无机材料学报 ›› 2021, Vol. 36 ›› Issue (1): 25-35.DOI: 10.15541/jim20200247
所属专题: 【结构材料】核用陶瓷
段涛1,丁艺1,罗世淋2,张胜泰3,刘建1
收稿日期:2020-05-11
修回日期:2020-08-10
出版日期:2021-01-20
网络出版日期:2020-07-10
作者简介:段涛(1976-), 男, 教授. E-mail: duant@swust.edu.cn
基金资助:DUAN Tao1,DING Yi1,LUO Shilin2,ZHANG Shengtai3,LIU Jian1
Received:2020-05-11
Revised:2020-08-10
Published:2021-01-20
Online:2020-07-10
About author:DUAN Tao(1976-), male, professor. E-mail: duant@swust.edu.cn
Supported by:摘要:
核工业生产、核能开发、核武器研制等不可避免会产生放射性废物, 高放废物是现存放射性废物中最难处理的废物之一。随着我国“积极发展核电”战略的实施, 放射性废物的安全有效处理处置成为关系到我国核能可持续发展的关键问题。人造岩石固化体(SYNROC)弥补了玻璃固化体低化学耐久性和亚稳态性能的缺点。本文在综述人造岩石固化的概念、候选矿物固化体分类的基础上, 重点介绍了SYNROC固化体快速合成方法、固核机理和长期稳定性评价等方面的最新研究进展。“道阻且长, 行则将至”。最后, 指出了SYNROC固化存在的不足, 并针对今后应重点关注的研究方向与发展趋势提出了建议。
中图分类号:
段涛, 丁艺, 罗世淋, 张胜泰, 刘建. 回归自然: 人造岩石固化核素的思考与进展[J]. 无机材料学报, 2021, 36(1): 25-35.
DUAN Tao, DING Yi, LUO Shilin, ZHANG Shengtai, LIU Jian. Radionuclides from Nature to Nature: Recent Progress in Immobilization of High Level Nuclear Wastes in SYNROC[J]. Journal of Inorganic Materials, 2021, 36(1): 25-35.
| SYNROC | Component mass fraction/% | Waste package capacity/% | ||||||
|---|---|---|---|---|---|---|---|---|
| TiO2 | UO2 | ZrO2 | Al2O3 | SiO2 | BaO | CaO | ||
| SYNROC-B | 74.1 | - | 6.6 | 5.4 | - | 5.6 | 11.0 | - |
| SYNROC-C | 57.1 | - | 5.4 | 4.4 | - | 4.4 | 8.9 | 30.0 |
| SYNROC-D | 18.8 | - | 6.6 | - | 6.6 | - | 5.3 | 62.7~65.0 |
| SYNROC-E | 87.6 | - | 3.0 | - | - | - | 2.2 | 7.0 |
| SYNROC-F | 40.6 | 47.6 | - | 0.9 | 1.1 | 9.5 | - | 50.0 |
表1 几种人造岩石固化体的化学成分
Table 1 Chemical composition of several artificial rock solidification bodies
| SYNROC | Component mass fraction/% | Waste package capacity/% | ||||||
|---|---|---|---|---|---|---|---|---|
| TiO2 | UO2 | ZrO2 | Al2O3 | SiO2 | BaO | CaO | ||
| SYNROC-B | 74.1 | - | 6.6 | 5.4 | - | 5.6 | 11.0 | - |
| SYNROC-C | 57.1 | - | 5.4 | 4.4 | - | 4.4 | 8.9 | 30.0 |
| SYNROC-D | 18.8 | - | 6.6 | - | 6.6 | - | 5.3 | 62.7~65.0 |
| SYNROC-E | 87.6 | - | 3.0 | - | - | - | 2.2 | 7.0 |
| SYNROC-F | 40.6 | 47.6 | - | 0.9 | 1.1 | 9.5 | - | 50.0 |
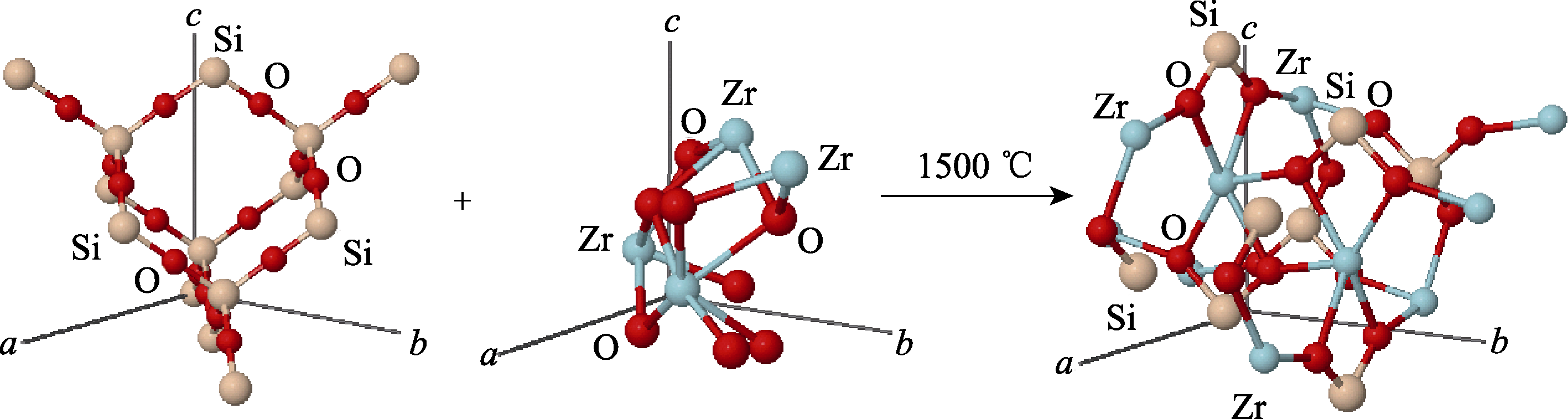
图3 氧化锆与氧化硅在1500 ℃、Po条件下反应生成锆英石球棍模型图
Fig. 3 Model diagram of zirconia and silicon oxide reacting under the condition of 1500 ℃ and Po to form zircon ball stick
| Irradiation method | Experimental program | Advantage | Disadvantage |
|---|---|---|---|
| Ion irradiation | Heavy ion beam bombardment | Irradiation damage at low level | Suppress vacancy defects |
| Electron irradiation | Accelerated voltage induction | Observe defect formation | Complex and expensive equipment |
