无机材料学报 ›› 2021, Vol. 36 ›› Issue (6): 615-622.DOI: 10.15541/jim20200437
所属专题: 【能源环境】光催化降解有机分子
安伟佳1( ), 李静1,2, 王淑瑶1, 胡金山1, 蔺在元2, 崔文权1(
), 李静1,2, 王淑瑶1, 胡金山1, 蔺在元2, 崔文权1( ), 刘利1, 解珺3, 梁英华1(
), 刘利1, 解珺3, 梁英华1( )
)
收稿日期:2020-08-10
修回日期:2020-11-12
出版日期:2021-06-20
网络出版日期:2020-12-10
通讯作者:
崔文权, 教授. E-mail: wkcui@ncst.edu.cn; 梁英华, 教授. E-mail: liangyh@ncst.edu.cn
作者简介:安伟佳(1989-), 男, 高级实验师, E-mail: anweijia@ncst.edu.cn
基金资助:
AN Weijia1( ), LI Jing1,2, WANG Shuyao1, HU Jinshan1, LIN Zaiyuan2, CUI Wenquan1(
), LI Jing1,2, WANG Shuyao1, HU Jinshan1, LIN Zaiyuan2, CUI Wenquan1( ), LIU Li1, XIE Jun3, LIANG Yinghua1(
), LIU Li1, XIE Jun3, LIANG Yinghua1( )
)
Received:2020-08-10
Revised:2020-11-12
Published:2021-06-20
Online:2020-12-10
Contact:
CUI Wenquan, professor. E-mail: wkcui@ncst.edu.cn; LIANG Yinghua, professor. E-mail: liangyh@ncst.edu.cn
About author:AN Weijia(1989-), male, senior laboratory technician. E-mail: anweijia@ncst.edu.cn
Supported by:摘要:
光催化-芬顿技术耦合可高效降解有机污染物。本研究采用溶剂热法制备了Fe(III)掺杂rGO/Bi2MoO6复合催化剂(Fe(III)/rGO/Bi2MoO6), 通过外加H2O2构建了光催化-芬顿协同体系, 可见光照射3 h后对苯酚的降解率(82%)远高于单独光催化(18%)或芬顿反应(48%), 进一步优化条件对苯酚可实现完全降解。这主要是通过Fe得失电子实现价态的转变, 并以此作为桥梁实现光催化-芬顿的协同作用。同时石墨烯的优异导电性能不仅克服了光催化中光生电子空穴难以分离的问题, 而且促进了Fe3+/Fe2+的循环反应, 促使芬顿反应产生更多的羟基自由基(?OH), 进一步提高了苯酚的降解效率。实验考察了Fe(III)含量、催化剂投加量、H2O2含量以及pH等因素对协同降解效果的影响。淬灭实验证明?OH是协同降解体系中最主要的活性物种, ?O2-和h+对降解活性也会产生一定的影响, 结合实验结果提出了Fe(III)/rGO/Bi2MoO6光催化-芬顿协同降解苯酚的机理。
中图分类号:
安伟佳, 李静, 王淑瑶, 胡金山, 蔺在元, 崔文权, 刘利, 解珺, 梁英华. Fe(III)/rGO/Bi2MoO6复合光催化剂制备及光催化芬顿协同降解苯酚[J]. 无机材料学报, 2021, 36(6): 615-622.
AN Weijia, LI Jing, WANG Shuyao, HU Jinshan, LIN Zaiyuan, CUI Wenquan, LIU Li, XIE Jun, LIANG Yinghua. Fe(III)/rGO/Bi2MoO6 Composite Photocatalyst Preparation and Phenol Degradation by Photocatalytic Fenton Synergy[J]. Journal of Inorganic Materials, 2021, 36(6): 615-622.
