无机材料学报 ›› 2021, Vol. 36 ›› Issue (6): 637-644.DOI: 10.15541/jim20200450
所属专题: 能源材料论文精选(2021); 【虚拟专辑】氢能材料(2020~2021)
朱云娜1( ), 陈必清1(
), 陈必清1( ), 程天舒2, 杜婵1, 张士民1, 赵静1
), 程天舒2, 杜婵1, 张士民1, 赵静1
收稿日期:2020-08-10
修回日期:2020-10-29
出版日期:2021-06-20
网络出版日期:2020-11-05
通讯作者:
陈必清, 教授. E-mail: chenbq2332@163.com
作者简介:朱云娜(1994-), 女, 硕士研究生. E-mail: zhuyunna11@163.com
基金资助:
ZHU Yunna1( ), CHEN Biqing1(
), CHEN Biqing1( ), CHENG Tianshu2, DU Chan1, ZHANG Shimin1, ZHAO Jing1
), CHENG Tianshu2, DU Chan1, ZHANG Shimin1, ZHAO Jing1
Received:2020-08-10
Revised:2020-10-29
Published:2021-06-20
Online:2020-11-05
Contact:
CHEN Biqing, professor. E-mail: chenbq2332@163.com
About author:ZHU Yunna(1994-), female, Master candidate. E-mail: zhuyunna11@163.com
Supported by:摘要:
本研究采用简单的一步化学沉积法制备非晶纳米Nd-Ni-B/NF稀土复合电极并研究其析氢(Hydrogen evolution reaction, HER)性能。通过各种测试方法对纳米电极材料进行物相分析和形貌表征,并探索其电催化析氢性能和稳定性。结果表明, 稀土Nd可提高电极的电催化析氢性能, 当硝酸钕浓度为3 g?L-1时, 恒温35 ℃下施镀1 h, 制备的Nd-Ni-B/NF电极析氢性能最佳。Nd-Ni-B/NF(Nickel foam)电极在1.0 mol?L-1KOH 溶液中, 20 mA?cm-2电流密度下的析氢过电位仅为180 mV, Tafel斜率为117 mV?dec-1, 析氢反应由Volmer-Heyrovsky步骤控制。此外, Nd-Ni-B/NF电极具有优越的电化学稳定性, 在持续电解12 h或2000次循环伏安测试后, 催化剂的活性没有明显衰减。
