无机材料学报 ›› 2021, Vol. 36 ›› Issue (5): 461-470.DOI: 10.15541/jim20200416
所属专题: 电致变色材料与器件; 【虚拟专辑】电致变色与热致变色材料; 电致变色专栏2021
• 专栏:电致变色材料与器件(特邀编辑:王金敏,刁训刚) • 上一篇 下一篇
收稿日期:2020-07-24
修回日期:2020-09-17
出版日期:2021-05-20
网络出版日期:2021-04-19
作者简介:王金敏(1975-), 男, 教授. E-mail:wangjinmin@sspu.edu.cn;jmwang@usst.edu.cn
基金资助:
WANG Jinmin1,2( ), HOU Lijun1, MA Dongyun1,2
), HOU Lijun1, MA Dongyun1,2
Received:2020-07-24
Revised:2020-09-17
Published:2021-05-20
Online:2021-04-19
About author:WANG Jinmin (1975-), male, professor. E-mail:wangjinmin@sspu.edu.cn;jmwang@usst.edu.cn
Supported by:摘要:
电致变色材料具有可逆的颜色转变特性, 在智能窗、显示器、防眩后视镜、电子纸、军事伪装等领域应用广泛。相对于其它种类的显示器件, 电致变色显示器件具有色彩丰富、对比度高、无视盲角、断电后仍显色等优点。作为一种典型的阴极着色电致变色材料, 氧化钼具有响应时间短和着色态更接近于人眼对光线的敏感波段等优点, 使得由氧化钼组成的电致变色器件具有重要的研究价值。本文简要介绍了电致变色、电致变色材料与器件的定义及其应用, 尤其电致变色技术最近在智能手机上得到了示范应用, 表明电致变色技术未来有良好的发展前景。然后, 详细综述了氧化钼薄膜的制备、氧化钼的改性、氧化钼电致变色器件的研究进展。最后提出了氧化钼电致变色薄膜与器件当前存在的问题和解决的途径, 并对其发展前景进行了展望。
中图分类号:
王金敏, 后丽君, 马董云. 氧化钼电致变色材料与器件[J]. 无机材料学报, 2021, 36(5): 461-470.
WANG Jinmin, HOU Lijun, MA Dongyun. Molybdenum Oxide Electrochromic Materials and Devices[J]. Journal of Inorganic Materials, 2021, 36(5): 461-470.

图3 水/溶剂热法应用领域(a),水/溶剂热法设备原理图(b)和水/溶剂热制备的一般步骤(c)[40]
Fig. 3 Application field of water/solvothermal method (a), schematic diagram of water/solvothermal method equipment (b), and general steps of water/solvothermal preparation (c)[40]

图5 施加+1.5/-1.5 V, 15/15 s后的六至十层MoO3膜的计时容量法曲线(a)和MoO3薄膜层数对其电荷密度的影响(b)[26]
Fig. 5 Chronocoulometry of six- to ten-layer MoO3 ?lm after applying +1.5/-1.5 V for 15/15 s (a) and effect of the number of MoO3 thin film layers on its charge density (b)[26] Colouful figures are available on roebsite

图7 具有多层堆叠结构的α-MoO3晶体在不同放大倍率下的SEM显微照片(a~c), 通过煅烧市售钼酸(MoO3·H2O)获得的MoO3晶体的SEM照片(d), 以及具有44层堆积的α-MoO3的SEM显微照片(e)[57]
