无机材料学报 ›› 2021, Vol. 36 ›› Issue (8): 856-864.DOI: 10.15541/jim20200663
所属专题: 【虚拟专辑】放射性污染物去除(2020~2021); 【能源环境】水体污染物去除
余祥坤1( ), 刘坤1, 李志鹏1, 赵雨露1, 沈锦优2, 茆平1(
), 刘坤1, 李志鹏1, 赵雨露1, 沈锦优2, 茆平1( ), 孙爱武1(
), 孙爱武1( ), 蒋金龙1
), 蒋金龙1
收稿日期:2020-11-19
修回日期:2021-01-04
出版日期:2021-08-20
网络出版日期:2021-03-01
通讯作者:
茆 平, 校聘副教授. E-mail: pingmao@hyit.edu.cn; 孙爱武, 教授. E-mail: sunaiwu@hyit.edu.cn
作者简介:余祥坤(1993-), 男, 硕士研究生. E-mail: yxk0079@163.com
基金资助:
YU Xiangkun1( ), LIU Kun1, LI Zhipeng1, ZHAO Yulu1, SHEN Jinyou2, MAO Ping1(
), LIU Kun1, LI Zhipeng1, ZHAO Yulu1, SHEN Jinyou2, MAO Ping1( ), SUN Aiwu1(
), SUN Aiwu1( ), JIANG Jinlong1
), JIANG Jinlong1
Received:2020-11-19
Revised:2021-01-04
Published:2021-08-20
Online:2021-03-01
Contact:
MAO Ping, associate professor. E-mail: pingmao@hyit.edu.cn; SUN Aiwu, professor. E-mail: sunaiwu@hyit.edu.cn
About author:YU Xiangkun(1993-), male, Master candidate. E-mail: yxk0079@163.com
Supported by:摘要:
放射性碘是核裂变必然产物。为解决现有铜基吸附剂的吸附容量低、铜利用率低、易脱附等不足, 基于凹凸棒石的廉价易得、可交换阳离子富足和层间结构等特性, 采用浸渍-还原法将单质Cu负载于凹凸棒石结构中, 制备得到Cu/凹凸棒石复合材料, 对样品进行表征并考察样品对液相碘离子的吸附性能。研究结果表明, 实验制备的Cu/凹凸棒石复合材料约有7.5%掺杂量的纳米铜颗粒负载于凹凸棒石层间结构中。与传统铜基吸附剂相比, 该材料具有高达116.1 mg/g的碘离子吸附量以及72%铜利用率; 并且可在中性、弱酸性以及SO42-、NO3-、Na+、Mg+等干扰离子共存的环境下使用。此外, 得益于铜负载凹凸棒石结构内, 该复合材料展现出较强的抗氧化性能, 且吸附后产物也具有较强的稳定性。
中图分类号:
余祥坤, 刘坤, 李志鹏, 赵雨露, 沈锦优, 茆平, 孙爱武, 蒋金龙. 铜/凹凸棒石复合材料高效吸附放射性碘离子性能[J]. 无机材料学报, 2021, 36(8): 856-864.
YU Xiangkun, LIU Kun, LI Zhipeng, ZHAO Yulu, SHEN Jinyou, MAO Ping, SUN Aiwu, JIANG Jinlong. Efficient Adsorption of Radioactive Iodide by Copper/Palygorskite Composite[J]. Journal of Inorganic Materials, 2021, 36(8): 856-864.
