无机材料学报 ›› 2021, Vol. 36 ›› Issue (6): 608-614.DOI: 10.15541/jim20200509
陈力驰1,2( ), 王耀功1,2, 王文江1,2, 麻晓琴1,2, 杨静远3(
), 王耀功1,2, 王文江1,2, 麻晓琴1,2, 杨静远3( ), 张小宁1,2
), 张小宁1,2
收稿日期:2020-09-01
修回日期:2020-10-12
出版日期:2021-06-20
网络出版日期:2020-12-01
通讯作者:
杨静远, 工程师. E-mail: yjytonghu@163.com
作者简介:陈力驰(1995-), 男, 博士研究生. E-mail: 734167430@qq.com
基金资助:
CHEN Lichi1,2( ), WANG Yaogong1,2, WANG Wenjiang1,2, MA Xiaoqin1,2, YANG Jingyuan3(
), WANG Yaogong1,2, WANG Wenjiang1,2, MA Xiaoqin1,2, YANG Jingyuan3( ), ZHANG Xiaoning1,2
), ZHANG Xiaoning1,2
Received:2020-09-01
Revised:2020-10-12
Published:2021-06-20
Online:2020-12-01
Contact:
YANG Jingyuan, engineer. E-mail: yjytonghu@163.com
About author:CHEN Lichi(1995-), male, PhD candidate. E-mail: 734167430@qq.com
Supported by:摘要:
量子限制效应使硅纳米线具有良好的场致发射特性, 结合多孔硅的准弹道电子漂移模型可提高场发射器件的性能。传统的金属辅助化学刻蚀法制备硅纳米线的效率较低, 本研究在传统方法的基础上引入恒流源, 提出电催化金属辅助化学刻蚀法, 高效制备了硅纳米线/多孔硅复合结构。在外加30 mA恒定电流的条件下, 硅纳米线的平均制备速率可达308 nm/min, 较传统方法提升了173%。研究了AgNO3浓度、刻蚀时间和刻蚀电流对复合结构形貌的影响规律; 测试了采用电催化金属辅助化学刻蚀法制备样品的场发射特性。结果显示样品的阈值场强为10.83 V/μm, 当场强为14.16 V/μm时, 电流密度为64 μA/cm2。
中图分类号:
陈力驰, 王耀功, 王文江, 麻晓琴, 杨静远, 张小宁. 电催化金属辅助化学刻蚀法制备硅纳米线/多孔硅复合结构[J]. 无机材料学报, 2021, 36(6): 608-614.
CHEN Lichi, WANG Yaogong, WANG Wenjiang, MA Xiaoqin, YANG Jingyuan, ZHANG Xiaoning. Preparation of Silicon Nanowires and Porous Silicon Composite Structure by Electrocatalytic Metal Assisted Chemical Etching[J]. Journal of Inorganic Materials, 2021, 36(6): 608-614.
