无机材料学报 ›› 2021, Vol. 36 ›› Issue (1): 1-8.DOI: 10.15541/jim20200215
所属专题: 能源材料论文精选(2021); 【虚拟专辑】氢能材料(2020~2021)
• 综述 • 下一篇
收稿日期:2020-04-22
修回日期:2020-07-10
出版日期:2021-01-20
网络出版日期:2020-08-01
作者简介:邓霁峰(1995-), 男, 博士研究生. E-mail:jfdeng@pku.edu.cn
基金资助:
DENG Jifeng1,2,CHEN Shunpeng1,WU Xiaojuan1,ZHENG Jie1,LI Xingguo1( )
)
Received:2020-04-22
Revised:2020-07-10
Published:2021-01-20
Online:2020-08-01
About author:DENG Jifeng(1995-), male, PhD candidate. E-mail:jfdeng@pku.edu.cn
Supported by:摘要:
水解制氢是一种常温常压下的现场制氢方式。由于水解制氢材料氢含量高, 储存容易, 运输方便, 安全可靠, 一直受到研究者们的关注。本文综述了近年来水解制氢材料的总体发展情况, 介绍了三类主要的水解制氢材料, 包括硼氢化物(NaBH4, NH3·BH3)、金属(Mg, Al)以及金属氢化物(MgH2), 对不同材料的制氢原理、主要问题、催化剂与材料设计进行了详细介绍, 比较了不同体系的特点与制氢成本, 并对水解制氢及水解制氢材料的现状和商业化面临的困难做了评价, 最后对未来的发展方向进行了展望。
中图分类号:
邓霁峰, 陈顺鹏, 武晓娟, 郑捷, 李星国. 水解制氢材料研究进展[J]. 无机材料学报, 2021, 36(1): 1-8.
DENG Jifeng, CHEN Shunpeng, WU Xiaojuan, ZHENG Jie, LI Xingguo. Recent Progress on Materials for Hydrogen Generation via Hydrolysis[J]. Journal of Inorganic Materials, 2021, 36(1): 1-8.
| Material | Molar mass/(g·mol-1) | Density/(g·cm-3) | ∆H[ | CM(H2)*/wt% | CM(H2) (H2O)#/wt% | CV(H2)†/(gH2·L-1) |
|---|---|---|---|---|---|---|
| NaBH4 | 37.83 | 1.07 | -47.15 | 21.32 | 7.34 | 228.09 |
| NH3·BH3 | 30.86 | 0.78 | -75.67 | 19.50 | 7.05 | 151.60 |
| Mg | 24.31 | 1.74 | -352.90 | 8.29 | 3.34 | 144.16 |
| Ca | 40.08 | 1.55 | -413.60 | 5.03 | 2.65 | 77.97 |
| Al | 26.98 | 2.70 | -284.40 | 11.21 | 3.73 | 302.62 |
| LiH | 7.95 | 0.82 | -111.20 | 25.36 | 7.06 | 207.97 |
| NaH | 24.00 | 1.40 | -83.70 | 8.40 | 4.80 | 117.60 |
| KH | 40.02 | 1.43 | -81.10 | 5.04 | 3.47 | 72.04 |
| MgH2 | 26.32 | 1.45 | -138.80 | 15.32 | 6.47 | 222.12 |
| CaH2 | 42.09 | 1.70 | -116.05 | 9.58 | 5.16 | 162.84 |
| AlH3 | 30.00 | 1.49 | -126.87 | 20.16 | 7.20 | 300.34 |
Table 1 Fundamental performances of materials for hydrogen generation via hydrolysis
| Material | Molar mass/(g·mol-1) | Density/(g·cm-3) | ∆H[ | CM(H2)*/wt% | CM(H2) (H2O)#/wt% | CV(H2)†/(gH2·L-1) |
|---|---|---|---|---|---|---|
| NaBH4 | 37.83 | 1.07 | -47.15 | 21.32 | 7.34 | 228.09 |
| NH3·BH3 | 30.86 | 0.78 | -75.67 | 19.50 | 7.05 | 151.60 |
| Mg | 24.31 | 1.74 | -352.90 | 8.29 | 3.34 | 144.16 |
| Ca | 40.08 | 1.55 | -413.60 | 5.03 | 2.65 | 77.97 |
| Al | 26.98 | 2.70 | -284.40 | 11.21 | 3.73 | 302.62 |
| LiH | 7.95 | 0.82 | -111.20 | 25.36 | 7.06 | 207.97 |
| NaH | 24.00 | 1.40 | -83.70 | 8.40 | 4.80 | 117.60 |
| KH | 40.02 | 1.43 | -81.10 | 5.04 | 3.47 | 72.04 |
| MgH2 | 26.32 | 1.45 | -138.80 | 15.32 | 6.47 | 222.12 |