| Neutron irradiation | Reactor irradiation | Large dosage, short time | Difficult to control temperature and dosage |
表2 辐照损伤的试验研究方法
Table 2 Experimental methods in detecting irradiation damage
| Irradiation method | Experimental program | Advantage | Disadvantage |
|---|---|---|---|
| Ion irradiation | Heavy ion beam bombardment | Irradiation damage at low level | Suppress vacancy defects |
| Electron irradiation | Accelerated voltage induction | Observe defect formation | Complex and expensive equipment |
| Neutron irradiation | Reactor irradiation | Large dosage, short time | Difficult to control temperature and dosage |
| SYNROC | Typical components | Density /(g·cm-3) | Strength | Long-term stability | Package capacity/wt% | Thermal conductivity /(W·m-1·℃-1) | Leaching rate /(g·cm-2·d-1) |
|---|---|---|---|---|---|---|---|
| Calcined product | CaF2 Al2O3 ZrO2 | 1.0-1.7 | Powder | Unstable | High | 0.13-0.20 | 0.1-10 |
| Borosilicate glass | SiO2 B2O3 Na2O Al2O3 | 2.5-2.8 | Hard and brittle | Yellow phase is formed, and there is a tendency to crystallize | 15-30 | 1.00-1.50 | 10-6-10-4 |
| Phosphate glass | P2O5 Al2O3 Na2O | 2.5-3.0 | Hard and brittle | Large tendency to crystallize | 15-30 | 1.00-1.50 | 10-5-10-3 |
| Synroc | TiO2 ZrO2 CaO Al2O3 BaO | 4.0-5.8 | Hard | Stable | 15-30 | 2.50-3.50 | 10-8-10-5 |
表3 高放废物不同固化方法
Table 3 Different curing methods for HLW
| SYNROC | Typical components | Density /(g·cm-3) | Strength | Long-term stability | Package capacity/wt% | Thermal conductivity /(W·m-1·℃-1) | Leaching rate /(g·cm-2·d-1) |
|---|---|---|---|---|---|---|---|
| Calcined product | CaF2 Al2O3 ZrO2 | 1.0-1.7 | Powder | Unstable | High | 0.13-0.20 | 0.1-10 |
| Borosilicate glass | SiO2 B2O3 Na2O Al2O3 | 2.5-2.8 | Hard and brittle | Yellow phase is formed, and there is a tendency to crystallize | 15-30 | 1.00-1.50 | 10-6-10-4 |
| Phosphate glass | P2O5 Al2O3 Na2O | 2.5-3.0 | Hard and brittle | Large tendency to crystallize | 15-30 | 1.00-1.50 | 10-5-10-3 |
| Synroc | TiO2 ZrO2 CaO Al2O3 BaO | 4.0-5.8 | Hard | Stable | 15-30 | 2.50-3.50 | 10-8-10-5 |
| [1] | HATCH L P . Ultimate disposal of radioactive wastes. Am. Sci., 1953,41(3):410-421. |
| [2] | RINGWOOD A, KESSON S, WARE N ,et al. Immobilisation of high level nuclear reactor wastes in SYNROC. Nature, 1979,278(5701):219-223. |
| [3] | SICKAFUS K E, MINERVINI L, GRIMES R W ,et al. Radiation tolerance of complex oxides. Science, 2000,289(5480):748-751. |
