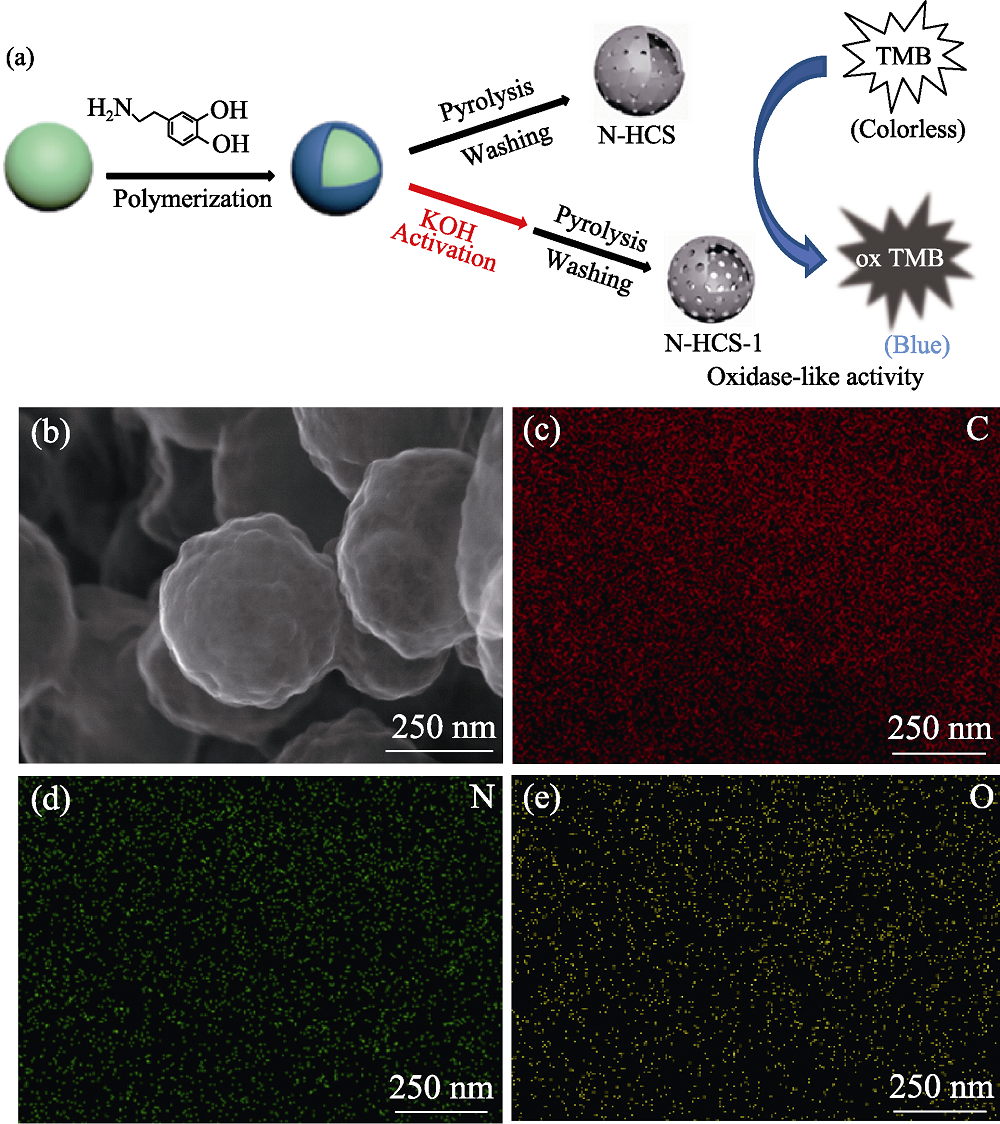
图1 N-HCSs的形貌和元素分析 (a) Schematic presentation for the oxide enzyme mimetic activity of N-HCSs; (b) STEM image of N-HCS; (c) C, (d) N and (e) O EDX mappings of N-HCS
Fig. 1 Morphologies and element mappings of N-HCSs
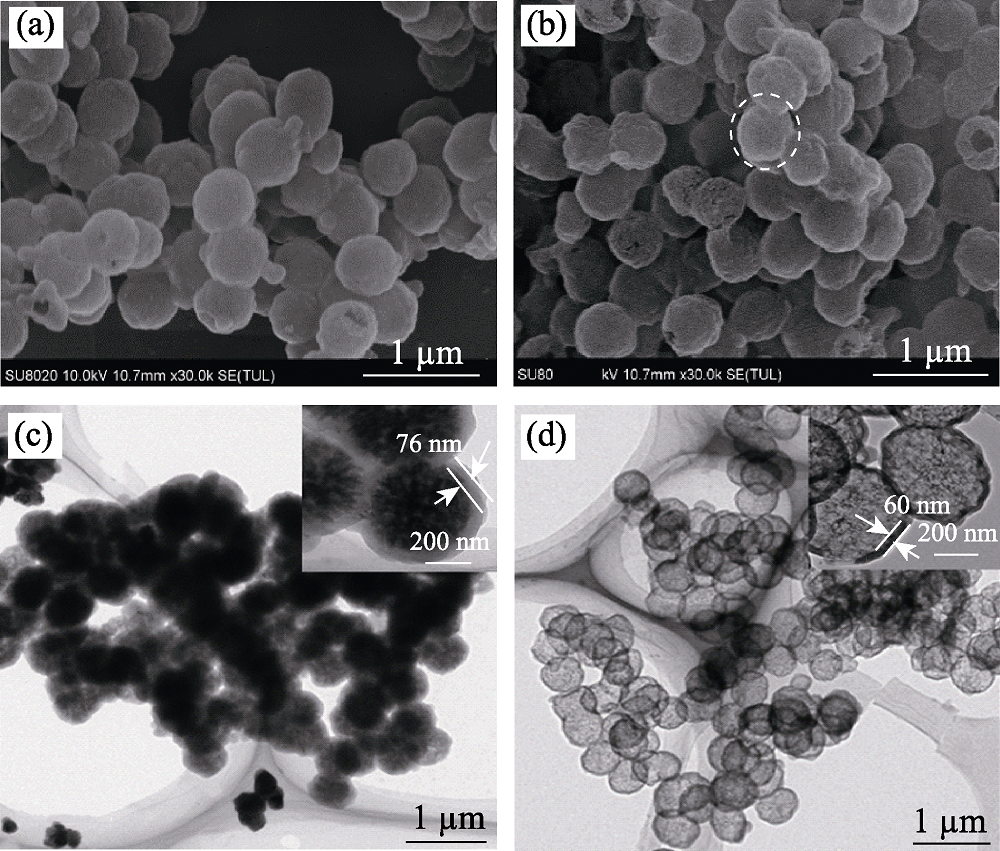
图2 (a)N-HCS和(b)N-HCS-1的SEM照片; (c)CaCO3@PDA和(d)N-HCS的TEM照片
Fig. 2 SEM images of (a) N-HCS and (b) N-HCS-1, and TEM images of (c) CaCO3@PDA and (d) N-HCS
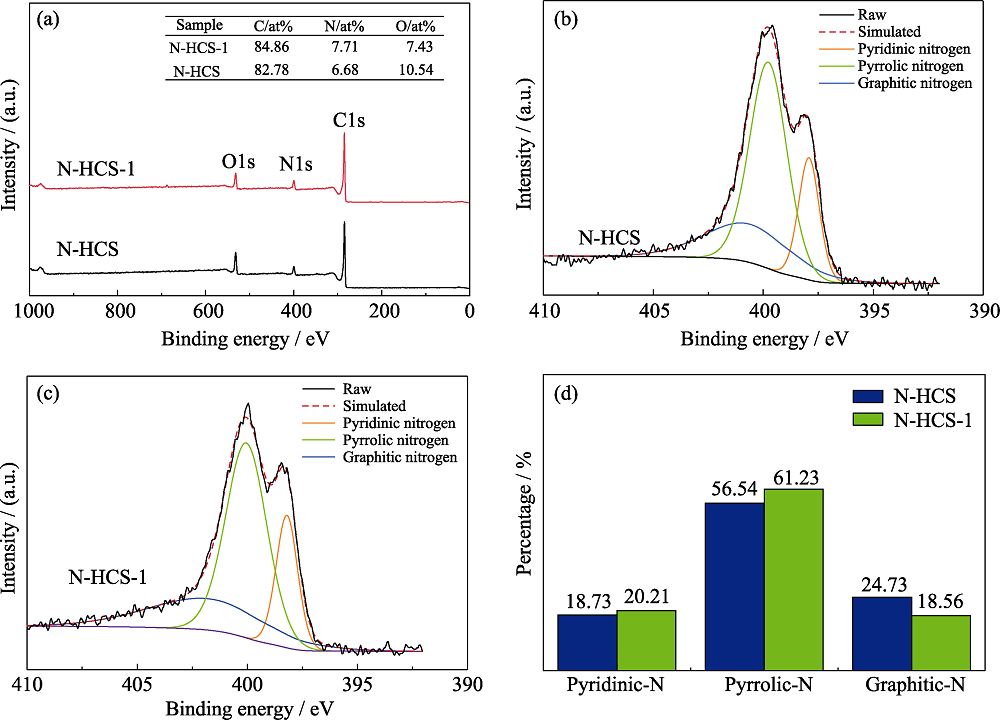
图3 (a)N-HCS和N-HCS-1的XPS全谱, (b)N-HCS和(c)N-HCS-1的N1s谱, (d)N-HCS和N-HCS-1的N元素组成