中图分类号:
朱云娜, 陈必清, 程天舒, 杜婵, 张士民, 赵静. 非晶Nd-Ni-B/NF稀土复合电极材料的制备及其析氢性能[J]. 无机材料学报, 2021, 36(6): 637-644.
ZHU Yunna, CHEN Biqing, CHENG Tianshu, DU Chan, ZHANG Shimin, ZHAO Jing. Amorphous Nd-Ni-B/NF Rare Earth Composites: Preparation and HER Electrocatalytic Performance[J]. Journal of Inorganic Materials, 2021, 36(6): 637-644.
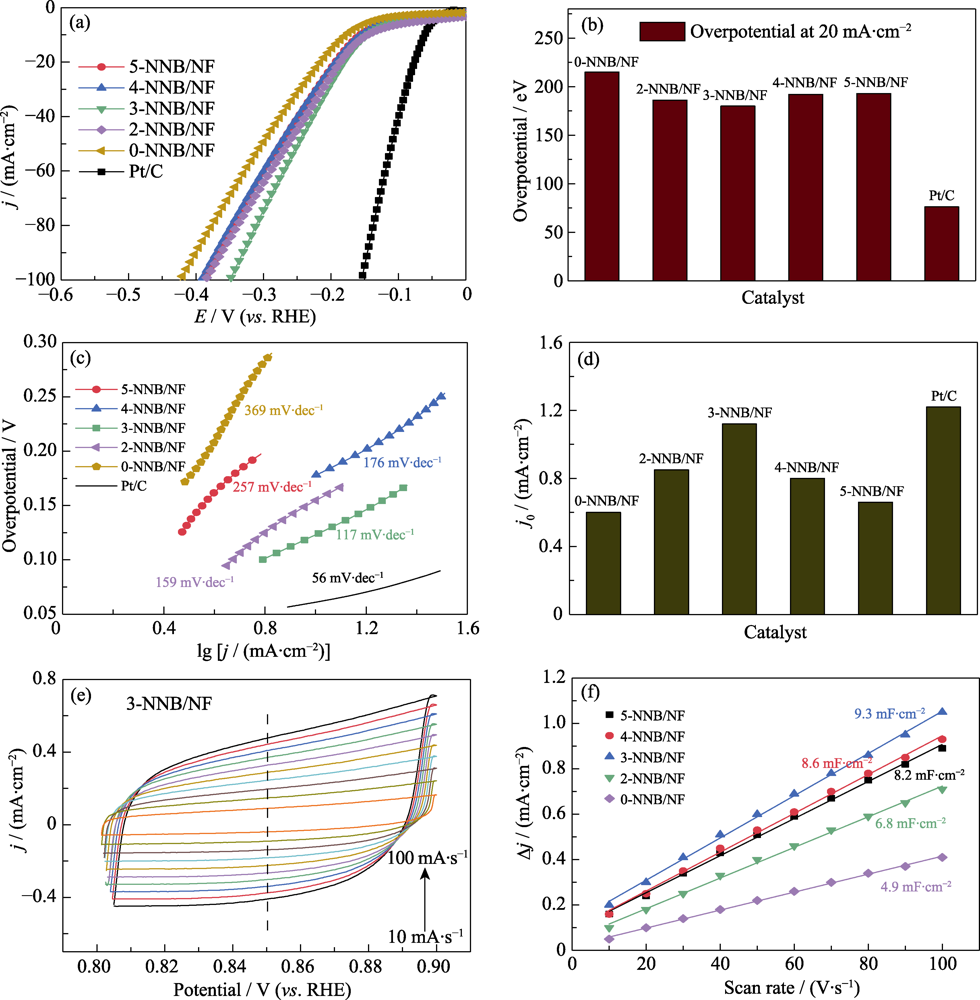
图5 (a)x-NNB/NF(x=0, 2, 3, 4, 5)和Pt/C电极的LSV曲线, (b)在20 mA?cm-2的过电位, (c)Tafel曲线(源于(a))和(d)根据Tafel曲线外推得到的交换电流密度(j0); (e)3-NNB/NF电极的CV曲线; (f)x-NNB/NF(x=0, 2, 3, 4, 5)电极的充电双电层库仑曲线
Fig. 5 (a) LSV curves, (b) overpotentials at 20 mA?cm-2, (c) Tafel curves (derived from (a)) and (d) exchange current densities (j0) derived from the Tafel curves of x-NNB/NF(x=0, 2, 3, 4, 5) and Pt/C electrodes; (e) CV curves of 3-NNB/NF electrode at different scan rates; (f) Coulomb curves of charge double-layer capacitance of x-NNB/NF(x=0, 2, 3, 4, 5) electrodes
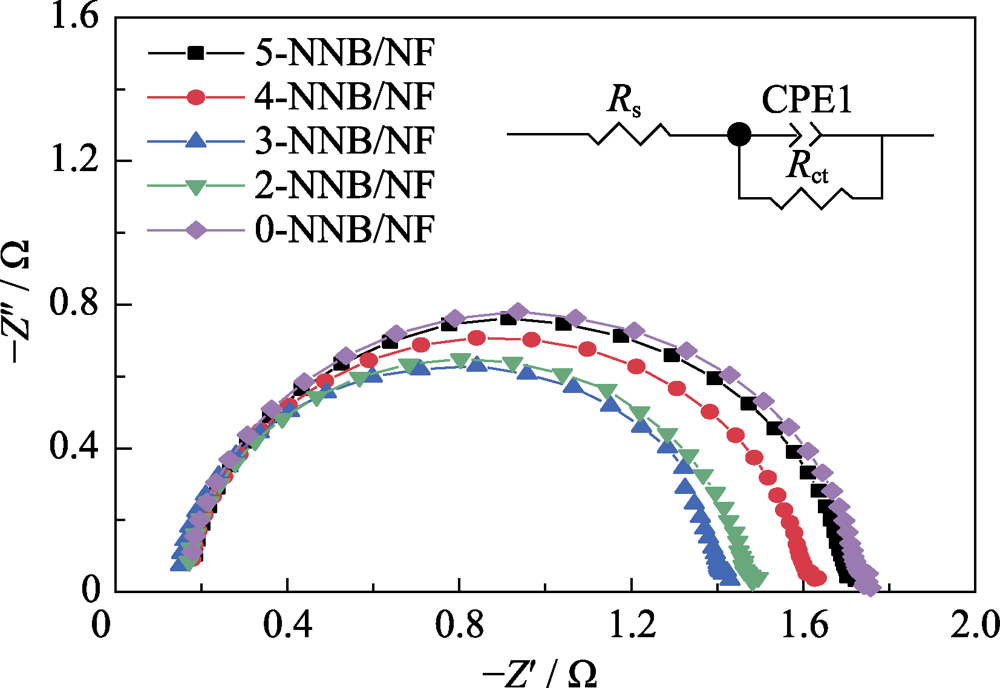
图6 x-NNB/NF(x=0, 2, 3, 4, 5)电极的电化学阻抗图谱, 插图为等效电路图
Fig. 6 Nyquist plots of x-NNB/NF(x=0, 2, 3, 4, 5) electrodes towards HER with inset showing the corresponding equivalent circuit