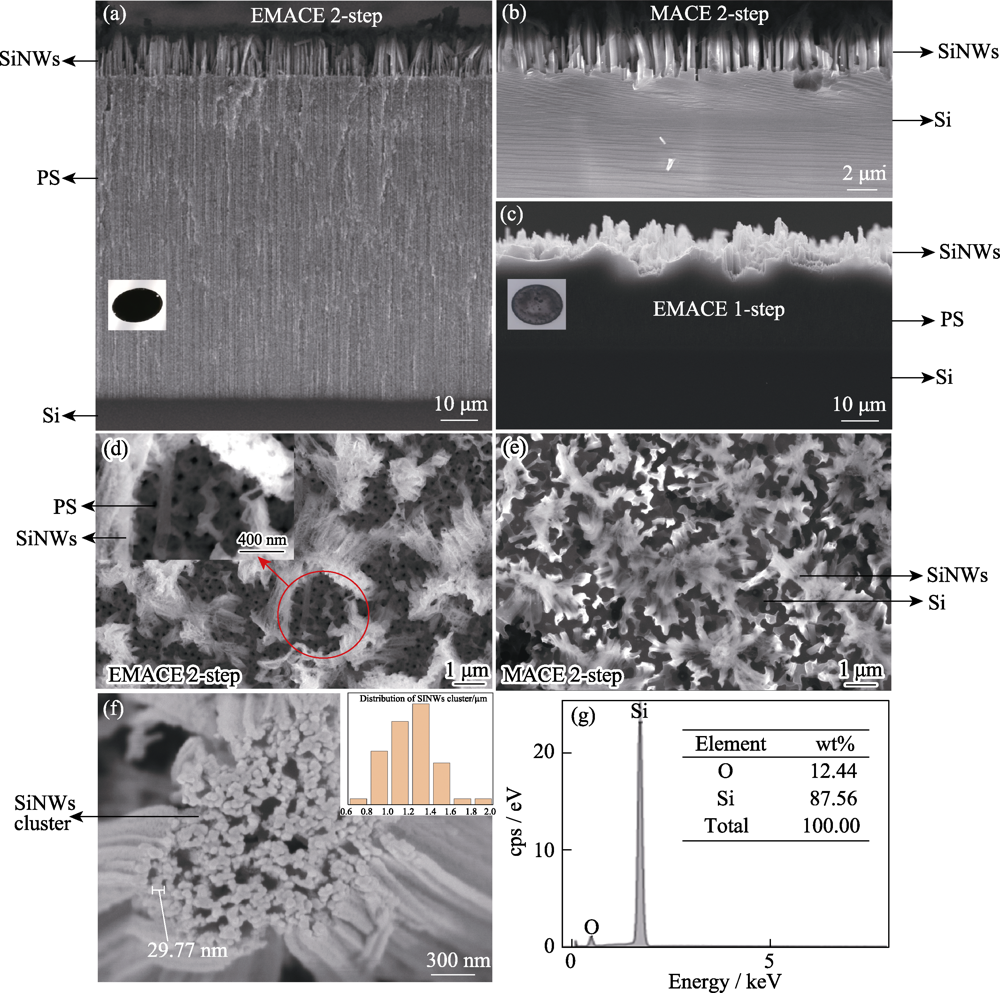
图2 采用不同方法制备样品的SEM照片
Fig. 2 SEM morphologies of samples prepared by different methods (a,d) EMACE 2-step method; (b,e) MACE 2-step method; (c) EMACE 1-step method; (f) High resolusion FESEM images of SiNWs clusters; (g) EDS of SiNWs clusters
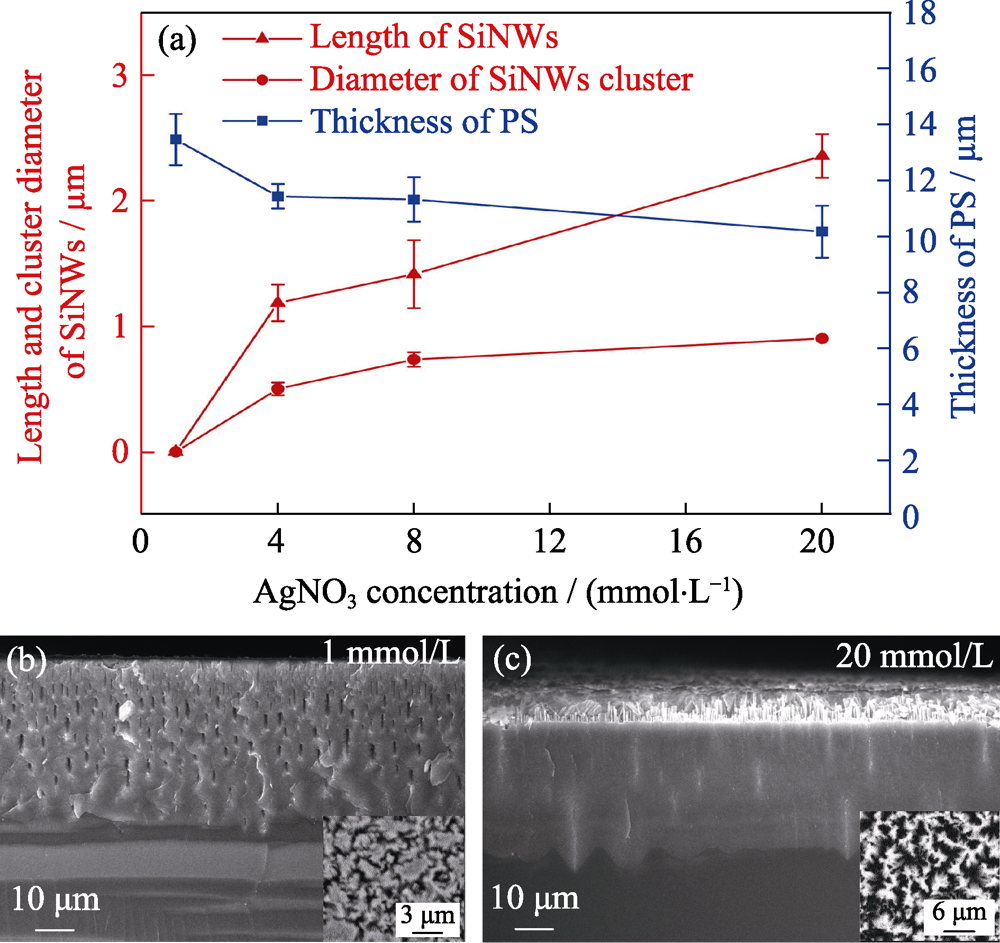
图3 AgNO3浓度对SiNWs/PS复合结构形貌的影响
Fig. 3 Effect of AgNO3 concentration on the morphology of SiNWs/PS composites (a) Changes of length of SiNWs, PS and cluster with AgNO3 concentration, and FESEM images of SiNWs/PS composites with AgNO3 concentration of (b) 1 mmol/L and (c) 20 mmol/L
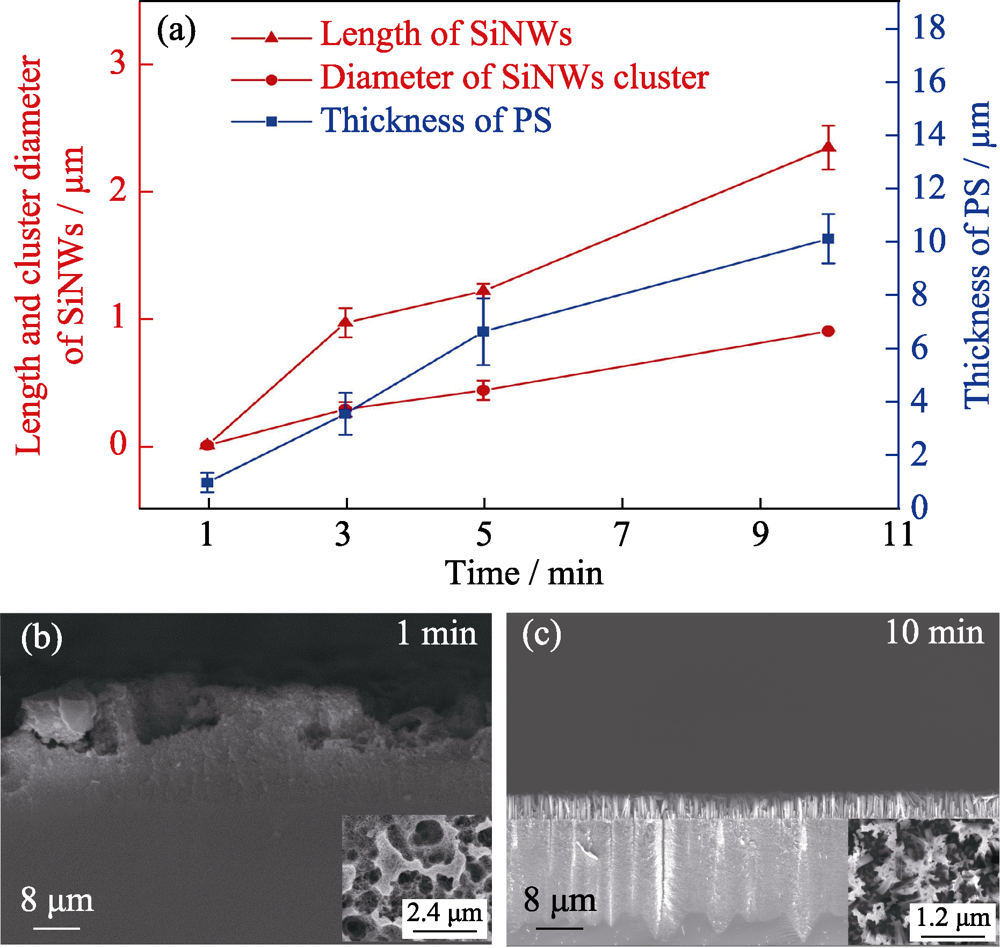
图4 刻蚀时间对SiNWs/PS复合结构形貌的影响