| [4] | RAK Z, EWING R C, BECKER U . Ferric garnet matrices for immobilization of actinides. J. Nucl. Mater., 2013,436(1/2/3):1-7. |
| [5] | CLARKE D R . Ceramic materials for the immobilization of nuclear waste. Annu. Rev. Mater. Sci., 1983,13(1):191-218. |
| [6] | ROBERT L E J . Radioactive Waste Management. Annu. Rev. Part Sci., 1990,40:79-112. |
| [7] | MONTEL J M . Minerals and design of new waste forms for conditioning nuclear waste. Cr. Geosci., 2011,343(2-3):230-236. |
| [8] | ZUR LOYE H C, BESMANN T, AMOROSO J ,et al. Hierarchical materials as tailored nuclear waste forms: a perspective. Chem Mater., 2018,30(14):4475-4488. |
| [9] | MORRISON G, SMITH M D, ZUR LOYE H C. Understanding the formation of salt-inclusion phases: an enhanced flux growth method for the targeted synthesis of salt-inclusion cesium halide uranyl silicates. J. Am. Chem. Soc., 2016,138(22):7121-7129 |
| [10] | BURNS P C, EWING R C, NAVROTSKY A . Nuclear fuel in a reactor accident. Science, 2012,335:1184-1188 |
| [11] | 卢喜瑞, 董发勤, 段涛 . 钆锆烧绿石固化錒系核素机理及稳定性. 北京: 科学出版社, 2016. |
| [12] | 顾忠茂 . 核废物处理技术. 北京: 原子能科学出版社, 2009. |
| [13] | ORLOVA A I, OJOVAN M I . Ceramic mineral waste-forms for nuclear waste immobilization. Materials, 2019,12:2638. |
| [14] | PROUST V, JEANNIN R, WHITE F D ,et al. Tailored perovskite waste forms for plutonium trapping. Inorg Chem., 2019,58(5):3026-3032. |
| [15] | FINKELDEI S, STENNETT M C, KOWALSKI P M ,et al. Insights into the fabrication and structure of plutonium pyrochlores. J Mater. Chem. A, 2020,8:2387-2403 |
| [16] | ADEL, MESBAH, NICOLAS,et al. Incorporation of thorium in the zircon structure type through the Th1-xErx(SiO4)1-x, 2016,55:11273-11282. |
| [17] | LI Y H, WANG Y Q, ZHOU M ,et al. Light ion irradiation effects on stuffed Lu2(Ti2. xLux)O7-x/2 (x=0, 0.4 and 0.67) structures Nucl. Instrum. Meth. B, 2011,269(18):2001-2005. |
| [18] | YANG D Y, XU C P, FU E G , , et al. Structure. Structure and radiation effect of Er-stuffed pyrochlore Er2(Ti2-xErx)O7-x/2(x=0-0.667). Nucl. Instrum. Meth. B, 2015, 356-357:69-74. |
| [19] | 李玉红, 许春萍 . Ne离子束辐照引起Gd2Ti2O7烧绿石体积肿胀效应研究. 原子核物理评论, 2011(3):68-72. |
| [20] | XU M, WU Y, WEI Y . Stable solidification of silica-based ammonium molybdophosphate absorbing cesium using allophane: mechenical property and leaching studies. J. Radioanal. Nucl. Ch., 2018,316:1313-1321. |
| [21] | WU Y, XU M, WEI Y ,et al. Stable solidification of silica-based ammonium molybdophosphate in ceramic matrices and its cesium- leaching properties. Chem Lett., 2018,47(2):179-182. |