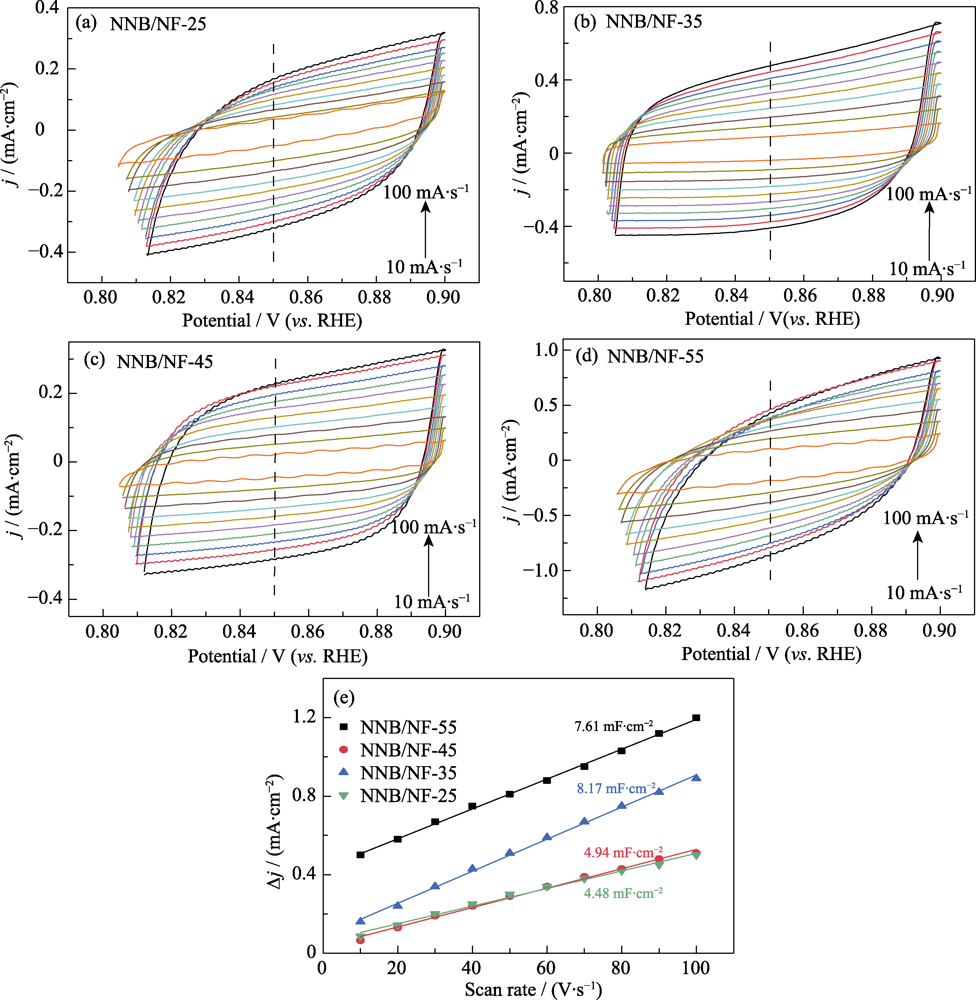
图8 NNB/NF-y(y=25, 35, 45, 55)电极(a~d)在不同扫描速率下的CV曲线和(e)充电双电层库仑曲线
Fig. 8 (a-e) CV curves at different scan rates and Coulomb curves of charge double-layer capacitance for NNB/NF-y (y=25, 35, 45, 55) electrodes
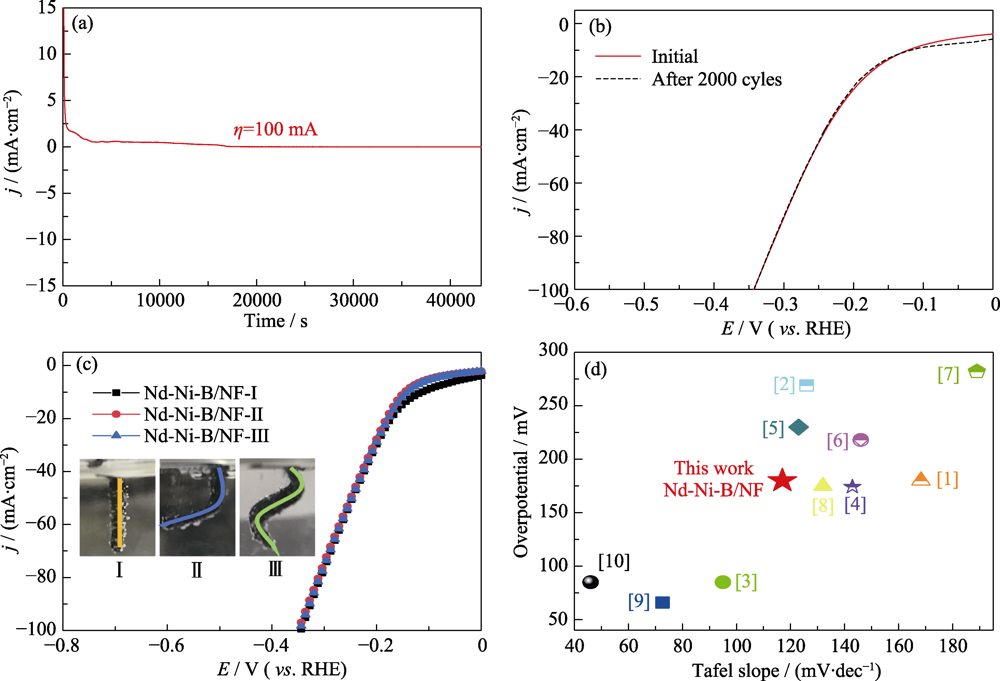
图9 (a)NNB/NF-35在100 mV电位下电解12 h的电流-时间曲线; (b)2000圈循环伏安扫描前后的LSV曲线; (c)不同形状的NNB/NF-35电极的极化曲线和数码照片(插图); (d)不同非贵金属电催化材料在碱性溶液中的HER活性对比
Fig. 9 (a) I-t curve for NNB/NF-35 under 100 mV static overpotential for stability test 12 h; (b) Polarization curves of NNB/NF-35 before and after 2000 CV sweeps; (c) LSV curves and digital photos (insets) of NNB/NF-35 electrodes with different shapes; (d) Comparison of HER activities for different non-noble electrocatalytic materials in alkaline solution
| Electrode | Rs/(Ω∙cm-2) | RP/(Ω∙cm-2) | Rct/(Ω∙cm-2) |
|---|---|---|---|
| 0-NNB/NF | 0.195 | 1.573 | 1.578 |
| 2-NNB/NF | 0.167 | 1.294 | 1.310 |
| 3-NNB/NF | 0.152 | 1.261 | 1.279 |
| 4-NNB/NF | 0.165 | 1.438 | 1.455 |
| 5-NNB/NF | 0.166 | 1.536 | 1.543 |