Fig. 3 (a) XPS full spectra of N-HCS and N-HCS-1; N1s spectra of (b) N-HCS and (c) N-HCS-1; (d) N species contents of N-HCS and N-HCS-1
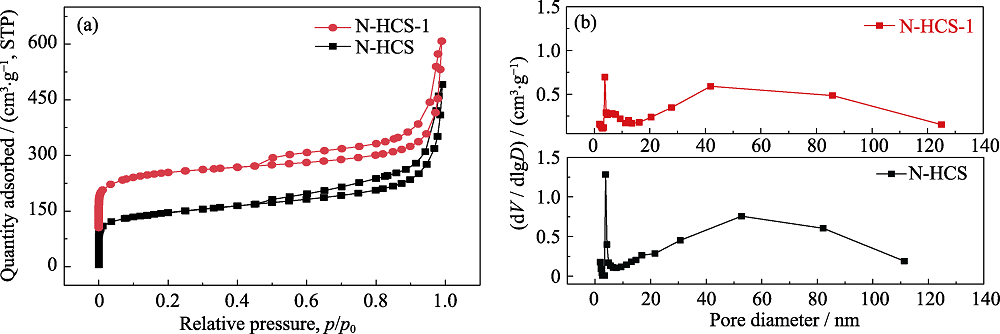
图4 (a)N-HCS和N-HCS-1的N2吸附-脱附等温曲线及(b)HK模型孔径分布图
Fig. 4 (a) Nitrogen absorption-desorption isotherm curves of N-HCS and N-HCS-1, (b) HK model corresponding pore size distribution curves of N-HCS and N-HCS-1
| Sample | Specfic suface/(m2•g-1) | Vt/ (cm3•g-1) | Vm/ (cm3•g-1) | Sm/ (m2•g-1) | (Sm/St)/ % | |
|---|---|---|---|---|---|---|
| Langmuir | BET | |||||
| N-HCS | 685.6 | 490.1 | 0.651 | 0.109 | 230.51 | 45.49 |
| N-HCS-1 | 730.0 | 518.4 | 0.732 | 0.111 | 228.16 | 44.01 |
表1 N-HCS和N-HCS-1材料的孔结构参数