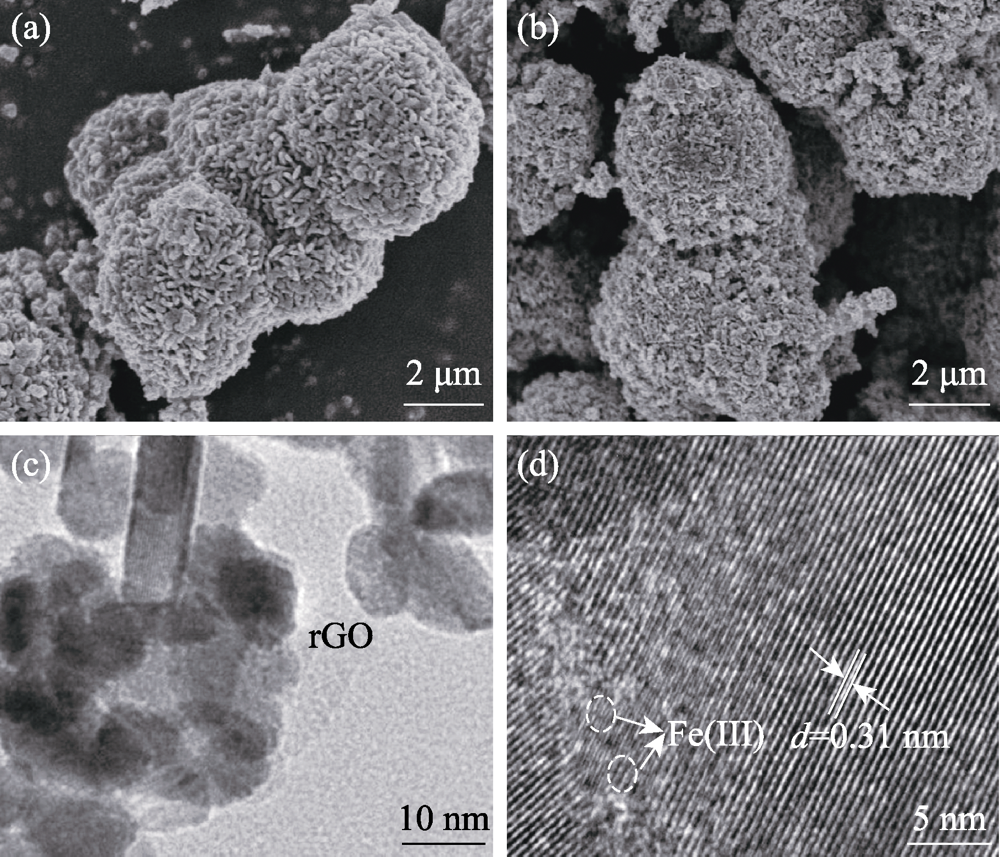
图1 (a)Bi2MoO6和(b)rGO/Bi2MoO6的SEM照片; Fe(III)/ rGO/Bi2MoO6的TEM(c)和HRTEM(d)照片
Fig. 1 (a) SEM images of Bi2MoO6 and (b) rGO/Bi2MoO6; TEM (c) and HRTEM (d) images of Fe(III)/rGO/Bi2MoO6
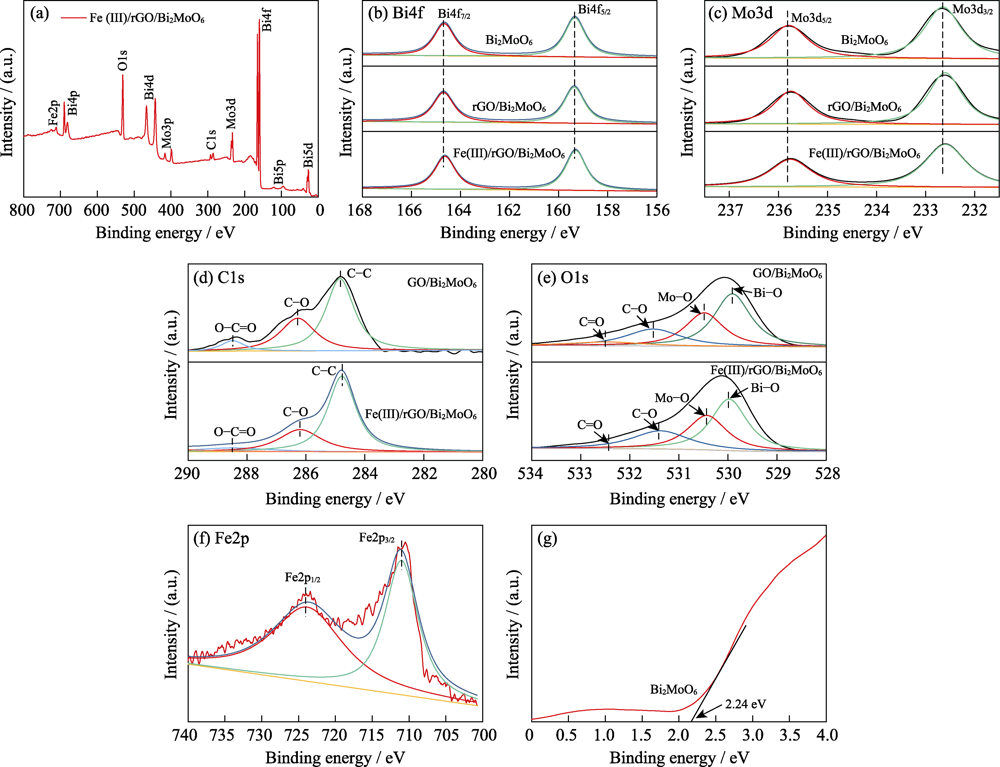
图3 Bi2MoO6, GO/Bi2MoO6和Fe(III)/rGO/Bi2MoO6样品的XPS谱图
Fig. 3 XPS spectra of the compared composites (a) Full spectrum analysis; (b-f) XPS spectra of various element; (g) XPS spectra of valence band in Bi2MoO6
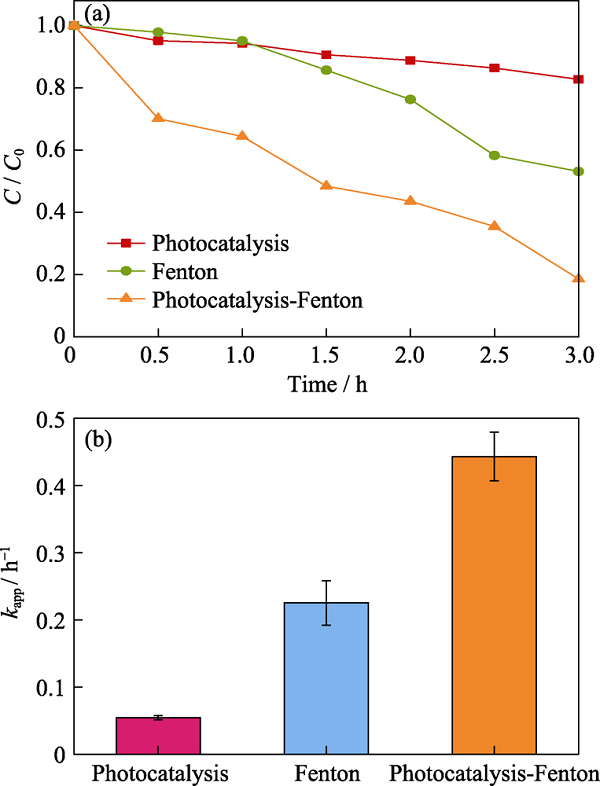
图4 (a)光催化、芬顿反应和光催化-芬顿协同降解苯酚活性对比; (b)不同条件下降解速率常数
Fig. 4 (a) Phenol degradation activity by photocatalysis, Fenton, and photocatalysis-Fenton synergy, and (b) degradation rate constant over different conditions