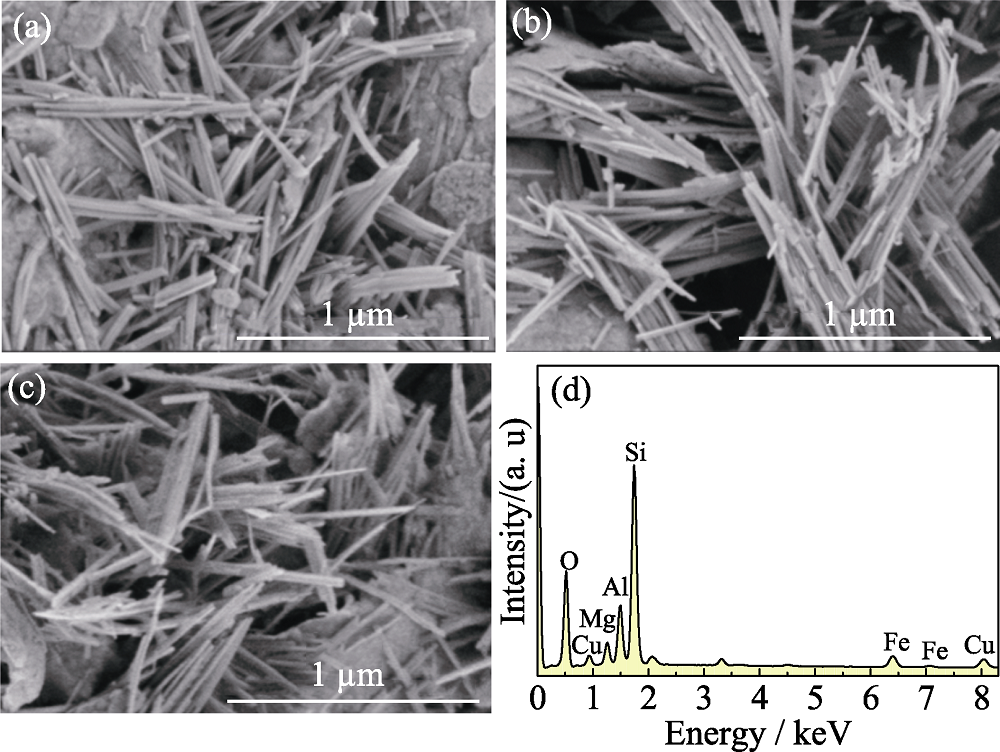
图1 样品(a)PAL, (b)Cu2+@PAL, (c)Cu@PAL的SEM照片及(d) Cu@PAL的EDX谱图
Fig. 1 SEM images of the samples (a) PAL, (b) Cu 2+@PAL, (c) Cu@PAL and (d) EDX pattern of Cu@PAL
| Composition | PAL | Cu@PAL |
|---|---|---|
| SiO2 | 60.96 | 59.87 |
| MgO | 9.36 | 8.12 |
| Al2O3 | 12.20 | 11.07 |
| Fe2O3 | 8.00 | 7.51 |
| CaO | 5.55 | 0.46 |
| CuO | 0.01 | 9.83 |
| Na2O | 0.12 | 0.01 |
| LOI | 3.80 | 3.13 |
表1 PAL和Cu@PAL的化学组成(XRF)/wt%
Table1 Chemical analysis (XRF) of PAL and Cu@PAL/wt%
| Composition | PAL | Cu@PAL |
|---|---|---|
| SiO2 | 60.96 | 59.87 |
| MgO | 9.36 | 8.12 |
| Al2O3 | 12.20 | 11.07 |
| Fe2O3 | 8.00 | 7.51 |
| CaO | 5.55 | 0.46 |
| CuO | 0.01 | 9.83 |
| Na2O | 0.12 | 0.01 |
| LOI | 3.80 | 3.13 |
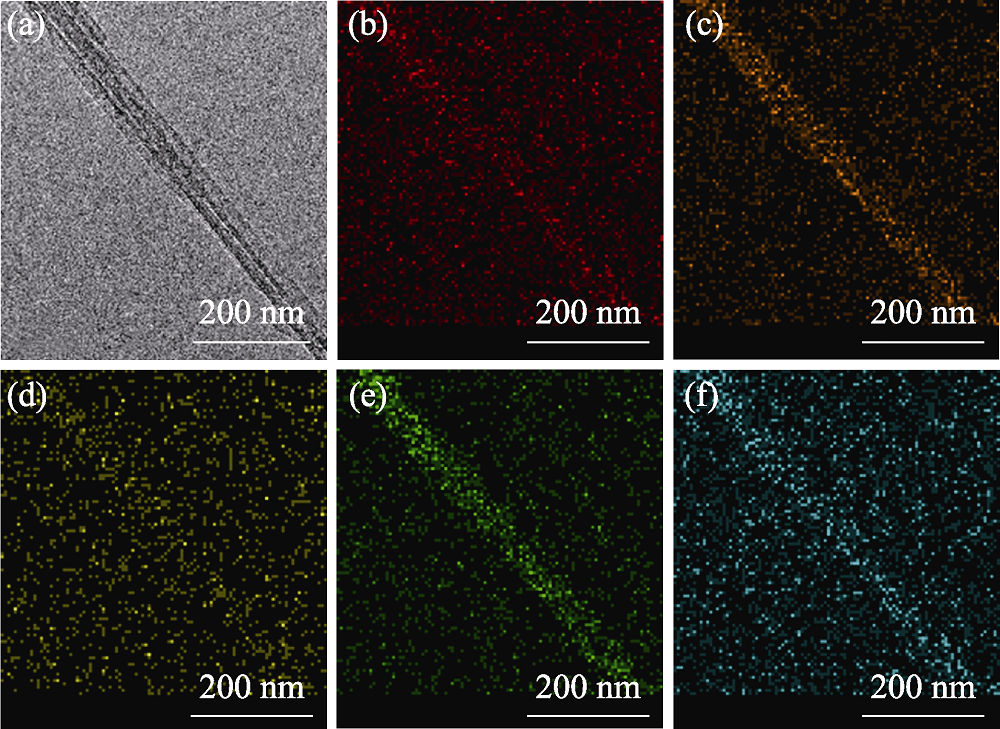
图3 Cu2+@PAL的(a) TEM照片及(b) N, (c) O, (d) Al, (e) Si, (f) Cu的元素分布图
Fig. 3 (a) TEM image of Cu2+@PAL and elemental mappings of (b) N, (c) O, (d) Al, (e) Si, and (f) Cu
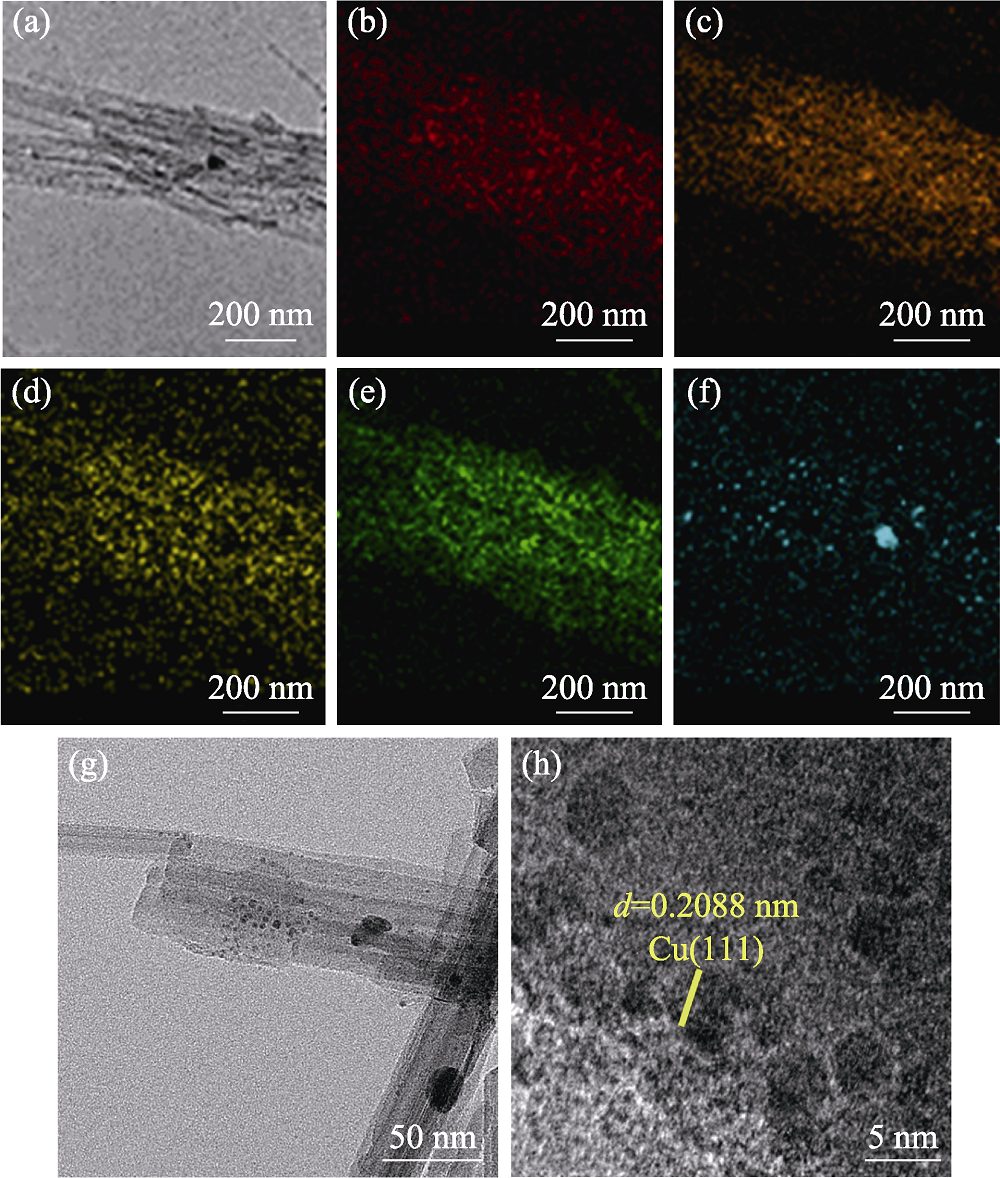
图4 Cu@PAL的(a) TEM照片及(b) N, (c) O, (d) Al, (e) Si, (f) Cu的元素分布图, 更高放大倍率TEM (g)和HRTEM (h) 照片