表1 x-NNB/NF(x=0, 2, 3, 4, 5)电极的电化学交流阻抗参数
Table S1 EIS parameters of x-NNB/NF(x=0, 2, 3, 4, 5) electrodes
| Electrode | Rs/(Ω∙cm-2) | RP/(Ω∙cm-2) | Rct/(Ω∙cm-2) |
|---|---|---|---|
| 0-NNB/NF | 0.195 | 1.573 | 1.578 |
| 2-NNB/NF | 0.167 | 1.294 | 1.310 |
| 3-NNB/NF | 0.152 | 1.261 | 1.279 |
| 4-NNB/NF | 0.165 | 1.438 | 1.455 |
| 5-NNB/NF | 0.166 | 1.536 | 1.543 |
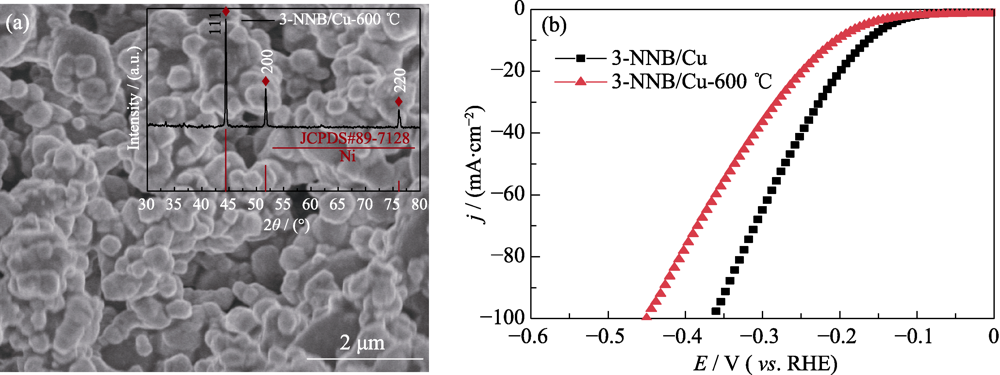
图S3 (a)3-NNB/Cu-600 ℃的SEM照片和XRD图谱及(b)3-NNB/Cu和3-NNB/Cu-600 ℃的LSV曲线
Fig. S3 (a) SEM image and XRD pattern of 3-NNB/Cu-600 ℃; (b) LSV curves of 3-NNB/Cu and 3-NNB/Cu-600 ℃
| Catalyst | Current density@Overpotential | Tafel slope/(mV∙dec-1) | Ref. |
|---|---|---|---|
| Nd-Ni-B/NF | 20 mA∙cm-2@180 mV | 117 | This work |
| Ni-Co/RuO2 | 50 mA∙cm-2@180 mV | 168.3 | [1] |
| Ni-Fe-S/Ti | 50 mA∙cm-2@126 mV | 269 | [2] |
| V-Ni2P NSAs/CC | 10 mA∙cm-2@85 mV | 95 | [3] |
| Ni-Fe-P | 10 mA∙cm-2@174.2 mV | 142.9 | [4] |
| Ni/NiS | 10 mA∙cm-2@230 mV | 123 | [5] |
| FeP Nanorod | 10 mA∙cm-2@218 mV | 146 | [6] |
| Ni0.5Co0.5/NC | 10 mA∙cm-2@282 mV | 189 | [7] |
| FeCoP Nanoarrays | 10 mA∙cm-2@175 mV | 132 | [8] |