| [22] | DING Y, LI Y J, JIANG Z D ,et al. Phase evolution and chemical stability of the Nd2O3-ZrO2-SiO2 system synthesized by a novel hydrothermal-assisted Sol-Gel process. J Nucl. Mater., 2018,510:10-18. |
| [23] | LI S, YANG X, LIU J ,et al. First-principles calculations and experiments for Ce 4+ effects on structure and chemical stabilities of Zr1-xCexSiO4. J. Nucl. Mater., 2019,514:276-283. |
| [24] | LU X, SHU X, CHEN S ,et al. Heavy-ion irradiation effects on U3O8 incorporated Gd2Zr2O7 waste forms. J Hazard Mater., 2018,357:424-430. |
| [25] | 张魁宝, 冠军, 尹丹 , 等. 钙钛锆石的自蔓延高温合成与热力学分析. 原子能科学技术, 2016,50(3):418-423. |
| [26] | HUANG Y, ZHANG H, ZHOU X ,et al. Synthesis and microstructure of fluorapatite-type Ca10-2xSmxNax(PO4)6F2 solid solutions for immobilization of trivalent minor actinide. J Nucl. Mater., 2017,485:105-112. |
| [27] | YANG J W, TANG B L, LUO S G . Immobilization of simulated actinides in pyrochlore-rich synroc. Journal of Nuclear & Radiochemistry, 2000,22(3):178-183. |
| [28] | 张华, 杨建文, 李宝军 , 等. 富烧绿石在模拟处置条件下的浸出行为研究. 中国原子能科学研究院年报, 2004,26(2):65-70. |
| [29] | 朱鑫璋, 罗上庚, 汤宝龙 , 等. 富钙钛锆石型人造岩石固化模拟锕系废物研究(Ⅰ). 核科学与工程, 1999(2):182-186. |
| [30] | 周慧, 张传智, 李宝军 , 等. 人造岩石固化模拟锝核素废物研究[C]// 第七届全国核化学与放射化学学术讨论会论文摘要集. 2005. |
| [31] | ZHAO X F, TENG Y C, YANG H ,et al. Comparison of microstructure and chemical durability of Ce0.9Gd0.1PO4 ceramics prepared by hot-press and pressureless sintering. Ceram Int., 2015,41(9):11062-11068. |
| [32] | TU H, DUAN T, DING Y ,et al. Preparation of zircon-matrix material for dealing with high-level radioactive waste with microwave. Mater Lett., 2014,131:171-173. |
| [33] | BARINOVA T V, PODBOLOTOV K B, BOROVINSKAYA I P ,et al. Self-propagating high-temperature synthesis of ceramic matrices for immobilization of actinide-containing wastes. Radiochemistry, 2014,56(5):554-559. |
| [34] | WANG L, SHUA X Y, YIA F C ,et al. Rapid fabrication and phase transition of Nd and Ce co-doped Gd2Zr2O7 ceramics by SPS. J Eur. Ceram. Soc., 2018,38(7):2863-2870. |
| [35] | LIU X F, NINA F, MARKUS A . Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chem. Soc. Rev., 2013,42:8237-8265. |
| [36] | APURV D, ROBERT V, OLIVIER G ,et al. Molten salt shielded synthesis of oxidation prone materials in air. Nat Mater., 2019,18:465-470. |
| [37] | LI Y B, SHAO H, LIN Z F ,et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat Mater., 2020, DOI: 10.1038/ s41563-020-0657-0. |