| CaH2 | 42.09 | 1.70 | -116.05 | 9.58 | 5.16 | 162.84 |
| AlH3 | 30.00 | 1.49 | -126.87 | 20.16 | 7.20 | 300.34 |
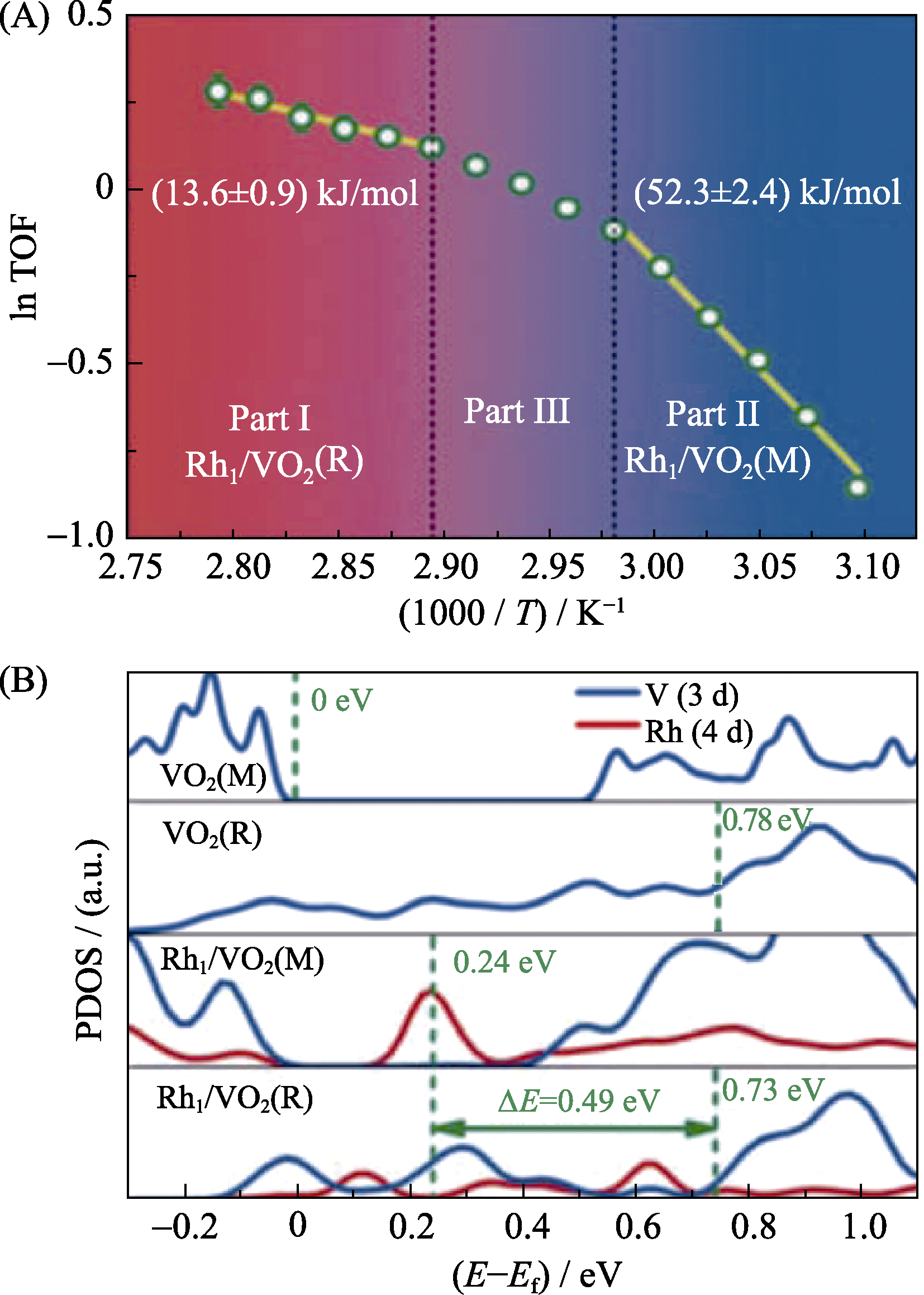
图3 Rh/VO2催化NH3·BH3水解的Arrhenius曲线(A),不同催化剂和基底的态密度计算结果(B)[42]
Fig. 3 Arrhenius plot for NH3·BH3 hydrolysis catalyzed by Rh/VO2 (A), calculated PDOS of several catalysts/supports (B)[42]
| Performance | Metal | Metal hydride | Borohydride |
|---|---|---|---|
| Hydrogen capacity of hydrolysis | Low | High | High (affected by dissolution) |
| Cost | Low | High | High |
| Complexities of system | Complex | Complex | Relatively simple |
| Degree of study on hydrolysis | Modest | Rare | Extensive |
| Storage condition | H2O & O2 free | H2O & O2 free | H2O free |
表2 主要制氢材料性能对比
Table 2 Performances of main materials for hydrogen production via hydrolysis
| Performance | Metal | Metal hydride | Borohydride |
|---|---|---|---|
| Hydrogen capacity of hydrolysis | Low | High | High (affected by dissolution) |
| Cost | Low | High | High |
| Complexities of system | Complex | Complex | Relatively simple |
| Degree of study on hydrolysis | Modest | Rare | Extensive |
| Storage condition | H2O & O2 free | H2O & O2 free | H2O free |
| Prices | Mg | Al | MgH2 | LiH | NaBH4 | NH3·BH3 |
|---|---|---|---|---|---|---|
| Price of material /(yuan∙kg-1) | 120 | 80 | 800 | 4300 | 700 | 60000 |
| Price of H2 /(yuan∙kg-1) | 1450 | 710 | 5200 | 16960 | 3280 | 307700 |
表3 常见水解制氢材料的价格及制氢成本估算
Table 3 Prices of materials for hydrogen generation via hydrolysis and their corresponding costs of H2
| Prices | Mg | Al | MgH2 | LiH | NaBH4 | NH3·BH3 |
|---|---|---|---|---|---|---|
| Price of material /(yuan∙kg-1) | 120 | 80 | 800 | 4300 | 700 | 60000 |
| Price of H2 /(yuan∙kg-1) | 1450 | 710 | 5200 | 16960 | 3280 | 307700 |
| [1] | EBERLE U, FELDERHOFF M, SCHUTH F . Chemical and physical solutions for hydrogen storage. Angew. Chemie. Int. Ed., 2009,48(36):6608-6630. |
| [2] | HE T, PACHFULE P, WU H , et al. Hydrogen carriers. Nat. Rev. Mater., 2016,1:16059. |
| [3] | SCHLAPBACH L, ZÜTTEL A. Hydrogen-storage materials for mobile applications. Nature, 2001,414:353-358. |
| [4] | GÜR T M . Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy & Environ. Sci., 2018,11(10):2696-2767. |
| [5] | STAMENKOVIC V R, STRMCNIK D, LOPES P P , et al. Energy and fuels from electrochemical interfaces. Nat. Mater., 2016,16:57-69. |
| [6] | DEAN J A. Lange's Handbook of Chemistry, 13th Edition. Beijing: Sci. Press, 1991,9:1-172. |