Table 1 Pore structure parameters of N-HCS and N-HCS-1
| Sample | Specfic suface/(m2•g-1) | Vt/ (cm3•g-1) | Vm/ (cm3•g-1) | Sm/ (m2•g-1) | (Sm/St)/ % | |
|---|---|---|---|---|---|---|
| Langmuir | BET | |||||
| N-HCS | 685.6 | 490.1 | 0.651 | 0.109 | 230.51 | 45.49 |
| N-HCS-1 | 730.0 | 518.4 | 0.732 | 0.111 | 228.16 | 44.01 |
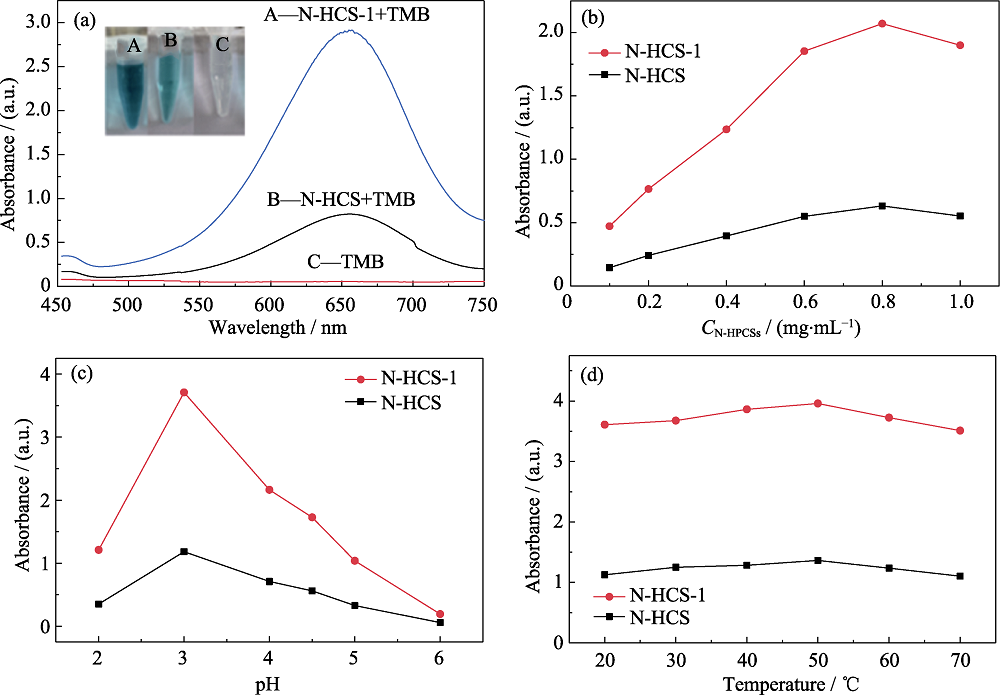
图5 (a)不同体系的紫外-可见吸收光谱, (b)催化剂浓度、(c)pH和(d)温度对N-HCSs氧化物模拟酶活性的影响
Fig. 5 (a) UV-Vis absorption spectra of different systems, and effects of (b) catalyst concentration, (c) pH and (d) temperature on the oxidase-like activity of N-HCSs
| Catalyst | Substrate | Km/ (mmol•L-1) | Vmax/(×10-8, mol•L-1•s-1) | Ref. |