| [38] | HU Z M, XIAO X, JIN H Y ,et al. Rapid mass production of two-dimensional metal oxides and hydroxides. via the molten salts method. Nat Commun., 2017,8:15630-15638. |
| [39] | WU Y J, HONG R Y, WANG L S ,et al. Molten-salt synthesis and characterization of Bi-substituted yttrium garnet nanoparticles. J Alloys Compd., 2009,481(1-2):96-99. |
| [40] | MATTHEW R . GILBERT. Molten salt synthesis of titanate pyrochlore waste-forms. Ceram. Int., 2016,42(4):5263-5270. |
| [41] | HAND M L, STENNETT M C, HYATT N C . Rapid low temperature synthesis of a titanate pyrochlore by molten salt mediated reaction. J. Euro. Ceram. Soc., 2012,32(12):3211-3219. |
| [42] | ADEL M, STEPHANIE S, NICOLAS C ,et al. Coffinite, USiO4, is abundant in nature: so why is it so difficult to synthesize. Inorg Chem., 2015,54(14):6687-6696. |
| [43] | WEBER W J . Self-radiation damage and recovery in Pu-doped zircon. Radiat. Eff. Defec. S, 1991,115(4):341-349. |
| [44] | BURAKOV B E, ANDERSON E B, ROVSHA V S , et al. Synthesis of Zircon for Immobilization of Actinides. MRS Proceedings: Cambridge University Press, 1995,412:33. |
| [45] | SZENKNECT S, COSTIN D T, CLAVIER N ,et al. From uranothorites to coffinite: a solid solution route to the thermodynamic properties of USiO4. Inorg Chem., 2013,52(12):6957-6968. |
| [46] | PROUST V, JEANNIN R, WHITE F D ,et al. Tailored perovskite waste forms for plutonium trapping. Inorg Chem., 2019,58(5):3026-3032. |
| [47] | DING Y, DAN H, LI J J ,et al. Structure evolution and aqueous durability of the Nd2O3-CeO2-ZrO2-SiO2 system synthesized by hydrothermal-assisted Sol-Gel route: a potential route for preparing ceramics waste forms. J Nucl. Mater., 2019,519:217-228. |
| [48] | WANG C, PING W, BAI Q ,et al. A general method to synthesize and sinter bulk ceramics in seconds. Science, 2020,368:521-526. |
| [49] | LU F, YAO T, XU J ,et al. Facile low temperature solid state synthesis of iodoapatite by high-energy ball milling. RSC Advances, 2014,4(73):38718. |
| [50] | CAO C, CHONG S, THIRION L ,et al. Wet chemical synthesis of apatite-based waste forms-a novel room temperature method for the immobilization of radioactive iodine. J Mater. Chem. A, 2017,5(27):14331-14342. |
| [51] | HASSAN M U, RYU H J . Cold sintering and durability of iodate- substituted calcium hydroxyapatite (IO-HAp) for the immobilization of radioiodine. J. Nucl. Mater., 2019,514:84-89. |
| [52] | YANG J H, PARK H S, AHN D H ,et al. Glass composite waste forms for iodine confined in bismuth-embedded SBA-15. J Nucl. Mater., 2016,480:150-158. |