| [7] | KIM H, OH T H, KWON S J . Simple catalyst bed sizing of a NaBH4 hydrogen generator with fast startup for small unmanned aerial vehicles. Int. J. Hydrogen Energy, 2016,41(2):1018-1026. |
| [8] | KIM T . NaBH4 (sodium borohydride) hydrogen generator with a volume-exchange fuel tank for small unmanned aerial vehicles powered by a PEM (proton exchange membrane) fuel cell. Energy, 2014,69:721-727. |
| [9] | LI H W, LI D P, ZHANG X D . Research and development of foreign submarine sodium borohydride hydrolysis hydrogen generation. Ship Sci. Technol., 2012,34(7):135-143. |
| [10] | OH T K . Conceptual design of small unmanned aerial vehicle with proton exchange membrane fuel cell system for long endurance mission. Energy Convers. Manag., 2018,176:349-356. |
| [11] | CHEN K, OUYANG L Z, ZHONG H , et al. Converting H + from coordinated water into H - enables super facile synthesis of LiBH4. Green Chem., 2019,21(16):4380-4387. |
| [12] | SEN B, ŞAVK A, KUYULDAR E , et al. Hydrogen liberation from the hydrolytic dehydrogenation of hydrazine borane in acidic media. Int. J. Hydrogen Energy, 2018,43(38):17978-17983. |
| [13] | KAUFMAN C M, SEN B . Hydrogen generation by hydrolysis of sodium tetrahydroborate: effects of acids and transition metals and their salts. Dalton Trans., 1984, ( 2):307-313. |
| [14] | LIU B H, LI Z P . A review: hydrogen generation from borohydride hydrolysis reaction. J. Power Sources, 2009,187(2):527-534. |
| [15] | BOZKURT G, ÖZER A, YURTCAN A B . Development of effective catalysts for hydrogen generation from sodium borohydride: Ru, Pt, Pd nanoparticles supported on Co3O4. Energy, 2019,180:702-713. |
| [16] | GENÇ A E¸ AKÇA A, KUTLU B . The catalytic effect of the Au(111) and Pt(111) surfaces to the sodium borohydride hydrolysis reaction mechanism: a DFT study. Int. J. Hydrogen Energy, 2018,43(31):14347-14359. |
| [17] | SEMIZ L, ABDULLAYEVA N, SANKIR M . Nanoporous Pt and Ru catalysts by chemical dealloying of Pt-Al and Ru-Al alloys for ultrafast hydrogen generation. J. Alloys Compd., 2018,744(5):110-115. |
| [18] | TUAN D D, LIN K Y A. Ruthenium supported on ZIF-67 as an enhanced catalyst for hydrogen generation from hydrolysis of sodium borohydride. Chem. Eng. J., 2018,351:48-55. |
| [19] | LI Y H, ZHANG X, ZHANG Q , et al. Activity and kinetics of ruthenium supported catalysts for sodium borohydride hydrolysis to hydrogen. RSC Adv., 2016,6(35):29371-29377. |
| [20] | HAO S, YANG L B, CUI L , et al. Self-supported spinel FeCo2O4 nanowire array: an efficient non-noble-metal catalyst for the hydrolysis of NaBH4 toward on-demand hydrogen generation. Nanotechnology, 2016,27(46):0957-4484. |