Fig. 7 SEM images of α-MoO3 crystals with a multi-layer stack structure at different magnifications (a-c), SEM image of MoO3 crystals obtained by calcination of commercial molybdic acid (MoO3·H2O) (d), and SEM image of α-MoO3 stacking with 44 layers (e)[57]
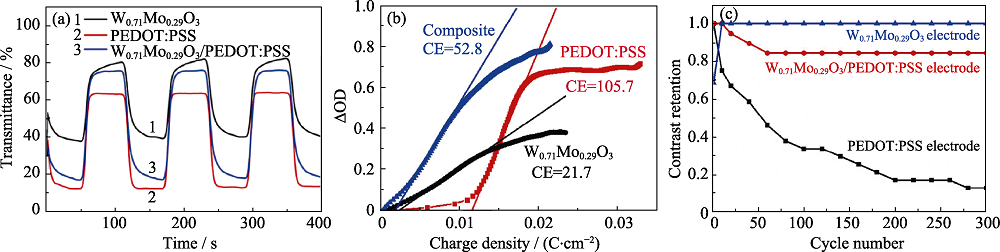
图9 W0.71Mo0.29O3薄膜、PEDOT:PSS薄膜和W0.71Mo0.29O3/PEDOT:PSS薄膜632.8 nm下的原位动力学特征曲线(a), 电极的着色效率曲线(b)和循环稳定性曲线(c)[66]
Fig. 9 In-situ kinetic properties measured at 632.8 nm for W0.71Mo0.29O3 film, PEDOT:PSS film and W0.71Mo0.29O3/PEDOT:PSS film (a), coloration efficiencies (b), and cycling stabilities (c) of the electrodes[66]
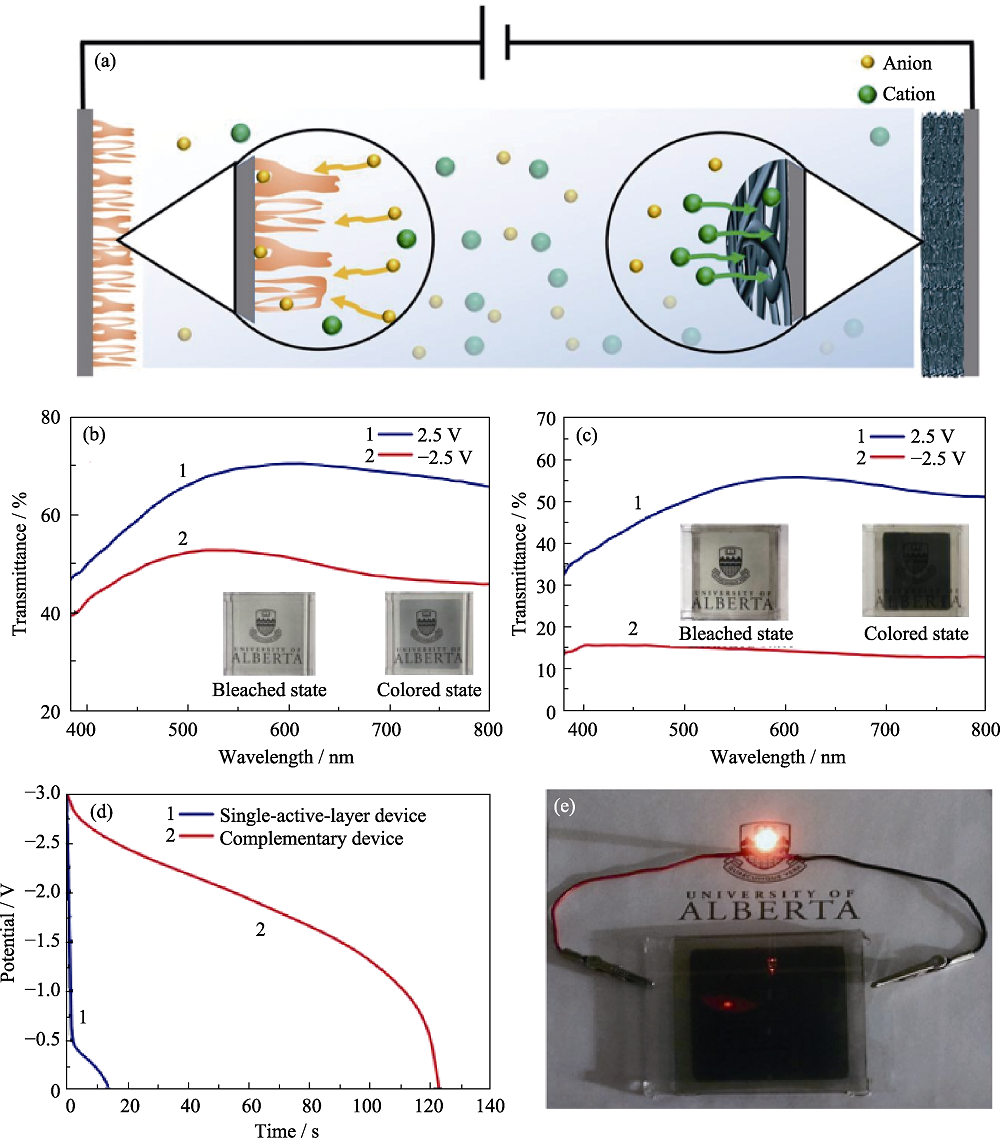
图10 互补型电致变色电池的示意图(a), 单活性层电致变色电池(b)和互补型电致变色电池的可见-近红外透射光谱(c), 单层器件和互补型器件的放电曲线(电流密度为0.05 mA·cm-2) (d), 以及互补型电致变色电池在-2.5 V着色后可以使LED点亮10 min以上(e)[70]
Fig. 10 Schematic diagram of a complementary electrochromic battery (a), visible-near-infrared transmission spectra of single active layer electrochromic battery (b) and complementary electrochromic batteries (c), discharge curves (current density is 0.05 mA·cm-2) of single-layer device and complementary device (d), and complementary electrochromic batteries lighting up the LED for 10 min after being colored at -2.5 V (e)[70]
| [1] |
WANG S Z, CAI S W, CAI W A, et al. Organic-inorganic hybrid electrochromic materials, polysilsesquioxanes containing triarylamine, changing color from colorless to blue. Scientific Reports, 2017,7(1):14627.
DOI URL PMID |
| [2] |
WANG Z, WANG X Y, CONG S, et al. Towards full-colour tunability of inorganic electrochromic devices using ultracompact fabry- perot nanocavities. Nature Communications, 2020,11(1):302.
DOI URL PMID |
| [3] |
WANG Z, WANG X, CONG S, et al. Fusing electrochromic technology with other advanced technologies: a new roadmap for future development. Materials Science and Engineering: R: Reports, 2020,140:100524.
DOI URL |
| [4] |