| [53] | MINERVINI L, GRIMES R W, SICKAFUS K E ,et al. Disorder in pyrochlore oxides. J. Am. Ceram. Soc., 2004,83(8):1873-1878. |
| [54] | LIAN J, HELEAN K B, KENNEDY B J ,et al. Effect of structure and thermodynamic stability on the response of lanthanide stannate pyrochlores to ion beam irradiation. J. Phys. Chem. B, 2006,110(5):2343-2350. |
| [55] | TU H, DUAN T, DING Y, LU X R ,et al. Phase and micro- structural evolutions of the CeO2-ZrO2-SiO2 system synthesized by the sol-gel process. Ceram. Int., 2015,6(41):8046-8050. |
| [56] | DING Y, LONG X G, PENG S M ,et al. Phase evolution and chemical durability of Nd-doped zircon ceramics designed to immobilize trivalent actinides. Ceram. Int., 2015,487:279-304. |
| [57] | CHAPMAN N A , MCKINLEY. The geological disposal of nuclear waste. London: Wiley&Sons, 1999. |
| [58] | STRACHAN D, TURCOTTE R, BARNES B . MCC-1: A standard leach test for nuclear waste forms. Nucl. Technol., 1982,56(2):306-312. |
| [59] | JANTZEN C M, BIBLER N E, BEAM D C , et al. Nuclear waste glass product consistency test (PCT): Version 7.0. Revision 3. Technical Report, Westinghouse Savannah River Co., Aiken, SC(United States), 1994. |
| [60] | SINGH D, MANDALIKA V, PARULEKAR S ,et al. Magnesium potassium phosphate ceramic for 99Tc immobilization. J. Nucl. Mater., 2006,348(3):272-282. |
| [61] | GRIFFITH C S, SEBESTA F, HANNA J V ,et al. Tungsten bronze- based nuclear waste form ceramics. Part 2: Conversion of granular microporous tungstate-polyacrylonitrile (PAN) composite adsorbents to leach resistant ceramics. J. Nucl. Mater., 2006,358(2/3):151-163. |
| [62] | FAN L, SHU X, LU X ,et al. Phase structure and aqueous stability of TRPO waste incorporation into Gd2Zr2O7 pyrochlore. Ceram. Int., 2015,41(9):11741-11747. |
| [63] | LI S Y, LIU J, YANG X ,et al. Effect of phase evolution and acidity on the chemical stability of Zr1-xNdxSiO4-x/2 ceramics. Ceram. Int., 2019,45(3):3052-3058. |
| [64] | NIKOLAEVA E V, BURAKOV B E . Investigation of Pu-doped ceramics using modified MCC-1 leach test. Mater. Res. Soc. Symp. Proc., 2002,713:429-432. |
| [65] | WEBER W J, WANG L, HESS N J ,et al. Radiation effects in nuclear waste materials. OSTI Tech. Rep., 1998,32(1-4):453-454. |
| [66] | LI Y H, WANG Y Q, VALDEZJ A ,et al. Swelling efects in Y2Ti2O7 pyrochlore irradiated with 400 keV Ne 2+ ions. Nucl. Instrum. Meth. B, 2012,274:182-187. |
| [67] | LI Y H, WANG Y Q, XU C P ,et al. Microstructural evolution of the pyrochlore compound Er2Ti2O7 induced by light ion irradiations. Nucl. Instrum. Meth. B, 2012,286:218-222. |