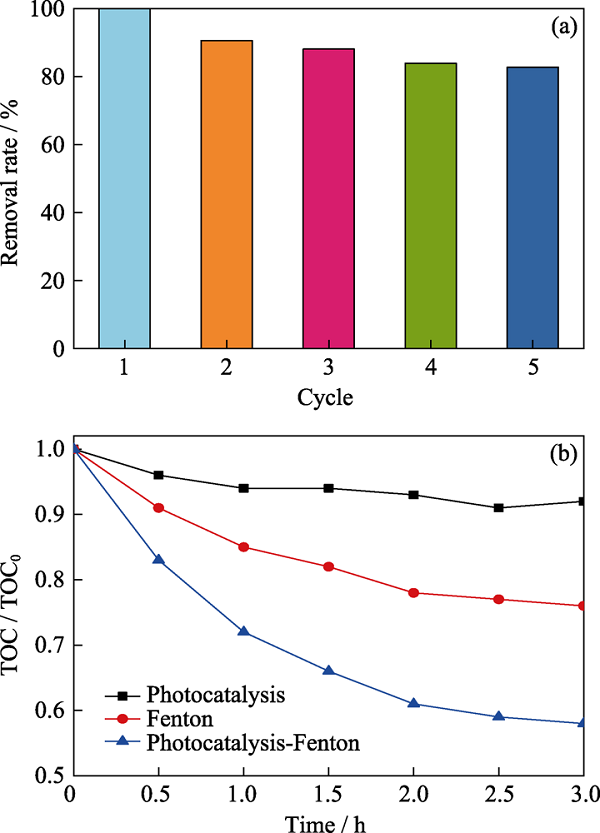
图5 (a)光催化-芬顿协同降解的稳定性, (b) 光催化、芬顿反应和光催化-芬顿协同降解苯酚的TOC结果
Fig. 5 (a) Photocatalysis-Fenton synergy degradation stability test, (b) TOC removal of phenol over photocatalysis, Fenton reaction, and photocatalysis-Fenton synergy
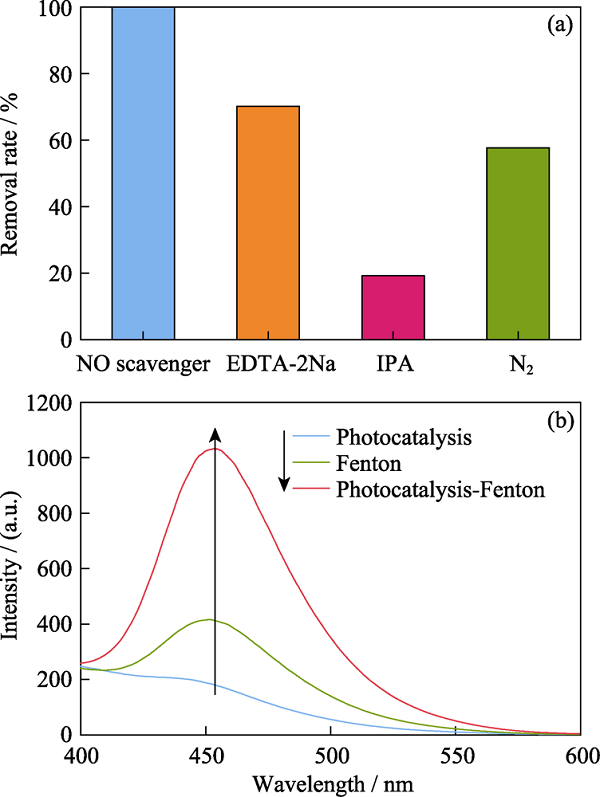
图7 (a)淬灭剂对降解性能的影响, (b)光催化、芬顿和光催化-芬顿协同生成?OH的浓度对比
Fig. 7 (a) Influence of degradation activity with the addition of quenchers, and (b) concentration of ?OH generated at photocatalysis, Fenton and photocatalysis-Fenton synergy

图S3 不同苯酚去除效率的活性对比图
Fig. S3 Comparison of the activity of different phenol removal efficiency (a) different proportions of Fe (III) (C0 = 5 ppm); (b) Different catalyst