| Ni2P-NiSe2 | 10 mA∙cm-2@66 mV | 72.6 | [9] |
| Ni-Co-P/NF | 10 mA∙cm-2@85 mV | 46 | [10] |
表S2 非贵金属催化剂在1.0 mol∙L-1 KOH介质中HER催化性能的对比
Table S2 Comparison of the electrocatalytic HER activity for representative nonprecious HER catalysts in 1.0 mol∙L-1 KOH electrolyte
| Catalyst | Current density@Overpotential | Tafel slope/(mV∙dec-1) | Ref. |
|---|---|---|---|
| Nd-Ni-B/NF | 20 mA∙cm-2@180 mV | 117 | This work |
| Ni-Co/RuO2 | 50 mA∙cm-2@180 mV | 168.3 | [1] |
| Ni-Fe-S/Ti | 50 mA∙cm-2@126 mV | 269 | [2] |
| V-Ni2P NSAs/CC | 10 mA∙cm-2@85 mV | 95 | [3] |
| Ni-Fe-P | 10 mA∙cm-2@174.2 mV | 142.9 | [4] |
| Ni/NiS | 10 mA∙cm-2@230 mV | 123 | [5] |
| FeP Nanorod | 10 mA∙cm-2@218 mV | 146 | [6] |
| Ni0.5Co0.5/NC | 10 mA∙cm-2@282 mV | 189 | [7] |
| FeCoP Nanoarrays | 10 mA∙cm-2@175 mV | 132 | [8] |
| Ni2P-NiSe2 | 10 mA∙cm-2@66 mV | 72.6 | [9] |
| Ni-Co-P/NF | 10 mA∙cm-2@85 mV | 46 | [10] |
| [1] |
GAO Q, ZHANG W, SHI Z, et al. Structural design and electronic modulation of transition-metal-carbide electrocatalysts toward efficient hydrogen evolution. Advanced Materials, 2019,31(2):1802880.