Fig. 4 Effect of etching time on the morphology of SiNWs/PS composites (a) Changes of length of SiNWs, PS and cluster with etching time, and FESEM images of SiNWs/PS composites with etching time of (b) 1 min and (c) 20 min
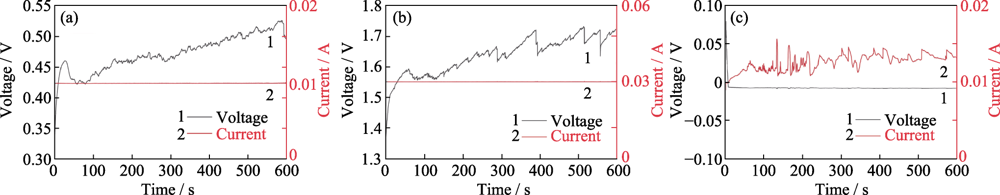
图8 刻蚀过程中阴阳极间的电压/电流随时间的变化
Fig. 8 Variation of voltage/current between anode and cathode with time during the etching (a) EMACE 2-step method at 10 mA; (b) EMACE 2-step method at 30 mA; (c) EMACE 1-step method at 10 mA
| [1] |
PIEDIMONTE P, MAZZETTA I, FUCILE S, et al. Silicon nanowires to detect electric signals from living cells. Materials Research Express, 2019,6(8):084005.
DOI URL |
| [2] |
DIMAGGIO E, PENNELLI G. Potentialities of silicon nanowire forests for thermoelectric generation. Nanotechnology, 2018,29(13):135401.
DOI URL |
| [3] |
PENNELLI G, ELYAMNY S, DIMAGGIO E, et al. Thermal conductivity of silicon nanowire forests. Nanotechnology, 2018,29(50):505402.
DOI URL |
| [4] |
MOKSHIN P V, JUNEJA S, PAVELYEV V S. Synthesis of silicon nanowires using plasma chemical etching process for solar cell applications. Journal of Physics: Conference Series, 2019,1368(2):022060.
DOI URL |
| [5] |
CHEN W H, CABARROCAS P R I. Rational design of nanowire solar cells: from single nanowire to nanowire arrays. Nanotechnology, 2019,30(19):194002.
DOI URL |
| [6] |
KUMAR V, SAXENA S K, KAUSHIK V, et al. Silicon nanowires prepared by metal induced etching (MIE): good field emitters. RSC Advances, 2014,4(101):57799-57803.
DOI URL |
| [7] |
ADAM T, HASHIM U. Silicon nanowire fabrication: silicon trimming via shallow anisotropic etching. Microelectronics International, 2014,31(2):78-85.