dosages (C0 = 5 ppm, 20% Fe (III)/rGO/Bi2MoO6); (c) different amounts of hydrogen peroxide (C0 = 5 ppm, 20% Fe (III)/rGO/Bi2MoO6, catalyst concentration: 1.0 g/L); (d) different pH (C0 = 5 ppm, 20 % Fe (III)/rGO/Bi2MoO6, catalyst concentration: 1.0 g/L, H2O2 = 19.5 mmol/L) *ppm=mg/L
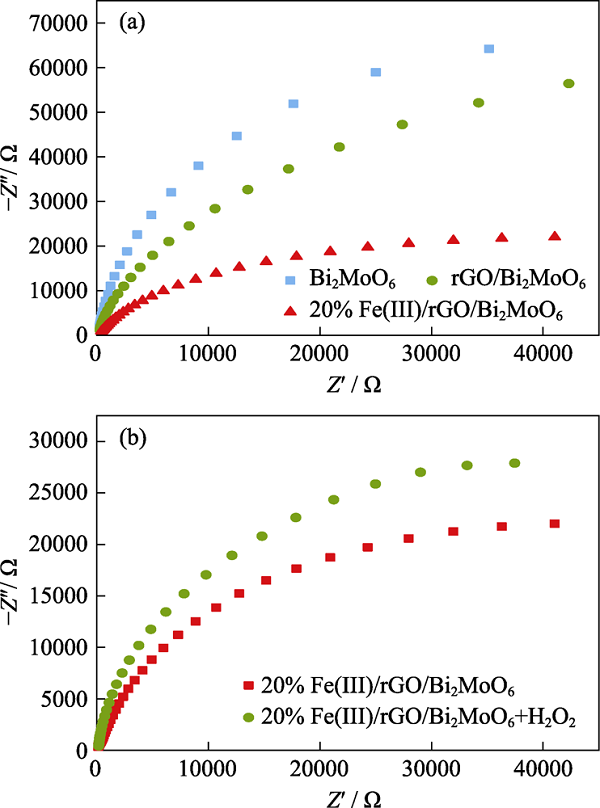
图S4 (a, b)不同催化剂的电化学阻抗谱; (b) Fe(III)/rGO/ Bi2MoO6和加入H2O2的电化学阻抗谱
Fig. S4 (a) EIS of different photocatalyst composites; (b) EIS of Fe(III)/rGO/Bi2MoO6 and Fe(III)/rGO/Bi2MoO6 + H2O2
| [1] |
ZHANG D S, CAI H, GAO K Y, et al. Preparation and visible-light photocatalytic degradation on metronidazole of Zn2SiO4-ZnO-biochar composites. Journal of Inorganic Materials, 2020,35(8):923-930.