| [21] | CAI H, LIU L, CHEN Q , et al. Ni-polymer nanogel hybrid particles: a new strategy for hydrogen production from the hydrolysis of dimethylamine-borane and sodium borohydride. Energy, 2016,99:129-135. |
| [22] | LUO C, FU F Y, YANG X J , et al. Highly efficient and selective Co@ZIF-8 nanocatalyst for hydrogen release from sodium borohydride hydrolysis. ChemCatChem, 2019,11(6):1643-1649. |
| [23] | MAHMOOD J, JUNG S M, KIM S J , et al.Cobalt oxide encapsulated in C2N-h2D network polymer as a catalyst for hydrogen evolution. Chem. Mater., 2015,27(13):4860-4864. |
| [24] | LIN K Y A, CHANG H A, CHEN B J . Multi-functional MOF-derived magnetic carbon sponge. J. Mater. Chem. A, 2016,4(35):13611-13625. |
| [25] | GUO S Q, WU Q Q, SUN J , et al. Highly stable and controllable CoB/Ni-foam catalysts for hydrogen generation from alkaline NaBH4 solution. Int. J. Hydrogen Energy, 2017,42(33):21063-21072. |
| [26] | CUI L, SUN X P, XU Y H , et al. Cobalt carbonate hydroxide nanowire array on Ti mesh: an efficient and robust 3D catalyst for on-demand hydrogen generation from alkaline NaBH4 solution. Chem. Eur. J., 2016,22(42):14831-14835. |
| [27] | PATEL N, MIOTELLO A . Progress in Co-B related catalyst for hydrogen production by hydrolysis of boron-hydrides: a review and the perspectives to substitute noble metals. Int. J. Hydrogen Energy, 2015,40(3):1429-1464. |
| [28] | GUO M S, CHENG Y, YU Y N , et al. Ni-Co nanoparticles immobilized on a 3D Ni foam template as a highly efficient catalyst for borohydride electrooxidation in alkaline medium. Appl. Surf. Sci., 2017,416:439-445. |
| [29] | MARCHIONNI A, BEVILACQUA M, FILIPPI J , et al. High volume hydrogen production from the hydrolysis of sodium borohydride using a cobalt catalyst supported on a honeycomb matrix. J. Power Sources, 2015,299(20):391-397. |
| [30] | ZHUANG D W, DAI H B, ZHONG Y J , et al. A new reactivation method towards deactivation of honeycomb ceramic monolith supported cobalt-molybdenum-boron catalyst in hydrolysis of sodium borohydride. Int. J. Hydrogen Energy, 2015,40(30):9373-9381. |
| [31] | SAHINER N . Soft and flexible hydrogel templates of different sizes and various functionalities for metal nanoparticle preparation and their use in catalysis. Prog. Polym. Sci., 2013,38(9):1329-1356. |