Fig. 4 (a) TEM image of Cu@PAL and elemental mappings of (b) N, (c) O, (d) Al, (e) Si, and (f) Cu; and high magnification TEM (g) and HRTEM (h) images of Cu@PAL
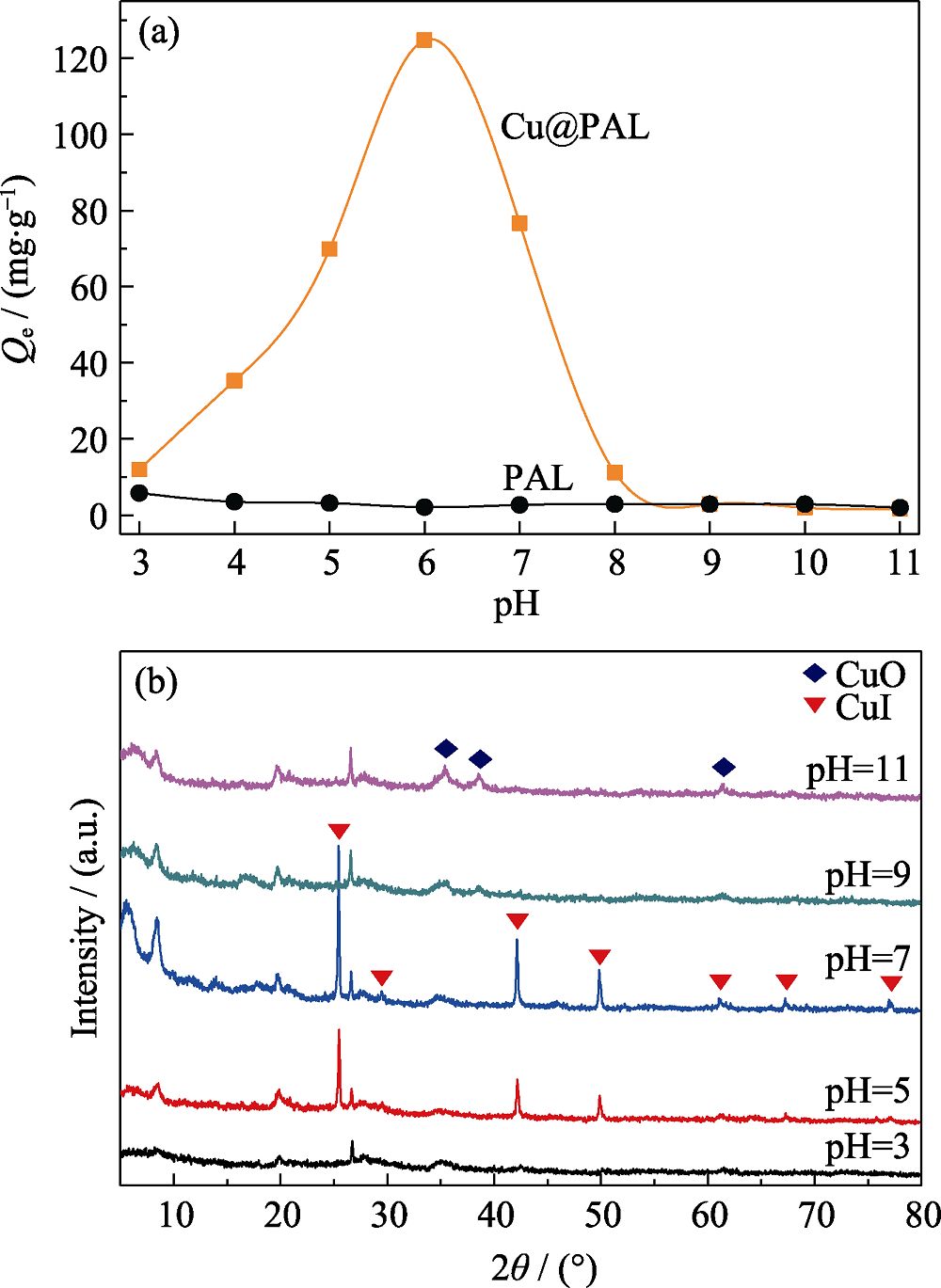
图8 (a)pH对Cu@PAL吸附性能的影响和(b)产物的XRD谱图
Fig. 8 (a) Adsorption capacity of I- anions on Cu@PAL and (b) XRD patterns of adsorption products in the solution with different pH
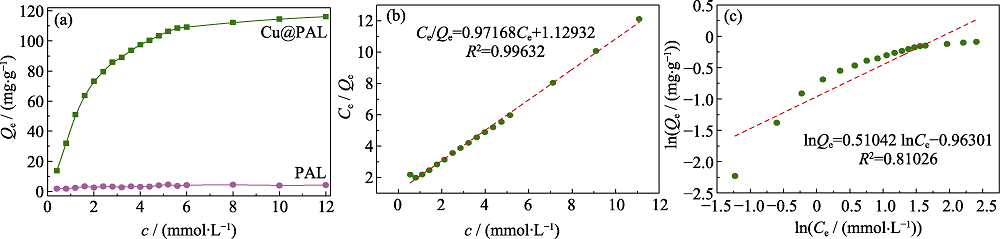
图9 (a)样品的等温吸附曲线, Cu@PAL等温吸附的(b) Langmuir模型和(c) Frenudlich模型模拟曲线
Fig. 9 (a) Adsorption isotherm of the samples, fitting curves of (b) Langmuir model and (c) Frenudlich model for adsorption isotherm of Cu@PAL
| Adsorbent | pH | Qe/ (mg∙g-1) | Utilization efficiency/% | Ref. |
|---|---|---|---|---|
| Cuprite sulfide | 7 | 6.1 | — | [ |
| Cu2O/Cu-C | 7 | 41.2 | 10.38 | [ |
| Hollow Cu/Cu2O | 7 | 33.0 | 1.85 | [ |
| Core-shell Cu/Cu2O | 7 | 22.9 | 1.4 | [ |
| Cu | 7 | 6.35 | 0.32 | [ |
| Cu/PAL | 7 | 116.1 | 71.98 | This work |
表2 铜基吸附剂碘离子吸附性能对比
Table 2 Comparison of several Cu based adsorbents for iodide adsorption
| Adsorbent | pH | Qe/ (mg∙g-1) | Utilization efficiency/% | Ref. |
|---|---|---|---|---|
| Cuprite sulfide | 7 | 6.1 | — | [ |
| Cu2O/Cu-C | 7 | 41.2 | 10.38 | [ |
| Hollow Cu/Cu2O | 7 | 33.0 | 1.85 | [ |