DOI URL |
| [2] |
AN W J, TIAN L Y, HU J S, et al. Efficient degradation of organic pollutants by catalytic ozonation and photocatalysis synergy system using double-functional MgO/g-C3N4 catalyst. Applied Surface Science, 2020,534:147518.
DOI URL |
| [3] |
AN W J, SUN K L, HU J S, et al. The Z-scheme Ag2CO3@g-C3N4 core-shell structure for increased photoinduced charge separation and stable photocatalytic degradation. Applied Surface Science, 2020,504:144345.
DOI URL |
| [4] |
HU J S, ZHANG P F, AN W J, et al. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Applied Catalysis B-Environmental, 2019,245:130-142.
DOI URL |
| [5] |
DU X, ZHAO T Y, XIU Z Y, et al. Nano-zero-valent iron and MnOx selective deposition on BiVO4 decahedron superstructures for promoted spatial charge separation and exceptional catalytic activity in visible-light-driven photocatalysis-Fenton coupling system. Journal of Hazardous Materials, 2019,377:330-340.
DOI URL |
| [6] |
WU Q S, YANG H P, KANG L, et al. Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide pH range: acceleration of Fe(II)/Fe(III) cycle under visible light irradiation. Applied Catalysis B-Environmental, 2020,263:118282.
DOI URL |
| [7] |
XU P, XU H, ZHENG D Y, et al. The efficiency and mechanism in a novel electro-Fenton process assisted by anodic photocatalysis on advanced treatment of coal gasification wastewater. Chemical Engineering Journal, 2019,361:968-974.
DOI URL |
| [8] | STEFFI T, ANOOP K V, VIKAS K S, et al. Once through continuous flow removal of metronidazole by dual effect of photo- Fenton and photocatalysis in a compound parabolic concentrator at pilot plant scale. Chemical Engineering Journal, 2020,388:124184. |
| [9] | XU Z M, ZHENG R, CHEN Y, et al. Ordered mesoporous Fe/TiO2 with light enhanced photo-Fenton activity. Chinese Journal of Catalysis, 2019,40:631-637. |
| [10] |
HU J S, ZHANG P F, CUI J F, et al. High-efficiency removal of phenol and coking wastewater via photocatalysis-Fenton synergy over a Fe-g-C3N4 graphene hydrogel 3D structure. Journal of Industrial and Engineering Chemistry, 2020,84:305-314.
DOI URL |
| [11] |
GUO L, ZHANG K L, HAN X X, et al. 2D/2D type-II Cu2ZnSnS4/Bi2WO6 heterojunctions to promote visible-light-driven photo-Fenton catalytic activity. Chinese Journal of Catalysis, 2020,41:503-513.
DOI URL |
| [12] |
XING M Y, XU W J, DONG C C, et al. Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes. Chem, 2018,4:1359-1372.
DOI URL |
| [13] |
ZHAO W H, WEI Z Q, ZHANG X D, et al. Magnetic recyclable MnFe2O4/CeO2/SnS2 ternary nano-photocatalyst for photo-Fenton degradation. Applied Catalysis A-General, 2020,593:117443.
DOI URL |
| [14] |
MENG Q Q, LÜ C D, SUN J X, et al. High-efficiency Fe-Mediated Bi2MoO6 nitrogen-fixing photocatalyst: reduced surface work function and ameliorated surface reaction. Applied Catalysis B-Environmental, 2019,256:117781.