HASANI A, LE Q V, NGUYEN T P, et al. Facile solution synthesis of tungsten trioxide doped with nanocrystalline molybdenum trioxide for electrochromic devices. Scientific Reports, 2017,7(1):13258.
DOI URL PMID |
| [5] |
LIN Y S, TSAI T H, HUNG S C, et al. Enhanced lithium electrochromism of atmospheric pressure plasma jet-synthesized tungsten/ molybdenum oxide films for flexible electrochromic devices. Journal of Solid State Electrochemistry, 2013,17(4):1077-1088.
DOI URL |
| [6] |
BECHINGER C, FERRERE S, ZABAN A, et al. Photoelectrochromic windows and displays. Nature, 1996,383(6601):608-610.
DOI URL |
| [7] |
XIONG K, EMILSSON G, MAZIZ A, et al. Plasmonic metasufaces with conjugated polymers for flexible electronic paper in color. Advanced Materials, 2016,28(45):9956-9960.
DOI URL PMID |
| [8] |
WANG J M, YU H Y, MA D Y. Progress in the preparation and application of nanostructured manganese dioxide. Journal of Inorganic Materials, 2020,35(12):1307-1314.
DOI URL |
| [9] |
CHANG C C, CHI P W, CHANDAN P, et al. Electrochemistry and rapid electrochromism control of MoO3/V2O5 hybrid nanobilayers. Materials, 2019,12(15):2475.
DOI URL |
| [10] |
XU T, WALTER E C, AGRAWAL A, et al. High-contrast and fast electrochromic switching enabled by plasmonics. Nature Communications, 2016,7(1):10479.
DOI URL |
| [11] |
PLATT J R. Electrochromism, a possible change of color producible in dyes by an electric field. The Journal of Chemical Physics, 1961,34(3):862-863.
DOI URL |
| [12] |
SUI Q, REN X T, DAI Y X, et al. Piezochromism and hydrochromism through electron transfer: new stories for viologen materials. Chemical Science, 2017,8(4):2758-2768.
URL PMID |
| [13] |
YU W H, ZHANG Y, KANG E T, et al. Electroless metallization of dielectric SiLK surfaces functionalized by viologen. Journal of The Electrochemical Society, 2003,150(8):F156-F163.