DOI URL |
| [2] |
DUAN J, CHEN S, ORTÍZ-LEDÓN C A, et al. Phosphorus vacancies that boost electrocatalytic hydrogen evolution by two orders of magnitude. Angewandte Chemie-International Edition, 2020,59(21):8181-8186.
DOI URL |
| [3] |
WAN X K, WU H B, GUAN B Y, et al. Confining sub-nanometer Pt clusters in hollow mesoporous carbon spheres for boosting hydrogen evolution activity. Advanced Materials, 2020,32(7):1901349.
DOI URL |
| [4] |
NWANEBU E O, YAO Y, OMANOVIC S. The influence of Ir content in (Ni0.4Co0.6)1-xIrx-oxide anodes on their electrocatalytic activity in oxygen evolution by acidic and alkaline water electrolysis. Journal of Electroanalytical Chemistry, 2020,865:114122.
DOI URL |
| [5] |
DONG X, YAN H, JIAO Y, et al. 3D hierarchical V-Ni-based nitride heterostructure as a highly efficient pH-universal electrocatalyst for the hydrogen evolution reaction. Journal of Materials Chemistry A, 2019,7(26):15823-15830.
DOI URL |
| [6] |
WANG H F, CHEN L, PANG H, et al. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chemical Society Reviews, 2020,49(5):1414-1448.
DOI URL |
| [7] |
BAYATSARMADI B, ZHENG Y, RUSSO V, et al. Highly active nickel-cobalt/nanocarbon thin films as efficient water splitting electrodes. Nanoscale, 2016,8(43):18507-18515.
DOI URL |
| [8] |
CHEN Z, QING H, ZHOU K, et al. Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Progress in Materials Science, 2020,108:100618.
DOI URL |
| [9] | PENG K, ZHOU J, GAO H, et al. Emerging one-/two-dimensional heteronanostructure integrating SiC nanowires with MoS2 nanosheets for efficient electrocatalytic hydrogen evolution. ACS Applied Materials & Interfaces, 2020,12(17):19519-19529. |
| [10] |
ZHANG P, WANG M, YANG Y, et al. Electroless plated Ni-Bx films as highly active electrocatalysts for hydrogen production from water over a wide pH range. Nano Energy, 2016,19:98-107.
DOI URL |
| [11] |
XU N, CAO G, CHEN Z, et al. Cobalt nickel boride as an active electrocatalyst for water splitting. Journal of Materials Chemistry A, 2017,5(24):12379-12384.
DOI URL |
| [12] | SHI J L, SHENG M Q, WU Q, et al. Preparation of electrode materials of amorphous Co-W-B/carbon cloth composite and their electro-catalytic performance for electrolysis of water. Chinese Journal of Materials Research, 2020,34(4):263-271. |
| [13] |
GAO W, WEN D, HO J C, et al. Incorporation of rare earth elements with transition metal-based materials for electrocatalysis: a review for recent progress. Materials Today Chemistry, 2019,12:266-281.
DOI URL |
| [14] |
RÖßNER L, ARMBRÜSTER M. Electrochemical energy conversion on intermetallic compounds: a review. ACS Catalysis, 2019,9(3):2018-2062.
DOI URL |
| [15] |
WU G, LI N, DAI C S, et al. Electrochemical preparation and characteristics of Ni-Co-LaNi5 composite coatings as electrode materials for hydrogen evolution. Materials Chemistry and Physics, 2004,83:307-314.
DOI URL |
| [16] |
ZHU Y, CHEN B, CHENG T, et al. Deposit amorphous Ni-Co-B-RE (RE=Ce, Gd and Nd) on nickel foam as a high performance and durable electrode for hydrogen evolution reaction. Journal of Electroanalytical Chemistry, 2020,878:114552.
DOI URL |
| [17] |
ROSALBINO F, DELSANTE S, BORZONE G, et al. Electrocatalytic behaviour of Co-Ni-R (R=Rare earth metal) crystalline alloys as electrode materials for hydrogen evolution reaction in alkaline medium. International Journal of Hydrogen Energy, 2008,33(22):6696-6703.