DOI URL |
| [15] |
XIE Y Y, SHANG X T, LIU D, et al. Non-noble metal thickness- tunable Bi2MoO6 nanosheets for highly efficient visible-light-driven nitrobenzene reduction into aniline. Applied Catalysis B-Environmental, 2019,259:118087.
DOI URL |
| [16] | WANG J L, DONG M R, ZHANG Q C, et al. Preparation of Bi2MoO6 microspheres with hollow structure and degradation performance of ofloxacin antibiotics. Chinese Journal of Inorganic Chemistry, 2020,36:827-834. |
| [17] |
XIANG S W, ZHANG Z Y, WU ZHI, et al. 3D Heterostructured Ti-Based Bi2MoO6/Pd/TiO2 photocatalysts for high-efficiency solar light driven photo electrocatalytic hydrogen generation. ACS Applied Energy Materials, 2019,2:558-568.
DOI URL |
| [18] | LV J L, ZHANG J F, LIU J, et al. Bi SPR-promoted Z-scheme Bi2MoO6/CdS-diethylenetriamine composite with effectively enhanced visible light photocatalytic hydrogen evolution activity and stability. ACS Sustainable Chemistry & Engineering, 2018,6:696-706. |
| [19] |
XIU Z Y, CAO Y, XING Z P, et al. Wide spectral response photothermal catalysis-Fenton coupling systems with 3D hierarchical Fe3O4/Ag/Bi2MoO6 ternary hetero-superstructural magnetic microspheres for efficient high-toxic organic pollutants removal. Journal of Colloid and Interface Science, 2019,533:24-33.
DOI URL |
| [20] |
JING K Q, WEN M, REN Y H, et al. Hierarchical Bi2MoO6 spheres in situ assembled by monolayer nanosheets toward photocatalytic selective oxidation of benzyl alcohol. Applied Catalysis B-Environmental, 2019,243:10-18.
DOI URL |
| [21] |
WANG S Y, DING X, YANG N, et al. Insight into the effect of bromine on facet-dependent surface oxygen vacancies construction and stabilization of Bi2MoO6 for efficient photocatalytic NO removal. Applied Catalysis B-Environmental, 2020,265:118585.
DOI URL |
| [22] |
HU J S, LI J, CUI J F, et al. Surface oxygen vacancies enriched FeOOH/Bi2MoO6 photocatalysis-Fenton synergy degradation of organic pollutants. Journal of Hazardous Materials, 2020,384:121399.
DOI URL |
| [23] | ZHU P F, CHEN Y J, DUAN M, et al. Construction and mechanism of a highly efficient and stable Z-scheme Ag3PO4/ reduced graphene oxide/Bi2MoO6 visible-light photocatalyst. Applied Catalysis B-Environmental, 2018,8:3818-3832. |
| [24] |
XUE C, LI H, AN H, et al. Cross-linked bond accelerated interfacial charge transfer in monolayer zinc indium sulfide (ZnIn2S4)/reduced graphene oxide (RGO) heterostructure for photocatalytic hydrogen production with mechanistic insight. ACS Catalysis, 2018,8:1532-1545.
DOI URL |
| [25] |
LIANG Y H, WANG X, AN W J, et al. A g-C3N4@ppy-rGO 3D structure hydrogel for efficient photocatalysis. Applied Surface Science, 2019,466:666-672.
DOI URL |
| [26] |
ZENG P, ZHANG Q G, PENG T Y, et al. One-pot synthesis of reduced graphene oxide-cadmium sulfide nanocomposite and its photocatalytic hydrogen production. Physical Chemistry Chemical Physics, 2011,13:21496-21502.
DOI URL |
| [27] |
XU Y S, ZHANG W D. Monodispersed Ag3PO4 nanocrystals loaded on the surface of spherical Bi2MoO6 with enhanced photocatalytic performance. Dalton Transactions, 2013,42:1094-1101.
DOI URL |
| [28] | YU H G, CAO G Q, CHEN FENG, et al. Enhanced photocatalytic performance of Ag3PO4 by simultaneous loading of Ag nanoparticles and Fe(III) cocatalyst. Applied Catalysis B-Environmental, 2014,160:658-665. |
| [29] |
YANG L, DU C Y, TAN S Y, et al. Improved photocatalytic properties of Fe(III) ion doped Bi2MoO6 for the oxidation of organic pollutants. Ceramics International, 2021,47(4):5786-5794.