| Core-shell Cu/Cu2O | 7 | 22.9 | 1.4 | [ |
| Cu | 7 | 6.35 | 0.32 | [ |
| Cu/PAL | 7 | 116.1 | 71.98 | This work |
| Langmuir model | Frenudlich model | ||||
|---|---|---|---|---|---|
| Qm/(mg∙g-1) | Kl | R2 | Kf | 1/n | R2 |
| 1.02915 | 0.86041 | 0.99632 | 0.38173 | 0.51042 | 0.81026 |
表3 Cu@PAL等温吸附模型的拟合参数
Table 3 Isotherm parameters for the adsorption of I- anions by Cu@PAL
| Langmuir model | Frenudlich model | ||||
|---|---|---|---|---|---|
| Qm/(mg∙g-1) | Kl | R2 | Kf | 1/n | R2 |
| 1.02915 | 0.86041 | 0.99632 | 0.38173 | 0.51042 | 0.81026 |
| Time/h | Qe/(mg∙g-1) | |
|---|---|---|
| Cu@PAL | Nano-Cu | |
| 0 | 74.2 | 234.7 |
| 12 | 69.4 | 133.8 |
| 24 | 68.1 | 110.4 |
| 48 | 64.1 | 104.6 |
| 72 | 60.2 | 96.3 |
| 144 | 58.7 | 88.4 |
表4 Cu@PAL和纳米铜暴露于空气中的吸附性能
Table 4 Adsorption properties of Cu@PAL and nano Cu exposed to air
| Time/h | Qe/(mg∙g-1) | |
|---|---|---|
| Cu@PAL | Nano-Cu | |
| 0 | 74.2 | 234.7 |
| 12 | 69.4 | 133.8 |
| 24 | 68.1 | 110.4 |
| 48 | 64.1 | 104.6 |
| 72 | 60.2 | 96.3 |
| 144 | 58.7 | 88.4 |
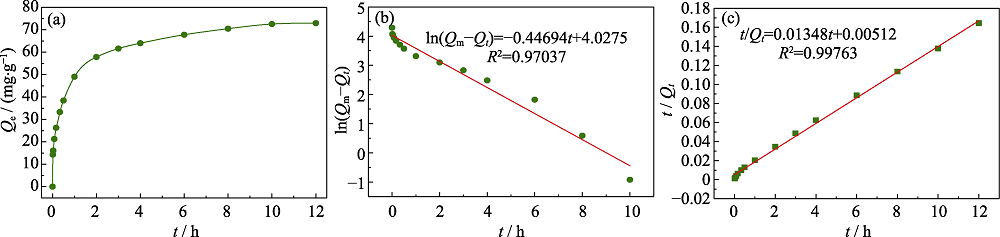
图11 Cu@PAL的吸附动力学曲线(a)以及吸附动力学曲线的伪一级动力学模型(b)和伪二级动力学模型(c)