DOI URL |
| [8] |
ACHARYA S, KOTTANTHARAYIL A. Poole-Frenkel transport in gold catalyzed VLS grown silicon nanowires. IEEE Transactions on Electron Devices, 2018,65(5):1685-1691.
DOI URL |
| [9] |
LESTER U VINZONS, LEI SHU, SENPO YIP, et al. Unraveling the morphological evolution and etching kinetics of porous silicon nanowires during metal-assisted chemical etching. Nanoscale Research Letters, 2017,12:385.
DOI URL |
| [10] |
CONG L T, NGOC LAM N T, GIANG N T, et al. N-type silicon nanowires prepared by silver metal-assisted chemical etching: fabrication and optical properties. Materials Science in Semiconductor Processing, 2019,90:198-204.
DOI URL |
| [11] |
VIRIDIANA ACA-LÓPEZ, ENRIQUE QUIROGA-GONZÁLEZ, ESTELA GÓMEZ-BAROJAS, et al. Effects of the doping level in the production of silicon nanowalls by metal assisted chemical etching. Materials Science in Semiconductor Processing, 2020,118:105206.
DOI URL |
| [12] |
ZHU Y F, ZHOU L, PAN C J, et al. Fabrication of silicon nanorod arrays via a facile metal-assisted chemical etching method. Journal of Materials Science Materials in Electronics, 2016,27(6):5833-5838.
DOI URL |
| [13] |
HUNG Y J, LEE S L. Manipulating the antireflective properties of vertically-aligned silicon nanowires. Solar Energy Materials & Solar Cells, 2014,130:573-581.
DOI URL |
| [14] |
LI L, FANG Y, XU C, et al. Fabricating vertically aligned sub-20 nm Si nanowire arrays by chemical etching and thermal oxidation. Nanotechnology, 2016,27(16):165303.
DOI URL |
| [15] |
HE LI, WANG WEN-JIANG, ZHANG XIAO-NING. Improvement of electron emission characteristics of porous silicon emitter by using cathode reduction and electrochemical oxidation. Applied Surface Science, 2017,399:592-598.
DOI URL |
| [16] |
KOMODA T, SHENG X, KOSHIDA N. Mechanism of efficient and stable surface-emitting cold cathode based on porous polycrystalline silicon films. Journal of Vacuum Science & Technology B, 1999,17(3):1076-1079.
DOI URL |
| [17] |
FENG W, ARAKI H, OZAKI M, et al. Field emission properties of the nonaligned multiwalled carbon nanotube films with different length. Japanese Journal of Applied Physics, 2005,44(1-7):L253-L255.
DOI URL |
| [18] |
ZHU K, VINZANT T B, NEALE N R, et al. Removing structural disorder from oriented TiO2 nanotube arrays: reducing the dimensionality of transport and recombination in dye-sensitized solar cells. Nano Letters, 2007,7(12):3739.
DOI URL |
| [19] |
HAN H, HUANG Z, LEE W. Metal-assisted chemical etching of silicon and nanotechnology applications. Nano Today, 2014,9(3):271-304.
DOI URL |
| [20] |
ABDULKADIR A, AZIZ A, PAKHURUDDIN M Z. Effects of silver nanoparticles layer thickness towards properties of black silicon fabricated by metal-assisted chemical etching for photovoltaics. SN Applied Sciences, 2020,2(4):515.
DOI URL |
| [21] |
ALHER M A, MOSLEH A, BANIHASHEMIAN S F. Investigation of silicon nanowires produced by metal-assisted chemical etching method. IOP Conference Series: Materials Science and Engineering, 2020,671(1):012028.
DOI URL |
| [22] |
CULLIS A G, CANHAM L T, CALCOTT P J. The structural and luminescence properties of porous silicon. Journal of Applied Physics, 1997,82(3):909-965.