DOI URL |
| [30] |
MENG X C, ZHANG Z S. Bi2MoO6 co-modified by reduced graphene oxide and palladium (PdPd2+ and Pd0) with enhanced photocatalytic decomposition of phenol. Applied Catalysis B-Environmental, 2017,209:383-393.
DOI URL |
| [31] |
WANG D J, SHEN H D, GUO L, et al. Design and construction of the sandwich-like Z-scheme multicomponent CdS/Ag/Bi2MoO6 heterostructure with enhanced photocatalytic performance in RhB photodegradation. New Journal of Chemistry, 2016,40(10):8614-8624.
DOI URL |
| [1] | 孙强强, 陈子璇, 杨子玥, 王毅梦, 曹宝月. 金属镍铜负载钒氧化物的高效电解产氢性能[J]. 无机材料学报, 2023, 38(6): 647-655. |
| [2] | 王磊, 李建军, 宁军, 胡天玉, 王洪阳, 张占群, 武琳馨. CoFe2O4@Zeolite催化剂活化过一硫酸盐对甲基橙的强化降解: 性能与机理[J]. 无机材料学报, 2023, 38(4): 469-476. |
| [3] | 陈士昆, 王楚楚, 陈晔, 李莉, 潘路, 文桂林. 磁性Ag2S/Ag/CoFe1.95Sm0.05O4 Z型异质结的制备及光催化降解性能[J]. 无机材料学报, 2022, 37(12): 1329-1336. |
| [4] | 李铁, 李玥, 王颖异, 张珽. 石墨烯-铁酸铋纳米晶复合材料的制备及其催化性能研究[J]. 无机材料学报, 2021, 36(7): 725-732. |
| [5] | 蔡苗, 陈子航, 曾实, 杜江慧, 熊娟. CuS纳米片修饰Bi5O7I复合材料用于光催化还原Cr(VI)水溶液[J]. 无机材料学报, 2021, 36(6): 665-672. |
| [6] | 熊金艳, 罗强, 赵凯, 张梦梦, 韩朝, 程刚. 界面电荷快速转移提升铜修饰氧化钨光催化性能[J]. 无机材料学报, 2021, 36(3): 325-331. |
| [7] | 兰青, 孙盛睿, 吴萍, 杨庆峰, 刘阳桥. 钴掺杂氧化铜/可见光协同活化PMS降解罗丹明B及其机理研究[J]. 无机材料学报, 2021, 36(11): 1171-1177. |
| [8] | 刘彩, 刘芳, 黄方, 王晓娟. 海藻基CDs-Cu-TiO2复合材料的制备及其光催化性能[J]. 无机材料学报, 2021, 36(11): 1154-1162. |
| [9] | 包峰, 常江. 硅酸钙纳米线复合电纺丝支架的制备及离子释放研究[J]. 无机材料学报, 2021, 36(11): 1199-1207. |
| [10] | 谢雪, 吴建荣, 蔡晓军, 郝俊年, 郑元义. 光热/pH响应B-CuS-DOX纳米药物用于化疗-光热协同治疗肿瘤[J]. 无机材料学报, 2021, 36(1): 81-87. |
| [11] | 李一敏,王成乐,李娟. 磷钼酸盐中金属离子对聚丙烯阻燃效率的提升[J]. 无机材料学报, 2020, 35(9): 1029-1033. |
| [12] | 张信聪,郭珂,彭莲莲,吴结宇,张富民,朱伟东,傅仰河. NH2-UiO-66机械催化降解染料的性能研究[J]. 无机材料学报, 2020, 35(9): 1023-1028. |
| [13] | 朱恩权,马玉花,艾尼瓦·木尼热,粟智. 膨润土负载红磷复合材料的吸附富集-定位光降解性能[J]. 无机材料学报, 2020, 35(7): 803-808. |
| [14] | 徐晶威,李政,王泽普,于涵,何祺,付念,丁帮福,郑树凯,闫小兵. 交错能带结构钕掺杂钒酸铋形貌与光催化性能调控[J]. 无机材料学报, 2020, 35(7): 789-795. |
| [15] | 王苹,李心宇,时占领,李海涛. Ag与Ag2O协同增强TiO2光催化制氢性能的研究[J]. 无机材料学报, 2020, 35(7): 781-788. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||