| [68] | SICKAFUS K E, GRIMES R W, VALDEZJ A ,et al. Radiation induced amorphization resistance and radiation tolerance in structurally related oxides. Nat. Mater., 2007,6(3):217-223. |
| [69] | UTSUNOMIYA S, YUDINTSEV S, EWING R . Radiation effects in ferrate garnet. J. Nucl. Mater., 2005,336(2/3):251-260. |
| [70] | DING Y, JIANG Z D, LI Y J ,et al. Effect of alpha-particles irradIation on the phase evolution and chemical stability of Nd-doped zircon ceramics. J. Alloys Compd., 2017,729:483-491. |
| [71] | YANG X Y, WANG S A, LU Y ,et al. Structures and energetics of point defects with charge states in zircon: a first-principles study. J. Alloys Compd., 2018,759:60-69. |
| [72] | FINCH R J, HANCHAR J M . Structure and chemistry of zircon and zircon-group minerals. Rev. Miner. Geochem., 2003,53:1-25. |
| [73] | GALUSKINA I O, GALUSKIN E V, ARMBRUSTER T , et al. Bitikleite-(SnAl) and bitikleite-, 2010,95(7):959-967. |
| [74] | 卢海萍, 王汝成, 陆现彩 . 锆石的结构与化学稳定性: 核废料处置矿物类比物研究. 地学前缘, 2003,10(2):403-409. |
| [1] | 汪波, 余健, 李存成, 聂晓蕾, 朱婉婷, 魏平, 赵文俞, 张清杰. Gd/Bi0.5Sb1.5Te3热电磁梯度复合材料的服役稳定性[J]. 无机材料学报, 2023, 38(6): 663-670. |
| [2] | 王世怡, 冯爱虎, 李晓燕, 于云. Fe3O4负载Ti3C2Tx对Pb(II)的吸附性能研究[J]. 无机材料学报, 2023, 38(5): 521-528. |
| [3] | 李悦, 张旭良, 景芳丽, 胡章贵, 吴以成. 铈掺杂硼酸钙镧晶体的生长与性能研究[J]. 无机材料学报, 2023, 38(5): 583-588. |
| [4] | 孙敬伟, 王洪磊, 孙楚函, 周新贵, 纪小宇. 碳源对先驱体转化法制备TaC陶瓷粉体微观结构及性能影响[J]. 无机材料学报, 2023, 38(2): 184-192. |
| [5] | 王红宁, 黄丽, 清江, 马腾洲, 黄维秋, 陈若愚. 有机-无机氧化硅空心球的合成及VOCs吸附应用[J]. 无机材料学报, 2022, 37(9): 991-1000. |
| [6] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [7] | 罗世淋, 张胜泰, 许保亮, 王凌坤, 段思逸菡, 丁艺, 赵倩, 段涛. YIG陶瓷对三价锕系模拟核素的固化行为研究[J]. 无机材料学报, 2022, 37(7): 757-763. |
| [8] | 杨勇, 郭啸天, 唐杰, 常浩天, 黄政仁, 胡秀兰. 非氧化物陶瓷光固化增材制造研究进展及展望[J]. 无机材料学报, 2022, 37(3): 267-277. |
| [9] | 周港怀, 刘耀, 石原, 刘绍军. 活性氧化铝催化剂载体的光固化浆料制备与成型[J]. 无机材料学报, 2022, 37(3): 297-302. |
| [10] | 王亚宁, 张玉琪, 宋索成, 陈若梦, 刘亚雄, 段玉岗. 氧化锆陶瓷扫描光固化成形与脱脂烧结工艺研究[J]. 无机材料学报, 2022, 37(3): 303-309. |
| [11] | 朱俊逸, 张成, 罗忠强, 曹继伟, 刘志远, 王沛, 刘长勇, 陈张伟. 脱脂工艺对光固化3D打印堇青石陶瓷性能的影响[J]. 无机材料学报, 2022, 37(3): 317-324. |
| [12] | 李乔磊, 顾玥, 于雪华, 张朝威, 邹明科, 梁静静, 李金国. 烧结温度对3D打印硅基陶瓷型芯表面形貌及粗糙度的影响[J]. 无机材料学报, 2022, 37(3): 325-332. |
| [13] | 刘国仟, 闫长海, 张可强, 金华, 何汝杰. 立体光刻增材制造中固含量对Al2O3陶瓷性能的影响[J]. 无机材料学报, 2022, 37(3): 353-360. |
| [14] | 刘鼎伟, 曾江涛, 郑嘹赢, 满振勇, 阮学政, 时雪, 李国荣. BiAlO3掺杂PZT陶瓷的高压电性能和低电场应变滞后[J]. 无机材料学报, 2022, 37(12): 1365-1370. |
| [15] | 王烈林, 谢华, 谢宇骐, 胡平涛, 尹雯, 任馨玥, 丁芸. Nd2Zr2O7烧绿石A、B位晶格固化钍的结构演化及化学稳定性研究[J]. 无机材料学报, 2022, 37(10): 1073-1078. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||