DOI URL |
| [14] |
MI S, WU J C, LIU J, et al. AIEE-active and electrochromic bifunctional polymer and device composed thereof synchronously achieving electrochemical fluorescence switching and electrochromic switching. ACS Applied Materials & Interfaces, 2015,7(49):27511-27517.
URL PMID |
| [15] |
CHEN X M, LIU H L, XU Z P, et al. Highly regiosymmetric homopolymer based on dioxythiophene for realizing water- processable blue-to-transmissive electrochrome. ACS Applied Materials & Interfaces, 2015,7(21):11387-11392.
DOI URL PMID |
| [16] |
DEB S K. A novel electrophotographic system. Applied Optics, 1969,8(Suppl 1):192-195.
DOI URL |
| [17] |
ATINAFU D G, DONG W, DU M. Controllable synthesis and surface modification of molybdenum oxide nanowires: a short review. Tungsten, 2019,1(4):258-265.
DOI URL |
| [18] |
HE Y C, LI T Z, ZHONG X L, et al. Lattice and electronic structure variations in critical lithium doped nickel oxide thin film for superior anode electrochromism. Electrochimica Acta, 2019,316:143-151.
DOI URL |
| [19] |
BULJA S, KOPF R, NOLAN K, et al. Tuneable dielectric and optical characteristics of tailor-made inorganic electro-chromic materials. Scientific Reports, 2017,7(1):13484.
URL PMID |
| [20] |
MA Y, ZHANG X, YANG M, et al. Controlled growth of MoO3 nanorods on transparent conducting substrates Materials Letters, 2014,136:146-149.
DOI URL |
| [21] |
ZHUO Q Q, TANG J J, SUN J, et al. High efficient reduction of graphene oxide via nascent hydrogen at room temperature. Materials, 2018,11(3):340.
DOI URL |
| [22] |
LI Y B, BANDO Y, GOLBERG D, et al. Field emission from MoO3 nanobelts. Applied Physics Letters, 2002,81(26):5048.
DOI URL |
| [23] |
CHOI H, HEO J H, HA S, et al. Facile scalable synthesis of MoO2 nanoparticles by new solvothermal cracking process and their application to hole transporting layer for CH3NH3PbI3 planar perovskite solar cells. Chemical Engineering Journal, 2017,310:179-186.
DOI URL |
| [24] |
TAO T, CHEN Q Y, HU H P, et al. MoO3 nanoparticles distributed uniformly in carbon matrix for supercapacitor applications Materials Letters, 2011,66(1):102-105.
DOI URL |
| [25] |
ZENG L, CHENG C Y. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts. Hydrometallurgy, 2009,98(1):1-9.
DOI URL |
| [26] |
LEMOS R M J, ALCÁZAR J C B, CARREÑO N L V, et al. Influence of molybdenum trioxide thin film thickness on its electrochemical properties. Molecular Crystals and Liquid Crystals, 2017,655(1):40-50.
DOI URL |
| [27] |
YANG P H, SUN P, MAI W J. Electrochromic energy storage devices. Materials Today, 2016,19(7):394-402.
DOI URL |
| [28] | ZHANG X, LI W J, LI Y, et al. Research progress of inorganic all-solid-state electrochromic devices. Materials Science and Technology, 2020,28(3):140-149. |
| [29] | MORTIMER R J, ROSSEINSKY D R, MONK P M S. Electrochromic Materials and Devices. Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 2015: 1-638. |
| [30] |
JIA H X, CAO X, JIN P S. Advances in inorganic all-solid-state electrochromic materials and devices. Journal of Inorganic Materials, 2020,35(5):511-524.
DOI URL |
| [31] |
ZHAO X H, WEI C, GAI Z Q, et al. Chemical vapor deposition and its application in surface modification of nanoparticles. Chemical Papers, 2020,74(3):767-778.
DOI URL |
| [32] |
GESHEVA K A, CZIRAKI A, IVANOVA T, et al. Crystallization of chemically vapor deposited molybdenum and mixed tungsten/ molybdenum oxide films for electrochromic application. Thin Solid Films, 2007,515(11):4609-4613.