DOI URL |
| [23] | FOWLER R H, NORDHEIM L. Electron emission in intense electric fields. Proceedings of the Royal Society of London, 1928,119(781):173-181. |
| [1] | 刘瑶, 尤勋海, 赵冰, 罗晓莹, 陈星. 功能纳米材料应用于电化学新冠病毒生物传感器的研究进展[J]. 无机材料学报, 2023, 38(1): 32-42. |
| [2] | 王如意, 徐国良, 杨蕾, 邓崇海, 储德林, 张苗, 孙兆奇. p-n异质结BiVO4/g-C3N4光阳极的制备及其光电化学水解性能[J]. 无机材料学报, 2023, 38(1): 87-96. |
| [3] | 阮景, 杨金山, 闫静怡, 游潇, 王萌萌, 胡建宝, 张翔宇, 丁玉生, 董绍明. 三维碳化硅纳米线增强碳化硅陶瓷基复合材料的电磁屏蔽性能[J]. 无机材料学报, 2022, 37(5): 579-584. |
| [4] | 阮景, 杨金山, 闫静怡, 游潇, 王萌萌, 胡建宝, 张翔宇, 丁玉生, 董绍明. 碳化硅纳米线增强多孔碳化硅陶瓷基复合材料的制备[J]. 无机材料学报, 2022, 37(4): 459-466. |
| [5] | 赵伟, 徐阳, 万颖杰, 蔡天逊, 穆金潇, 黄富强. 金属氰胺化合物的结构、合成及电化学储能应用[J]. 无机材料学报, 2022, 37(2): 140-151. |
| [6] | 李昆儒, 胡省辉, 张正富, 郭玉忠, 黄瑞安. 源于溪木贼的高性能锂离子电池三维多孔生物质硅/碳复合负极材料[J]. 无机材料学报, 2021, 36(9): 929-935. |
| [7] | 刘芳芳, 传秀云, 杨扬, 李爱军. 氮/硫共掺杂对纤水镁石模板碳纳米管电化学性能的影响[J]. 无机材料学报, 2021, 36(7): 711-717. |
| [8] | 张晓君, 李佳乐, 邱吴劼, 杨淼森, 刘建军. 钠离子电池正极材料P2-Nax[Mg0.33Mn0.67]O2的电化学活性研究[J]. 无机材料学报, 2021, 36(6): 623-628. |
| [9] | 朱勇, 顾军, 于涛, 何海佟, 姚睿. 铂钴合金纳米电催化剂的制备及性能研究[J]. 无机材料学报, 2021, 36(3): 299-305. |
| [10] | 李陇彬, 薛玉冬, 胡建宝, 杨金山, 张翔宇, 董绍明. 碳化硅纳米线增韧碳化硅纤维/碳化硅基体损伤行为研究[J]. 无机材料学报, 2021, 36(10): 1111-1117. |
| [11] | 张亚萍,雷宇轩,丁文明,于濂清,朱帅霏. 双铁电复合材料的制备及其光电化学性能研究[J]. 无机材料学报, 2020, 35(9): 987-992. |
| [12] | 丁昇, 宁锴, 袁斌霞, 潘卫国, 尹诗斌, 刘建峰. 碱性溶液中不同微观结构的Fe-N/C催化剂氧还原性能的稳定性对比研究[J]. 无机材料学报, 2020, 35(8): 953-958. |
| [13] | 湛菁,徐昌藩,龙怡宇,李启厚. 聚丙烯酰胺凝胶法制备Bi2Mn4O10及其电化学性能[J]. 无机材料学报, 2020, 35(7): 827-833. |
| [14] | 郑坤, 罗永春, 邓安强, 杨洋, 张海民. A2B7型La0.3Y0.7Ni3.4-xMnxAl0.1储氢合金微观结构和电化学性能研究[J]. 无机材料学报, 2020, 35(5): 549-555. |
| [15] | 唐帅,张文泰,钱军余,鲜鹏,莫小山,黄楠,万国江. 锌在林格氏液中的体外长期腐蚀降解行为[J]. 无机材料学报, 2020, 35(4): 461-468. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||