Fig. 11 Absorption kinetic curve (a), fitting curves of the pseudo first order kinetic model (b) and pseudo second order kinetic model (c) for adsorption kinetic of Cu@PAL
| Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|
| Qm/ (mg∙g-1) | k1/(g∙(mmol∙ g-1)-1) | R2 | Qm/ (mg∙g-1) | k2/(g∙(mmol∙ g-1)-1) | R2 |
| 56.10 | 0.4469 | 0.9704 | 74.1840 | 0.03549 | 0.9976 |
表5 Cu@PAL的吸附动力学拟合参数
Table 5 Kinetic parameters for the adsorption of I- by Cu@PAL
| Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|
| Qm/ (mg∙g-1) | k1/(g∙(mmol∙ g-1)-1) | R2 | Qm/ (mg∙g-1) | k2/(g∙(mmol∙ g-1)-1) | R2 |
| 56.10 | 0.4469 | 0.9704 | 74.1840 | 0.03549 | 0.9976 |
| Adsorbent | cNaCl/(mol∙L-1) | Desorption efficiency/% |
|---|---|---|
| Cu@PAL | 0 | 21.3 |
| 0.1 | 32.1 | |
| Nano-Cu | 0 | 87.4 |
| 0.1 | 94.1 |
表6 Cu@PAL和纳米Cu吸附后产物的脱附解析率
Table 6 Leaching or desorption efficiencies of Cu@PAL and nano-Cu after adsorption
| Adsorbent | cNaCl/(mol∙L-1) | Desorption efficiency/% |
|---|---|---|
| Cu@PAL | 0 | 21.3 |
| 0.1 | 32.1 | |
| Nano-Cu | 0 | 87.4 |
| 0.1 | 94.1 |
| [1] | KBERGER T. Progress of renewable electricity replacing fossil fuels. Global Energy Interconnection , 2018, 1(1):48-52. |