DOI URL |
| [33] | IVANOVA T, GESHEVA K A, POPKIROV G, et al. Electrochromic properties of atmospheric CVD MoO3 and MoO3-WO3 films and their application in electrochromic devices. Materials Science & Engineering B, 2005,119(3):232-239. |
| [34] |
ARROYO-HERNÁNDEZ M, ÁLVARO R, SERRANO S, et al. Catalytic growth of ZnO nanostructures by r.f. magnetron sputtering. Nanoscale Research Letters, 2011,6(1):1-6.
URL PMID |
| [35] |
USHA N, SIVAKUMAR R, SANJEEVIRAJA C. Structural, optical and electrochromic properties of Nb2O5:MoO3 (95:5, 90:10, and 85:15) thin films prepared by RF magnetron sputtering technique. Materials Letters, 2018,229(15):189-192.
DOI URL |
| [36] |
ALVARADO J A, MALDONADO A, JUAREZ H, et al. Characterization of nanostructured ZnO thin films deposited through vacuum evaporation. Beilstein Journal of Nanotechnology, 2015,6:971-975.
DOI URL PMID |
| [37] |
VARNAMKHASTI M G, FALLAH H R, ZADSAR M. Effect of heat treatment on characteristics of nanocrystalline ZnO films by electron beam evaporation. Vacuum, 2012,86(7):871-875.
DOI URL |
| [38] | MIYATA N, SUZUKI T, OHYAMA R. Physical properties of evaporated molybdenum oxide films. Thin Solid Films, 1996,281:218-222. |
| [39] | DIXIT D, MADHURI K V. Effect of oxygen partial pressure on the growth of molybdenum trioxide thin films. Materials Today: Proceedings, 2019,19:2688-2692. |
| [40] |
YANG G J, PARK S J. Conventional and microwave hydrothermal synthesis and application of functional materials: a review. Materials, 2019,12(7):1-18.
DOI URL |
| [41] |
QURESHI N, ARBUJ S, SHINDE M, et al. Swift tuning from spherical molybdenum microspheres to hierarchical molybdenum disulfide nanostructures by switching from solvothermal to hydrothermal synthesis route. Nano Convergence, 2017,4(1):25.
DOI URL PMID |
| [42] | SHI E W, XIA C T, WANG B G, et al. Development application and of hydrothermal method. Journal of Inorganic Materials, 1996,11(2):193-206. |
| [43] |
ZHOU E, TIAN L L, CHENG Z F, et al. Design of NiO flakes@CoMoO4 nanosheets core-shell architecture on Ni foam for high- performance supercapacitors. Nanoscale Research Letters, 2019,14(1):1-11.
URL PMID |
| [44] |
YAO B, HUANG L, ZHANG J, et al. Flexible transparent molybdenum trioxide nanopaper for energy storage. Advanced Materials, 2016,28(30):6353-6358.
DOI URL PMID |
| [45] |
HE S H, LI W D, FENG L, et al. Rational interaction between the aimed gas and oxide surfaces enabling high-performance sensor: the case of acidic α-MoO3 nanorods for selective detection of triethylamine Journal of Alloys and Compounds, 2019,783:574-582.
DOI URL |
| [46] |
JITTIARPORN P, BADILESCU S, SAWAFTA M N A, et al. Electrochromic properties of Sol-Gel prepared hybrid transition metal oxides-a short review. Journal of Science: Advanced Materials and Devices, 2017,2(3):286-300.
DOI URL |
| [47] |
WANG Y H, BOUCHNEB M, ALAUZUN J G, et al. Tuning texture and morphology of mesoporous TiO2 by non-hydrolytic Sol- Gel syntheses. Molecules, 2018,23(11):3006.
DOI URL |
| [48] |
DHANASANKAR M, PURUSHOTHAMAN K K, MURALIDHARAN G. Enhanced electrochromism in cerium doped molybdenum oxide thin films. Materials Research Bulletin, 2010,45(12):1969-1972.