| [32] | Ai L H, GAO X Y, JIANG J . In situ synthesis of cobalt stabilized on macroscopic biopolymer hydrogel as economical and recyclable catalyst for hydrogen generation from sodium borohydride hydrolysis. J. Power Sources, 2014,257(1):213-220. |
| [33] | PRASAD D, PATIL K N, CHAITRA C R , et al. Sulfonic acid functionalized PVA/PVDF composite hollow microcapsules: highly phenomenal & recyclable catalysts for sustainable hydrogen production. Appl. Surf. Sci., 2019,488:714-727. |
| [34] | LI Q M, CHEN Y B, LEE D J , et al. Preparation of Y-zeolite/CoCl2 doped PVDF composite nanofiber and its application in hydrogen production. Energy, 2012,38(1):144-150. |
| [35] | ZHAO L W, LI Q, SU Y , et al. A novel enteromorpha based hydrogel for copper and nickel nanoparticle preparation and their use in hydrogen production as catalysts. Int. J. Hydrogen Energy, 2017,42(10):6746-6756. |
| [36] | SCHLESINGER H I, BROWN H C, FINHOLT A E . The preparation of sodium borohydride by the high temperature reaction of sodium hydride with borate esters. J. Am. Chem. Soc., 1953,75:205-209. |
| [37] | KOJIMA Y, HAGA T . Recycling process of sodium metaborate to sodium borohydride. Int. J. Hydrogen Energy, 2003,28:989-993. |
| [38] | LANG C G, JIA Y, LIU J W , et al. NaBH4 regeneration from NaBO2 by high-energy ball milling and its plausible mechanism.Int. J. Hydrogen Energy, 2017,42(18):13127-13135. |
| [39] | OUYANG L Z, CHEN W, LIU J W , et al.Enhancing the regeneration process of consumed NaBH4 for hydrogen storage. Adv. Energy Mater., 2017,7(19):1700299. |
| [40] | SANYAL U, DEMIRCI U B, JAGIRDAR B R , et al. Hydrolysis of ammonia borane as a hydrogen source: fundamental issues and potential solutions towards implementation. ChemSusChem, 2011,4(12):1731-1739. |
| [41] | DEMIRCI U B . Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrogen Energy, 2017,42(15):9978-10013. |
| [42] | WANG L B, LI H L, ZHANG W B , et al. Supported rhodium catalysts for ammonia-borane hydrolysis: dependence of the catalytic activity on the highest occupied state of the single rhodium atoms. Angew. Chemie. Int. Ed., 2017,56(17):4712-4718. |
| [43] | HOU C C, LI Q, WANG C J , et al. Ternary Ni-Co-P to boost the hydrolytic dehydrogenation of ammonia-borane. Energy & Environ. Sci., 2017,10(8):1770-1776. |
| [44] | PIGHIN S A, URRETAVIZCAYA G, BOBET J L . Nanostructured Mg for hydrogen production by hydrolysis obtained by MgH2 milling and dehydriding. J. Alloys Compd., 2020,827:154000. |
| [45] | TAN Z H, OUYANG L Z, LIU J W , et al. Hydrogen generation by hydrolysis of Mg-Mg2Si composite and enhanced kinetics performance from introducing of MgCl2 and Si. Int. J. Hydrogen Energy, 2018,43(5):2903-2912. |