| [2] |
TRUESDALE V W, NAUSCH G, BAKER A. The distribution of iodine in the Baltic Sea during summer. Mar. Chem. , 2001, 74(2):87-98.
DOI URL |
| [3] |
GMEZ-GUZM N J M, HOLM E, NIAGOLOVA N, et al. Influence of releases of 129I and 137Cs from European reprocessing facilities in Fucus vesiculosus and seawater from the Kattegat and Skagerrak areas. Chemosphere , 2014, 108:76-84.
DOI URL |
| [4] |
LI C, WEI Y, WANG X, et al. Efficient and rapid adsorption of iodide ion from aqueous solution by porous silica spheres loaded with calcined Mg-Al layered double hydroxide. Journal of the Taiwan Institute of Chemical Engineers , 2018, 85:193-200.
DOI URL |
| [5] |
HOSKINS J S, KARANFIL T, SERKIZ S M. Removal and sequestration of iodide using silver-impregnated activated carbon. Environ. Sci. Technol. , 2002, 36(4):784-789.
DOI URL |
| [6] |
THEISS F L, AYOKO G A, FROST R L. Iodide removal using LDH technology. Chemical Engineering Journal , 2016, 296:300-309.
DOI URL |
| [7] |
INOUE H. Effects of Co-ions on transport of iodide ions through a non-conventional anion exchange paper membrane. J. Membr. Sci. , 2004, 228(2):209-215.