DOI URL |
| [49] | ZHANG J Y, YU Z R, DU J H. Fabrication and electrochromic properties of NiO electrodeposit films. Journal of Chinese Electron Microscopy Society, 1997,16(4):451-452. |
| [50] | WEN Y Y, ZHONG X H, HONG Y Z, et al. Fabrication of molybdenum oxides/carbon nanotube composite fibers by electrochemical deposition and its electrochemical behavior. Journal of the Chinese Ceramic Society, 2012,40(8):1220-1223. |
| [51] |
ZHUZHEL’SKII D V, YALDA K D, SPIRIDONOV V N, et al. Electrochemical deposition of molybdenum oxide into films of poly (3,4-ethylenedioxythiophene) conducting polymer on glassy carbon substrates. Russian Journal of Applied Chemistry, 2016,89(8):1252-1260.
DOI URL |
| [52] |
KÄRBER E, KATERSKI A, ACIK O I, et al. Low-cost plasmonic solar cells prepared by chemical spray pyrolysis. Beilstein Journal of Nanotechnology, 2014,5(1):2398-2402.
DOI URL |
| [53] |
DUNDAR I, KRICHEVSKAYA M, KATERSKI A, et al. TiO2 thin films by ultrasonic spray pyrolysis as photocatalytic material for air purification. Royal Society Open Science, 2019,6(2):181578.
DOI URL PMID |
| [54] |
CHO J S. Large scale process for low crystalline MoO3-carbon composite microspheres prepared by one-step spray pyrolysis for anodes in lithium-ion batteries. Nanomaterials, 2019,9(4):539.
DOI URL |
| [55] |
MOUSAVI-ZADEH S H, RAHMANI M B. Synthesis and ethanol sensing characteristics of nanostructured MoO3:Zn thin films. Surface Review and Letters, 2018,25(4):1850046.
DOI URL |
| [56] | YU H, LI Y, ZHAO L, et al. Novel MoO3-TiO2 composite nanorods films with improved electrochromic performance. Materials Letters, 2016,169:65-68. |
| [57] |
MARTíN-RAMOS P, FERNÁNDEZ-COPPEL I, AVELLA M, et al. α-MoO3 crystals with a multilayer stack structure obtained by annealing from a lamellar MoS2/g-C3N4 nanohybrid. Nanomaterials, 2018,8(7):559.
DOI URL |
| [58] | ZHANG Y Z, HUANG Y S, CAO Y Z, et al. Synthesis and electro- photochromic properties of lithium-doped MoO3 films Chinese Journal of Liquid Crystals and Displays, 2002,17(3):163-168. |
| [59] | MAHAJAN S S, MUJAWAR S H, SHINDE P S, et al. Structural, morphological, optical and electrochromic properties of Ti-doped MoO3 thin films Solar Energy Materials and Solar Cells, 2009,93(2):183-187. |
| [60] | LAYEGH M, GHODSI F E, HADIPOUR H. Experimental and theoretical study of Fe doping as a modifying factor in electrochemical behavior of mixed-phase molybdenum oxide thin films. Applied Physics A: Materials Science & Processing, 2019,126(1):372-387. |
| [61] | KAMOUN OLFA, MAMI A, AMARA M A, et al. Nanostructured Fe, Co-codoped MoO3 thin films Micromachines, 2019,10(2):138. |
| [62] | ZUO Y, MA D Y, XU Z P, et al. Hydrothermal growth, device preparation and electrochromic properties of nano-molybdenum oxide film. Journal of Shanghai Second Polytechnic University, 2017,34(2):81-86. |
| [63] | LIU S, XU Z P, MA D Y, et al. Preparation of MoO3 thin film by MoS2 oxidation method, device assembly and electrochromic properties. Journal of Shanghai Second Polytechnic University, 2018,35(2):111-116. |
| [64] |
KARTEN K, HEIN A, CIOBARU M, et al. Complementary hybrid electrodes for high contrast electrochromic devices with fast response. Nature Communications, 2019,10(1):4874.
DOI URL PMID |
| [65] | ZHANG G, ZHANG W Z, WANG S M. Preparation of molybdenum oxide/polypyrrole composite membrane and study on its discoloration properties. Journal of Xi'an University of Technology, 2018,38(1):1-6, 13. |
| [66] |
LI H Z, MCRAE L, ELEZZABI A Y. Solution-processed interfacial PEDOT:PSS assembly into porous tungsten molybdenum oxide nanocomposite films for electrochromic applications. ACS Applied Materials & Interfaces, 2018,10(12):10520-10527.