| [46] | JIANG J, OUYANG L Z, WANG H , et al. Controllable hydrolysis performance of MgLi alloys and their hydrides. ChemPhysChem, 2019,20(10):1316-1324. |
| [47] | GAN D Y, LIU Y N, ZHANG J G , et al. Kinetic performance of hydrogen generation enhanced by AlCl3 via hydrolysis of MgH2 prepared by hydriding combustion synthesis. Int. J. Hydrogen Energy, 2018,43(22):10232-10239. |
| [48] | ZHAO Z L, ZHU Y F, LI L Q . Efficient catalysis by MgCl2 in hydrogen generation via hydrolysis of Mg-based hydride prepared by hydriding combustion synthesis. Chem. Comm., 2012,48(44):5509-5511. |
| [49] | ZHENG J, YANG D C, LI W , et al. Promoting H2 generation from the reaction of Mg nanoparticles and water using cations. Chem. Comm., 2013,49(82):9437-9439. |
| [50] | HUANG X N, GAO T, PAN X L , et al. A review: feasibility of hydrogen generation from the reaction between aluminum and water for fuel cell applications. J. Power Sources, 2013,229:133-140. |
| [51] | DENG Z Y, FERREIRA J M F, TANAKA Y, et al. Physicochemical mechanism for the continuous reaction of γ-Al2O3-modified aluminum powder with water. J. Am. Chem. Soc., 2007,90(5):1521-1526. |
| [52] | LIU Y A, WANG X H, LIU H Z , et al. Improved hydrogen generation from the hydrolysis of aluminum ball milled with hydride. Energy, 2014,72:421-426. |
| [53] | FAN M Q, WANG Y, TIAN G L , et al. Hydrolysis of AlLi/NaBH4 system promoted by Co powder with different particle size and amount as synergistic hydrogen generation for portable fuel cell. Int. J. Hydrogen Energy, 2013,38(25):10857-10863. |
| [54] | FAN M Q, LIU S, SUN W Q , et al. Controllable hydrogen generation and hydrolysis mechanism of AlLi/NaBH4 system activated by CoCl2 solution. Renew. Energ., 2012,46:203-209. |
| [55] | FAN M Q, LIU S, SUN W Q , et al.Hydrogen generation from Al/NaBH4 hydrolysis promoted by Li-NiCl2 additives. Int. J. Hydrogen Energy, 2011,36(24):15673-15680. |
| [56] | FAN M Q, WANG Y, TANG R , et al. Hydrogen generation from Al/NaBH4 hydrolysis promoted by Co nanoparticles and NaAlO2 solution. Renew. Energ., 2013,60:637-642. |
| [57] | HUANG X N, LV C J, HUANG Y X , et al. Effects of amalgam on hydrogen generation by hydrolysis of aluminum with water. Int. J. Hydrogen Energy, 2011,36(23):15119-15124. |
| [58] | REN R M, ORTIZ A L, MARKMAITREE T , et al. Stability of lithium hydride in argon and air. J. Phys. Chem. B, 2006,110(21):10567-10575. |