DOI URL |
| [18] |
GAO Z, YANG Z, LI Y, et al. Improving the phase stability and cycling performance of Ce2Ni7-type RE-Mg-Ni alloy electrodes by high electronegativity element substitution. Dalton Transactions, 2018,47(46):16453-16460.
DOI URL |
| [19] |
WANG M F, XIAO D H, ZHOU P F, et al. Effects of rare earth yttrium on microstructure and properties of Mg-Al-Zn alloy. Journal of Alloys and Compounds, 2018,742:232-239.
DOI URL |
| [20] |
LI H, LI H, DAI W, et al. Preparation of the Ni-B amorphous alloys with variable boron content and its correlation to the hydrogenation activity. Applied Catalysis A: General, 2003,238(1):119-130.
DOI URL |
| [21] |
XIA W S, FAN Y, JIANG Y S, et al. Local surface state of amorphous NiP and NiB alloy catalysts. Applied Surface Science, 1996,103(1):1-9.
DOI URL |
| [22] |
JIN H, LIU X, JIAO Y, et al. Constructing tunable dual active sites on two-dimensional C3N4@MoN hybrid for electrocatalytic hydrogen evolution. Nano Energy, 2018,53:690-697.
DOI URL |
| [23] | ZHANG L, YIN J, WEI K, et al. Fabrication of hierarchical SrTiO3@MoS2 heterostructure nanofibers as efficient and low-cost electrocatalysts for hydrogen-evolution reactions. Nanotechnology, 2020,31(20):205604. |
| [24] | KHANI H, GRUNDISH N S, WIPF D O, et al. Graphitic-shell encapsulation of metal electrocatalysts for oxygen evolution, oxygen reduction, and hydrogen evolution in alkaline solution. Advanced Energy Materials, 2020,10(1):1903215. |
| [25] |
WANG Y, ZOU K, WANG D, et al. Highly efficient hydrogen evolution from the hydrolysis of ammonia borane solution with the Co-Mo-B/NF nanocatalyst. Renewable Energy, 2020,154:453-460.
DOI URL |
| [26] |
SUN H, XU X, YAN Z, et al. Superhydrophilic amorphous Co-B-P nanosheet electrocatalysts with Pt-like activity and durability for the hydrogen evolution reaction. Journal of Materials Chemistry A, 2018,6(44):22062-22069.
DOI URL |
| [27] |
YU P, WANG F, SHIFA T A, et al. Earth abundant materials beyond transition metal dichalcogenides: a focus on electrocatalyzing hydrogen evolution reaction. Nano Energy, 2019,58:244-276.
DOI URL |
| [28] | SHI J, SHENG M, WU Q, et al. Preparation of electrode materials of amorphous Co-W-B/carbon cloth composite and their electro-catalytic performance for electrolysis of water. Chinese Journal of Materials Research, 2020,34(4):263-271. |
| [29] |
CHAUDHARI N, JIN H, KIM B, et al. Nanostructured materials on 3D nickel foam as electrocatalysts for water splitting. Nanoscale, 2017,9(34):12231-12247.
DOI URL |
| [30] |
ZHAO Y, ZHAO M, DING X, et al. One-step colloid fabrication of nickel phosphides nanoplate/nickel foam hybrid electrode for high-performance asymmetric supercapacitors. Chemical Engineering Journal, 2019,373:1132-1143.
DOI URL |
| [31] |
ZHU J, HU L, ZHAO P, et al. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chemical Reviews, 2020,120(2):851-918.
DOI URL |
| [32] |
DU Z, JANNATUN N, YU D, et al. C60-decorated nickel-cobalt phosphide as an efficient and robust electrocatalyst for hydrogen evolution reaction. Nanoscale, 2018,10(48):23070-23079.
DOI URL |
| [33] |
WU Z Y, HU B C, WU P, et al. Mo2C nanoparticles embedded within bacterial cellulose-derived 3D N-doped carbon nanofiber networks for efficient hydrogen evolution. NPG Asia Materials, 2016,8(7):e288-e288.
DOI URL |
| [34] |
VIJ V, SULTAN S, HARZANDI A M, et al. Nickel-based electrocatalysts for energy-related applications: oxygen reduction, oxygen evolution, and hydrogen evolution reactions. ACS Catalysis, 2017,7(10):7196-7225.
DOI URL |
| [35] |
SHARMA L, KHUSHWAHA H S, MATHUR A, et al. Role of molybdenum in Ni-MoO2 catalysts supported on reduced graphene oxide for temperature dependent hydrogen evolution reaction. Journal of Solid State Chemistry, 2018,265:208-217.