DOI URL |
| [8] |
LIU S, WANG N, ZHANG Y, et al. Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O-Ag/TiO2 composites under visible light irradiation. J. Hazard Mater. , 2015, 284:171-181.
DOI URL |
| [9] |
MAO P, LIU Y, JIAO Y, et al. Enhanced uptake of iodide on Ag@Cu2O nanoparticles. Chemosphere , 2016, 164:396-403.
DOI URL |
| [10] |
CHOI M H, JEONG S W, SHIM H E, et al. Efficient bioremediation of radioactive iodine using biogenic gold nanomaterial-containing radiation-resistant bacterium, Deinococcus radiodurans R1. Chemical Communications , 2017, 53(28):3937-3940.
DOI URL |
| [11] |
ZHANG W, LI Q, MAO Q, et al. Cross-linked chitosan microspheres: an efficient and eco-friendly adsorbent for iodide removal from waste water. Carbohydr. Polym. , 2019, 209:215-222.
DOI URL |
| [12] |
MAO P, QI L, LIU X, et al. Synthesis of Cu/Cu2O hydrides for enhanced removal of iodide from water. J. Hazard Mater. , 2017, 328:21-28.
DOI URL |
| [13] | LIU L, LIU W, ZHAO X, et al. Selective capture of iodide from solutions by microrosette-like δ-Bi2O3. ACS Applied Materials & Interfaces , 2014, 6(18):16082-16090. |
| [14] |
MAILEN J C, HORNER D E. Removal of iodine from reactor fuel solutions as insoluble PdI2. Nucl. Technol. , 1977, 33(3):260-263.
DOI URL |
| [15] |
BALSLEY S D, BRADY P V, KRUMHANSL J L, et al. Iodide retention by metal sulfide surfaces: cinnabar and chalcocite. Environ. Sci. Technol. , 1996, 30(10):3025-3027.
DOI URL |
| [16] | YAQUAN W, FENG Y, JIANG J, et al. Designing of recyclable attapulgite for wastewater treatments: a review. ACS Sustainable Chemistry & Engineering , 2018, 7(2):1855-1869. |
| [17] |
CHEN H, ZHAO Y, WANG A. Removal of Cu(II) from aqueous solution by adsorption onto acid-activated palygorskite. J. Hazard Mater. , 2007, 149(2):346-354.
DOI URL |
| [18] | 王爱勤, 王文波, 郑易安, et al. 凹凸棒石棒晶束解离及其纳米化功能复合材料. 北京: 科学出版社, 2014. |
| [19] | ZHOU Y, HE J, WANG H, et al. Carbon nanofiber yarns fabricated from co-electrospun nanofibers. Materials & Design , 2016, 95:591-598. |
| [20] |
MAO P, JIANG J, PAN Y, et al. Enhanced uptake of iodide from solutions by hollow Cu-based adsorbents. Materials , 2018, 11(5):769.
DOI URL |
| [21] |
MAO P, YU X, LIU K, et al. Rapid and reversible adsorption of radioactive iodide from wastewaters by green and low-cost palygorskite-based microspheres. J. Radioanal Nucl. Chem. , 2020, 325(1):303-313.
DOI URL |
| [22] |
ZHANG Y, YU C, HU P, et al. Mechanical and thermal properties of palygorskite poly(butylene succinate) nanocomposite. Applied Clay Science , 2016, 119:96-102.
DOI URL |
| [23] |
CARAZO E, BORREGO-S NCHEZ A, GARC A-VILL N F, et al. Adsorption and characterization of palygorskite-isoniazid nanohybrids. Applied Clay Science , 2018, 160:180-185.
DOI URL |
| [24] |
BA N, ZHU L, LI H, et al. 3D rod-like copper oxide with nanowire hierarchical structure: ultrasound assisted synthesis from Cu2(OH)3NO3 precursor, optical properties and formation mechanism. Solid State Sciences , 2016, 53:23-29.
DOI URL |
| [25] |
YAO S, ZHANG H, CHEN Z, et al. Promotion of graphitic carbon oxidation via stimulating CO2 desorption by calcium carbonate. J. Hazard Mater. , 2019, 363:10-15.
DOI URL |
| [26] |
THOMMES M, KANEKO K, NEIMARK A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. , 2015, 87(9/10):1051-1069.
DOI URL |
| [27] |
LEF VRE G, BESSI RE J, EHRHARDT J J, et al. Immobilization of iodide on copper(I) sulfide minerals. Journal of Environmental Radioactivity , 2003, 70(1/2):73-83.