| [59] | DUAN C W, HU L X, MA J L . Ionic liquids as an efficient medium for the mechanochemical synthesis of α-AlH3 nano-composites. J. Mater. Chem. A, 2018,6(15):6309-6318. |
| [60] | CHEN J, FU H, XIONG Y F , et al.MgCl2 promoted hydrolysis of MgH2 nanoparticles for highly efficient H2 generation.Nano Energy, 2014,10:337-343. |
| [61] | HUANG M H, OUYANG L Z, WANG H , et al. Hydrogen generation by hydrolysis of MgH2 and enhanced kinetics performance of ammonium chloride introducing. Int. J. Hydrogen Energy, 2015,40(18):6145-6150. |
| [62] | TEGEL M, SCHÖNE S, KIEBACK B, et al. An efficient hydrolysis of MgH2-based materials. Int. J. Hydrogen Energy, 2017,42(4):2167-2176. |
| [63] | LU C, MA Y L, LI F , et al.Visualization of fast “hydrogen pump” in core-shell nanostructured Mg@Pt through hydrogen-stabilized Mg3Pt. J. Mater. Chem. A, 2019,7(24):14629-14637. |
| [64] | SIFER N, GARDNER K . An analysis of hydrogen production from ammonia hydride hydrogen generators for use in military fuel cell environments. J. Power Sources, 2004,132:135-138. |
| [1] | 王世怡, 冯爱虎, 李晓燕, 于云. Fe3O4负载Ti3C2Tx对Pb(II)的吸附性能研究[J]. 无机材料学报, 2023, 38(5): 521-528. |
| [2] | 游钧淇, 李策, 杨栋梁, 孙林锋. 氧化物双介质层忆阻器的设计及应用[J]. 无机材料学报, 2023, 38(4): 387-398. |
| [3] | 王如意, 徐国良, 杨蕾, 邓崇海, 储德林, 张苗, 孙兆奇. p-n异质结BiVO4/g-C3N4光阳极的制备及其光电化学水解性能[J]. 无机材料学报, 2023, 38(1): 87-96. |
| [4] | 陈瀚翔, 周敏, 莫曌, 宜坚坚, 李华明, 许晖. CoN/g-C3N4 0D/2D复合结构及其光催化制氢性能研究[J]. 无机材料学报, 2022, 37(9): 1001-1008. |
| [5] | 张笑宇, 刘永盛, 李然, 李耀刚, 张青红, 侯成义, 李克睿, 王宏志. 基于Cu3(HHTP)2薄膜的离子液体电致变色电极[J]. 无机材料学报, 2022, 37(8): 883-890. |
| [6] | 洪佳辉, 马冉, 仵云超, 文涛, 艾玥洁. MOFs自牺牲模板法制备CoNx/g-C3N4纳米材料用作高效光催化还原U(VI)[J]. 无机材料学报, 2022, 37(7): 741-749. |
| [7] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [8] | 孙炼, 顾全超, 杨雅萍, 王洪磊, 余金山, 周新贵. 二维过渡金属硫属化合物氧还原反应催化剂的研究进展[J]. 无机材料学报, 2022, 37(7): 697-709. |
| [9] | 安琳, 吴淏, 韩鑫, 李耀刚, 王宏志, 张青红. 非贵金属Co5.47N/N-rGO助催化剂增强TiO2光催化制氢性能[J]. 无机材料学报, 2022, 37(5): 534-540. |
| [10] | 王虹力, 王男, 王丽莹, 宋二红, 赵占奎. 功能化石墨烯担载型AuPd纳米催化剂增强甲酸制氢反应[J]. 无机材料学报, 2022, 37(5): 547-553. |
| [11] | 曹志军, 李在均. 钌-生物质碳人工酶的制备及在比色检测杀虫剂毒死蜱残留中的应用[J]. 无机材料学报, 2022, 37(5): 554-560. |
| [12] | 马慧, 陶疆辉, 王艳妮, 韩玉, 王亚斌, 丁秀萍. 硅钛杂化介孔球负载金纳米粒子及其催化性能调控[J]. 无机材料学报, 2022, 37(4): 404-412. |
| [13] | 付永胜, 毕敏, 李春, 孙敬文, 汪信, 朱俊武. 非贵金属/碳氮复合材料电催化析氧反应的研究进展[J]. 无机材料学报, 2022, 37(2): 163-172. |
| [14] | 赵伟, 徐阳, 万颖杰, 蔡天逊, 穆金潇, 黄富强. 金属氰胺化合物的结构、合成及电化学储能应用[J]. 无机材料学报, 2022, 37(2): 140-151. |
| [15] | 李文博, 黄民松, 李月明, 李驰麟. 双盐镁电池CoS2正极材料的电化学性能研究[J]. 无机材料学报, 2022, 37(2): 173-181. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||