DOI URL |
| [36] |
ANANTHARAJ S, NODA S. Amorphous catalysts and electrochemical water splitting: an untold story of harmony. Small, 2020,16(2):1905779.
DOI URL |
| [37] |
DENG B, ZHOU L, JIANG Z, et al. High catalytic performance of nickel foam supported Co2P-Ni2P for overall water splitting and its structural evolutions during hydrogen/oxygen evolution reactions in alkaline solutions. Journal of Catalysis, 2019,373:81-92.
DOI URL |
| [1] | 孙强强, 陈子璇, 杨子玥, 王毅梦, 曹宝月. 金属镍铜负载钒氧化物的高效电解产氢性能[J]. 无机材料学报, 2023, 38(6): 647-655. |
| [2] | 季邦, 赵文锋, 段洁利, 马立哲, 付兰慧, 杨洲. 泡沫镍网负载TiO2/WO3薄膜对乙烯的光催化降解[J]. 无机材料学报, 2020, 35(5): 581-588. |
| [3] | 付亚康,翁杰,刘耀文,张科宏. 钛网表面含hBMP-2的复合涂层制备及hBMP-2的释放研究[J]. 无机材料学报, 2020, 35(2): 173-178. |
| [4] | 李兆, 孙强强, 陈索倩, 周春生, 曹静, 王永锋, 王亚楠. 水热合成镍铜复合磷化物及其电催化析氢与肼氧化性能[J]. 无机材料学报, 2020, 35(10): 1149-1156. |
| [5] | 张亚萍, 丁文明, 朱海丰, 黄承兴, 于濂清, 王永强, 李哲, 徐飞. 电还原MoSe2修饰TiO2纳米管光电化学性能研究[J]. 无机材料学报, 2019, 34(8): 797-802. |
| [6] | 王远, 林杰, 常郑, 林天全, 钱猛, 黄富强. 基于三维石墨烯衬底的纳米片状水合氧化钌:电化学沉积制备以及柔性超级电容器储能应用[J]. 无机材料学报, 2019, 34(4): 455-460. |
| [7] | 苏琨, 张亚茹, 陆飞, 张君, 王熙. 铂修饰二氧化钛纳米片的制备及其光电催化析氢反应研究[J]. 无机材料学报, 2019, 34(11): 1200-1204. |
| [8] | 王辉, 俞有幸. KOH碱化处理对Fe3N纳米颗粒电催化制氢性能影响[J]. 无机材料学报, 2018, 33(6): 653-658. |
| [9] | 祁星耀, 周清锋, 崔芒伟, 杨永珍, 蒋海伟, 梁 伟, 康利涛. 原位氧化法制备Zn-Ni氢氧化物纳米片及其电荷存储特性研究[J]. 无机材料学报, 2017, 32(4): 372-378. |
| [10] | 何 玲, 李乾坤, 李文生, 崔 帅. 三基色镍基自敏复合涂层的制备及其性能研究[J]. 无机材料学报, 2017, 32(1): 56-62. |
| [11] | 周 辉, 韩满贵, 唐中开, 吴燕辉. N型多孔硅/镍微米管复合材料的制备及其磁性能研究[J]. 无机材料学报, 2016, 31(8): 855-859. |
| [12] | 叶晓丹, 胡建冠, 杨 倩, 郑遗凡, 黄宛真. NiO/AC非对称电容器电极材料的制备及性能研究[J]. 无机材料学报, 2014, 29(3): 250-256. |
| [13] | 胡 锋, 张羊换, 张 胤, 侯忠辉1, 董忠平, 邓磊波. 球磨CeMg12+100wt%Ni+Ywt%TiF3(Y=0、3、5)合金微观结构及电化学储氢性能[J]. 无机材料学报, 2013, 28(2): 217-223. |
| [14] | 石锦罡, 姚 辉, 陈名海, 刘 宁, 李清文. 铜包裹碳化硅颗粒复合粉体的化学原位沉积制备与表征[J]. 无机材料学报, 2012, 27(8): 795-799. |
| [15] | 魏浩铭, 陈 令, 巩海波, 曹丙强. 氧化锌纳米棒形貌对ZnO/Cu2O异质结太阳能电池光伏性能的影响[J]. 无机材料学报, 2012, 27(8): 833-837. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||