|---|---|---|---|---|
| N-PCS | TMB | 0.095 | 5.20 | [4] |
| His@AuNCs | TMB | 0.041 | 6.21 | [31] |
| Pt Au DNPs | TMB | 0.22 | 2.82 | [32] |
| Acr+-Mes | TMB | 0.129 | 2.68 | [33] |
| N-HCS | TMB | 0.1049 | 4.69 | This work |
| N-HCS-1 | TMB | 0.0825 | 5.98 | This work |
表2 N-HCSs与其它氧化物模拟酶动力学参数的比较
Table 2 Comparison of the Km and Vmax between N-HCSs and other oxidase-like
| Catalyst | Substrate | Km/ (mmol•L-1) | Vmax/(×10-8, mol•L-1•s-1) | Ref. |
|---|---|---|---|---|
| N-PCS | TMB | 0.095 | 5.20 | [4] |
| His@AuNCs | TMB | 0.041 | 6.21 | [31] |
| Pt Au DNPs | TMB | 0.22 | 2.82 | [32] |
| Acr+-Mes | TMB | 0.129 | 2.68 | [33] |
| N-HCS | TMB | 0.1049 | 4.69 | This work |
| N-HCS-1 | TMB | 0.0825 | 5.98 | This work |
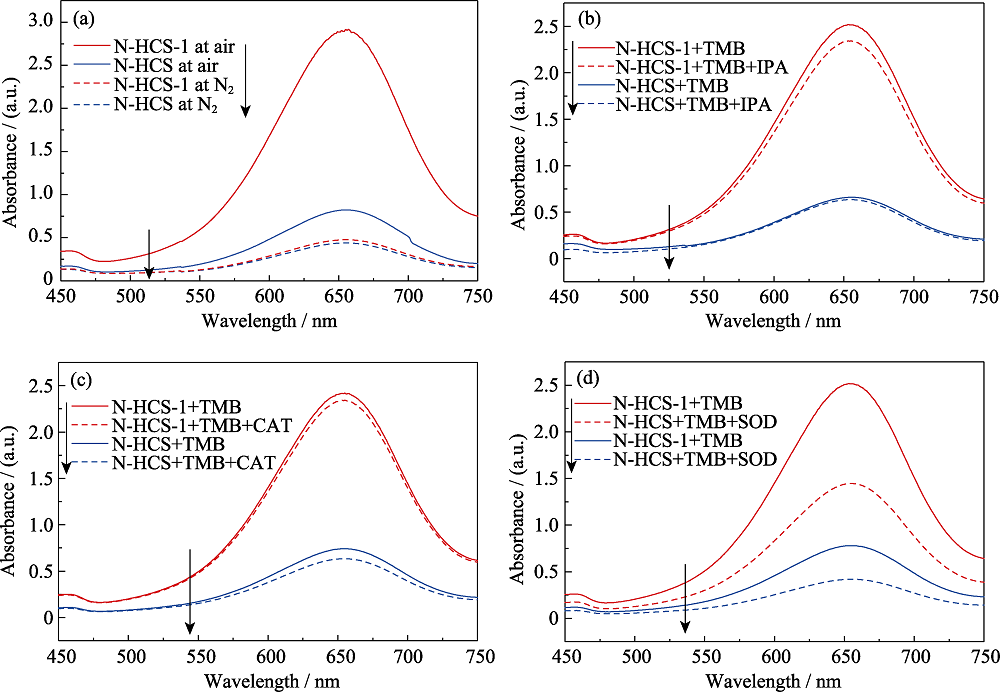
图7 在体系中(a)通入氮气后的吸收光图谱, 及加入不同活性氧清除剂(b)异丙醇(IPA)、(c)过氧化氢酶(CAT)、(d)超氧化物歧化酶(SOD)对N-HCS和N-HCS-1类氧化物酶活性的影响
Fig. 7 (a) Absorption spectra of the solution containing TMB and N-HCS and N-HCS-1 under N2-saturated conditions, and effect of scavengers (b) IPA, (c) CAT and (d) SOD on the catalytic oxidation of TMB by the N-HCS and N-HCS-1
| [1] |
LIN Y, REN J, QU X, et al. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Accounts of Chemical Research, 2014,47(4):1097-1105.