DOI URL PMID |
| [67] |
WANG W Q, WANG X L, XIA X H, et al. Enhanced electrochromic and energy storage performance in mesoporous WO3 film and its application in a bi-functional smart window. Nanoscale, 2018,10(17):8162-8169.
DOI URL PMID |
| [68] |
WANG J M, ZHANG L, YU L, et al. A bi-functional device for self-powered electrochromic window and self-rechargeable transparent battery application. Nature Communications, 2014,5:4921.
DOI URL PMID |
| [69] |
CONG S, TIAN Y, LI Q W, et al. Single-crystalline tungsten oxide quantum dots for fast pseudocapacitor and electrochromic applications. Advanced Materials, 2014,26(25):4260-4267.
DOI URL PMID |
| [70] | LI H Z, MCRAE L, FIRBY C J, et al. Nanohybridization of molybdenum oxide with tungsten molybdenum oxide nanowires for solution-processed fully reversible switching of energy storing smart windows. Nano Energy, 2018,47:130-139. |
| [71] | YANG B, MA D Y, ZHENG E M, et al. A self-rechargeable electrochromic battery based on electrodeposited polypyrrole film. Solar Energy Materials and Solar Cells, 2019,192:1-7. |
| [1] | 孔国强, 冷明哲, 周战荣, 夏池, 沈晓芳. Sb掺杂O3型Na0.9Ni0.5Mn0.3Ti0.2O2钠离子电池正极材料[J]. 无机材料学报, 2023, 38(6): 656-662. |
| [2] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| [3] | 汪波, 余健, 李存成, 聂晓蕾, 朱婉婷, 魏平, 赵文俞, 张清杰. Gd/Bi0.5Sb1.5Te3热电磁梯度复合材料的服役稳定性[J]. 无机材料学报, 2023, 38(6): 663-670. |
| [4] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [5] | 陈强, 白书欣, 叶益聪. 热管理用高导热碳化硅陶瓷基复合材料研究进展[J]. 无机材料学报, 2023, 38(6): 634-646. |
| [6] | 林俊良, 王占杰. 铁电超晶格的研究进展[J]. 无机材料学报, 2023, 38(6): 606-618. |
| [7] | 杨颖康, 邵怡晴, 李柏良, 吕志伟, 王路路, 王亮君, 曹逊, 吴宇宁, 黄荣, 杨长. Cl掺杂对CuI薄膜发光性能增强研究[J]. 无机材料学报, 2023, 38(6): 687-692. |
| [8] | 李悦, 张旭良, 景芳丽, 胡章贵, 吴以成. 铈掺杂硼酸钙镧晶体的生长与性能研究[J]. 无机材料学报, 2023, 38(5): 583-588. |
| [9] | 马晓森, 张丽晨, 刘砚超, 汪全华, 郑家军, 李瑞丰. 13X@SiO2合成及其甲苯吸附性能[J]. 无机材料学报, 2023, 38(5): 537-543. |
| [10] | 牛嘉雪, 孙思, 柳鹏飞, 张晓东, 穆晓宇. 铜基纳米酶的特性及其生物医学应用[J]. 无机材料学报, 2023, 38(5): 489-502. |
| [11] | 张硕, 付前刚, 张佩, 费杰, 李伟. C/C多孔体的高温热处理对C/C-SiC复合材料摩擦磨损行为的影响[J]. 无机材料学报, 2023, 38(5): 561-568. |
| [12] | 苑景坤, 熊书锋, 陈张伟. 聚合物前驱体转化陶瓷增材制造技术研究趋势与挑战[J]. 无机材料学报, 2023, 38(5): 477-488. |
| [13] | 陈鑫力, 李岩, 王伟胜, 石智文, 竺立强. 明胶/羧化壳聚糖栅控氧化物神经形态晶体管[J]. 无机材料学报, 2023, 38(4): 421-428. |
| [14] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [15] | 杨洋, 崔航源, 祝影, 万昌锦, 万青. 柔性神经形态晶体管研究进展[J]. 无机材料学报, 2023, 38(4): 367-377. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||