DOI URL |
| [28] |
ZHANG X, GU P, LI X, et al. Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chemical Engineering Journal , 2017, 322:129-139.
DOI URL |
| [29] |
HAQ Z, BANCROFT G M, FYFE W S, et al. Sorption of iodide on copper. Environ. Sci. Technol. , 1980, 14(9):1106-1110.
DOI URL |
| [30] |
WU C K, YIN M, O'BRIEN S, et al. Quantitative analysis of copper oxide nanoparticle composition and structure by X-ray photoelectron spectroscopy. Chemistry of Materials , 2006, 18(25):6054-6058.
DOI URL |
| [1] | 孙强强, 陈子璇, 杨子玥, 王毅梦, 曹宝月. 金属镍铜负载钒氧化物的高效电解产氢性能[J]. 无机材料学报, 2023, 38(6): 647-655. |
| [2] | 吴爱军, 朱敏, 朱钰方. 含铜硅酸钙纳米棒复合水凝胶用于肿瘤治疗和皮肤伤口愈合性能研究[J]. 无机材料学报, 2022, 37(11): 1203-1216. |
| [3] | 郭李娜, 何雪冰, 吕琳, 吴丹, 原弘. 调控CuO表面性质选择性电催化还原CO2制HCOOH[J]. 无机材料学报, 2022, 37(1): 29-37. |
| [4] | 张维维, 陆晨, 应国兵, 张建峰, 江莞. AlN表面处理及级配填充对覆铜板绝缘层性能的影响规律与机制研究[J]. 无机材料学报, 2021, 36(8): 847-855. |
| [5] | 王恩典, 常江. 钼掺杂铜硅钙石的制备及其抗菌性能和细胞相容性研究[J]. 无机材料学报, 2021, 36(7): 738-744. |
| [6] | 李锐,王浩,付强,田子玉,王建旭,马小健,杨剑,钱逸泰. 构建亲锂铜基3D集流体实现金属锂的均匀沉积[J]. 无机材料学报, 2020, 35(8): 882-888. |
| [7] | 李昆强,乔玉琴,刘宣勇. 钛表面铜离子注入对细菌和细胞行为的影响[J]. 无机材料学报, 2020, 35(2): 158-164. |
| [8] | 孙顶, 丁彦妍, 孔令炜, 张玉红, 郭秀娟, 魏立明, 张力, 张立新. Mg掺杂Cu2ZnSnS4的第一性原理研究[J]. 无机材料学报, 2020, 35(11): 1290-1294. |
| [9] | 孙付通, 冯爱虎, 陈兵兵, 于云, 杨红. 铜基底预处理对CVD法生长石墨烯薄膜的影响[J]. 无机材料学报, 2020, 35(10): 1177-1182. |
| [10] | 李兆, 孙强强, 陈索倩, 周春生, 曹静, 王永锋, 王亚楠. 水热合成镍铜复合磷化物及其电催化析氢与肼氧化性能[J]. 无机材料学报, 2020, 35(10): 1149-1156. |
| [11] | 何端鹏,高鸿,张静静,吴杰,刘泊天,王向轲. 氮化铝覆铜板在空间热场下热学性能的模拟仿真及实验验证[J]. 无机材料学报, 2019, 34(9): 947-952. |
| [12] | 张盛, 蒋亿, 纪媛媛, 杜莹, 盛振环, 殷竟洲, 李乔琦, 张莉莉. 凹凸棒石/g-C3N4复合材料的制备及其电催化析氧性能研究[J]. 无机材料学报, 2019, 34(8): 803-810. |
| [13] | 张峰, 张凯立, 周明明, 陈超, 蔡志威, 魏国辉, 姜兴茂, 张诚, 劳伦·鲁尔曼, 吕耀康. 基于纳米银负载氧化石墨烯的新型聚乙烯复合材料[J]. 无机材料学报, 2019, 34(6): 633-640. |
| [14] | 林正得, 舒圣程, 李傲, 吴明亮, 杨明阳, 韩钰, 祝志祥, 陈保安, 丁一, 张强, 王强, 戴丹. 石墨烯增强铜基复合材料的研究进展[J]. 无机材料学报, 2019, 34(5): 469-477. |
| [15] | 徐信, 王书荣, 马逊, 杨帅, 李耀斌, 杨洪斌. 硫化物靶与单质靶制备Cu2ZnSnS4薄膜的比较研究[J]. 无机材料学报, 2019, 34(5): 529-534. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||