DOI URL PMID |
| [2] |
GAO L, ZHUANG J, NIE L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnology, 2007,2(9):577-583.
DOI URL PMID |
| [3] |
WEI X, CHEN J, ALI M C, et al. Cadmium cobaltite nanosheets synthesized in basic deep eutectic solvents with oxidase-like, peroxidase-like, and catalase-like activities and application in the colorimetric assay of glucose. Microchimica Acta, 2020,187(6):314-325.
URL PMID |
| [4] |
FAN K, XI J, FAN L, et al. In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nature Communications, 2018,9:1440.
URL PMID |
| [5] |
CHEN Z, YIN J J, ZHOU Y T, et al. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano, 2012,6(5):4001-4012.
DOI URL PMID |
| [6] |
WEI H, WANG E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chemical Society Reviews, 2013,42(14):6060-6093.
DOI URL PMID |
| [7] | ZHANG X, MAO X, LI S Q, et al. Tuning the oxidase mimics activity of manganese oxides via control of their growth conditions for highly sensitive detection of glutathione. Sensors and Actuators B-Chemical, 2018,258:80-87. |
| [8] |
WANG Y, ZHANG Z, JIA G, et al. Elucidating the mechanism of the structure-dependent enzymatic activity of Fe-N/C oxidase mimic. Chemical Communications, 2019,55(36):5271-5274.
DOI URL PMID |
| [9] |
HE W, HAN X, JIA H, et al. AuPt alloy nanostructures with tunable composition and enzyme-like activities for colorimetric detection of bisulfide. Scientific Reports, 2017,7:40103.
DOI URL PMID |
| [10] |
GE C, FANG G, SHEN X, et al. Facet energy versus enzyme-like activities: the unexpected protection of palladium nanocrystals against oxidative damage. ACS Nano, 2016,10(11):10436-10445.
DOI URL PMID |
| [11] | HU Y, GAO X J, ZHU Y, et al. Nitrogen-doped carbon nanomaterials as highly active and specific peroxidase mimics. Chemistry of Materials, 2018,30(18):6431-6439. |
| [12] | MU J, LI J, ZHAO X, et al. Cobalt-doped graphitic carbon nitride with enhanced peroxidase-like activity for wastewater treatment. RSC Advances, 2016,6(42):35568-35576. |
| [13] | AHMWD A, JOHN P, NAWAZ M H, et al. Zinc-doped mesoporous graphitic carbon nitride for the colorimetric detection of hydrogen peroxide. ACS Nano Materials, 2019,2(8):5156-5168. |
| [14] |
ZHANG H, LIANG X, HAN L, et al. “Non-naked” gold with glucose oxidase-like activity: a nanozyme for tandem catalysis. Small, 2018,14(44):e1803256.
URL PMID |
| [15] | LI R, LEI C, ZHAO X E, et al. A label-free fluorimetric detection of biothiols based on the oxidase-like activity of Ag+ ions. Sectrochimica Acta art A: Molecular & Biomolecular Spectroscopy, 2018,188:20-25. |
| [16] |
FENG J Y, HUANG P, WU F Y, et al. Gold-platinum bimetallic nanocluster with enhanced peroxidase-like activity and its integrated agarose hydrogel-based sensing platform for colorimetric analysis of glucose level in serum. Analyst., 2017,142(21):4106-4115.
URL PMID |
| [17] |
SHEN X, LIU W, GAO X, et al. Mechanisms of oxidase and superoxide dismutation-like activities of gold, silver, platinum, and palladium, and their alloys: a general way to the activation of molecular oxygen. Journal of the American Chemical Society, 2015,137(50):15882-15891.
URL PMID |
| [18] | HUANG L, ZHANG W, CHEN K, et al. Facet-selective response of trigger molecule to CeO2 {110} for up-regulating oxidase-like activity. Chemical Engineering Journal, 2017,330(17):746-752. |
| [19] | ZHAO M, TAO Y, HUANG W, et al. Reversible pH switchable oxidase-like activities of MnO2 nanosheets for a visual molecular majority logic gate. Physical Chemistry Chemical, 2018,20(45):28644-28648. |
| [20] | FAN Y, SHI W, ZHANG X, et al. Mesoporous material-based manipulation of the enzyme-like activity of CoFe2O4 nanoparticles. Journal of Materials Chemistry A, 2014,2(8):2482-2486. |
| [21] | DU J, LIU L, YU Y, et al. Hollow carbon sphere with tunable structure by encapsulation pyrolysis synchronous deposition for cefalexin adsorption. Journal of Inorganic Materials, 2020,35(5):608-616. |
| [22] | WANG N, SONG H, REN H, et al. Partly nitrogenized nickel oxide hollow spheres with multiple compositions for remarkable electrochemical performance. Chemical Engineering Journal, 2019,358:531-539. |
| [23] |
GONG K, DU F, XIA Z, et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science, 2009,323(5915):760-764.
DOI URL PMID |
| [24] |
ZHANG Y, YANG H, CHI Q, et al. Nitrogen-doped carbon supported nickel nanoparticles: a robust catalyst to bridge the hydrogenation of nitriles and the reductive amination of carbonyl compounds for the synthesis of primary amines. ChemSusChem, 2019,12(6):1246-1255.
DOI URL PMID |
| [25] |
FAN L, XU X, ZHU C, et al. Tumor catalytic-photothermal therapy with yolk-shell Gold@Carbon nanozymes. ACS Applied Materials & Interfaces, 2018,10(5):4502-4511.
URL PMID |
| [26] |
LIANG H W, ZHUANG X, SEBASTIAN B, et al. Hierarchically porous carbons with optimized nitrogen doping as highly active electro-catalysts for oxygen reduction. Nature Communications, 2014,5:4973.
URL PMID |
| [27] |
HE X, LIU P, LIU J, et al. Facile synthesis of hierarchical N-doped hollow porous carbon whiskers with ultrahigh surface area via synergistic inner-outer activation for casein hydrolysate adsorption. Journal of Materials Chemistry B, 2017,5:9211-9218.
URL PMID |
| [28] | MISTAR E M, ALFATAH T, SUPARDAN M D. Synthesis and characterization of activated carbon from Bambusa vulgaris striata using two-step KOH activation. Journal of Materials Research and Technology, 2020,9(3):6278-6286. |
| [29] | WU T, MA Z, LI P, et al. Bifunctional colorimetric biosensors via regulation of the dual nanoenzyme activity of carbonized FeCo-ZIF. Sensors & Actuators B Chemical, 2019,290:357-363. |
| [30] |
WANG H, LI S, SI Y, et al. Recyclable enzyme mimic of cubic Fe3O4 nanoparticles loaded on graphene oxide-dispersed carbon nanotubes with enhanced peroxidase-like catalysis and electrocatalysis. Journal of Materials Chemistry B, 2016,2:4442-4448.
URL PMID |
| [31] |
LIU L, DU J, LIU W E, et al. Enhanced His@AuNCs oxidase-like activity by reduced graphene oxide and its application for colorimetric and electrochemical detection of nitrite. Analytical & Bioanalytical Chemistry, 2019,411(10):2189-2200.
URL PMID |
| [32] | CHENG Q, YANG Y, YANG L L, et al. Pt-Au dendritic nanoparticles with high oxidase-like activity for detection of ascorbic acid. Journal of Inorganic Materials, 2020,35(10):1169-1176. |
| [33] |
DU J, WANG J, HUANG W, et al. Visible light-activatable oxidase mimic of 9-mesityl-10-methylacridinium ion for colorimetric detection of biothiols and logic operations. Analytical Chemistry, 2018,90(16):9959-9965.
URL PMID |
| [34] |
WANG Y, ZHANG Z, JIA G, et al. Elucidating the mechanism of the structure-dependent enzymatic activity of Fe-N/C oxidase mimics. Chemical Communications, 2019,55:5271-5274.
DOI URL PMID |
| [35] |
CHEN M, WANG Z, SHU J, et al. Mimicking a natural enzyme system: cytochrome c oxidase-like activity of Cu2O nanoparticles by receiving flectrons from cytochrome. Inorganic Chemistry, 2017,56(16):9400-9403.
URL PMID |
| [36] | LIU J, HU X, HOU S, et al. Au@Pt core/shell nanorods with peroxidase and ascorbate oxidase-like activities for improved detection of glucose. Sensors & Actuators B Chemical, 2012,166/167:708-714. |
| [1] | 高娃, 熊宇杰, 吴聪萍, 周勇, 邹志刚. 基于超薄纳米结构的光催化二氧化碳选择性转化[J]. 无机材料学报, 2022, 37(1): 3-14. |
| [2] | 许云青,王海增. EDTA辅助水热法制备不同形貌的氟化镁钠[J]. 无机材料学报, 2019, 34(9): 933-937. |
| [3] | 鱼银虎, 汪涛, 廖秋平, 缪润杰, 潘剑锋, 张度宝. 低温固相反应合成纳米级TiB2-TiC复合粉体[J]. 无机材料学报, 2016, 31(3): 324-328. |
| [4] | 鱼银虎, 汪 涛, 张洪敏, 张度宝, 潘剑锋. PTFE促发TiC陶瓷粉体低温固相合成研究[J]. 无机材料学报, 2015, 30(3): 272-276. |
| [5] | 豆志河, 张廷安, 文 明, 史冠勇, 赫冀成. 燃烧合成法制备NdB6超细粉体及反应机理[J]. 无机材料学报, 2014, 29(7): 711-716. |
| [6] | 史晓睿, 王 群, 吕羚源, 李 洋, 俞 潇, 陈 刚. 碲化铜纳米材料的液相可控合成及其电导率[J]. 无机材料学报, 2012, 27(4): 433-438. |
| [7] | 穆云超, 梁宝岩, 郭基凤. 金刚石表面形成Ti3SiC2的反应机理[J]. 无机材料学报, 2012, 27(10): 1099-1104. |
| [8] | 徐秀华, 肖汉宁, 郭文明, 高朋召, 彭苏华. 常压固相反应合成 LaB6 粉末及其反应机理[J]. 无机材料学报, 2011, 26(4): 417-421. |
| [9] | 吴 皓1,2, 陈 诚1, 蒋丹宇2, 李 强1. 纳米铌镁酸铅机械化学法低温快速合成[J]. 无机材料学报, 2010, 25(5): 541-545. |
| [10] | 刘学建,李会利,黄政仁,王士维,江东亮. 高温固相反应工艺制备AlON粉体[J]. 无机材料学报, 2009, 24(6): 1159-1162. |
| [11] | 徐顺建,乔冠军,王红洁,李涤尘,卢天健. 微孔碳陶瓷化反应机理的研究[J]. 无机材料学报, 2009, 24(2): 291-296. |
| [12] | 李江鸿,张红波,熊翔,肖鹏,赵磊,黄伯. 含钽树脂先驱体转变生成TaC的过程研究[J]. 无机材料学报, 2007, 22(5): 973-978. |
| [13] | 符史流,尹涛,柴飞. Ca2SnO4:Eu3+的固相反应形成机理及发光性质研究[J]. 无机材料学报, 2007, 22(4): 647-651. |
| [14] | 唐爱东,黄可龙. Li1.15-xNi0.33Co0.33Mn0.33O2+δ溶胶-凝胶法合成、表征及合成机理[J]. 无机材料学报, 2006, 21(2): 397-402. |
| [15] | 刘金华,王大志. 乌贼骨及其水热改性制备羟基磷灰石的研究[J]. 无机材料学报, 2006, 21(2): 433-440. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||