无机材料学报 ›› 2021, Vol. 36 ›› Issue (1): 9-24.DOI: 10.15541/jim20200240
所属专题: 封面文章; 【生物材料】肿瘤治疗
程晓昆1,2,张越1,吕海军1,刘歆颖2,侯森林3,陈爱兵1( )
)
收稿日期:2020-05-06
修回日期:2020-06-04
出版日期:2021-01-20
网络出版日期:2020-07-10
作者简介:程晓昆(1985-), 女, 博士研究生. E-mail: kunkuner007@163.com
基金资助:
CHENG Xiaokun1,2,ZHANG Yue1,Lü Haijun1,LIU Xinying2,HOU Senlin3,CHEN Aibing1( )
)
Received:2020-05-06
Revised:2020-06-04
Published:2021-01-20
Online:2020-07-10
About author:CHENG Xiaokun (1985-), female, PhD candidate. E-mail: kunkuner007@163.com
Supported by:摘要:
抗肿瘤药物靶向传递系统是提高传统化疗药物疗效, 并降低其毒副作用的重要手段。以多孔碳纳米材料为药物载体, 根据肿瘤组织微环境特点, 构建抗肿瘤药物靶向传递系统是实现靶向治疗方案的有效方式。本文围绕基于多孔碳纳米材料的抗肿瘤药物靶向传递系统的构建及应用进行综述, 描述了多孔碳纳米材料适宜载药的设计、合成及功能化修饰; 通过理论与实例相结合的方式, 介绍了提高多孔碳纳米材料载药量和实现联合给药的有效策略; 从内源和外源性敏感刺激的角度, 重点分析了多孔碳纳米材料基于肿瘤微环境构建的靶向传递系统的机制和应用; 阐述了多孔碳纳米材料作为抗肿瘤药物载体面临的生物相容性和生物降解性的问题, 并分析了可能的解决途径; 展望了多孔碳纳米材料在构建肿瘤药物靶向传递系统应用中的前景及发展方向, 为研发靶向、可控的抗肿瘤药物传递系统提供了理论依据和例证支持。
中图分类号:
程晓昆, 张越, 吕海军, 刘歆颖, 侯森林, 陈爱兵. 多孔碳纳米材料构建抗肿瘤药物靶向传递系统的研究进展[J]. 无机材料学报, 2021, 36(1): 9-24.
CHENG Xiaokun, ZHANG Yue, Lü Haijun, LIU Xinying, HOU Senlin, CHEN Aibing. Porous Carbon Nanomaterials Based Tumor Targeting Drug Delivery System: a Review[J]. Journal of Inorganic Materials, 2021, 36(1): 9-24.
| Preparation | Basic steps | Ref. |
|---|---|---|
| Hard template method | Impregnate the preformed hard template with carbon source, then remove the template after pyrolysis at a high temperature | [29-30] |
| Soft template method | Through surfactant assembly, the template is removed after pyrolysis at high temperature | [31-33] |
| Direct pyrolysis method | Direct pyrolysis of carbon precursors, such as MOF, biomass, ionic liquid or polymer | [34-37] |
| Chemical vapor deposition method | Introducing two or more gaseous carbon precursors into the tubular quartz reactor, carbon materials obtained through pyrolysis | [38-40] |
表1 多孔碳纳米材料的制备方法
Table 1 Preparation of porous carbon nanomaterials
| Preparation | Basic steps | Ref. |
|---|---|---|
| Hard template method | Impregnate the preformed hard template with carbon source, then remove the template after pyrolysis at a high temperature | [29-30] |
| Soft template method | Through surfactant assembly, the template is removed after pyrolysis at high temperature | [31-33] |
| Direct pyrolysis method | Direct pyrolysis of carbon precursors, such as MOF, biomass, ionic liquid or polymer | [34-37] |
| Chemical vapor deposition method | Introducing two or more gaseous carbon precursors into the tubular quartz reactor, carbon materials obtained through pyrolysis | [38-40] |
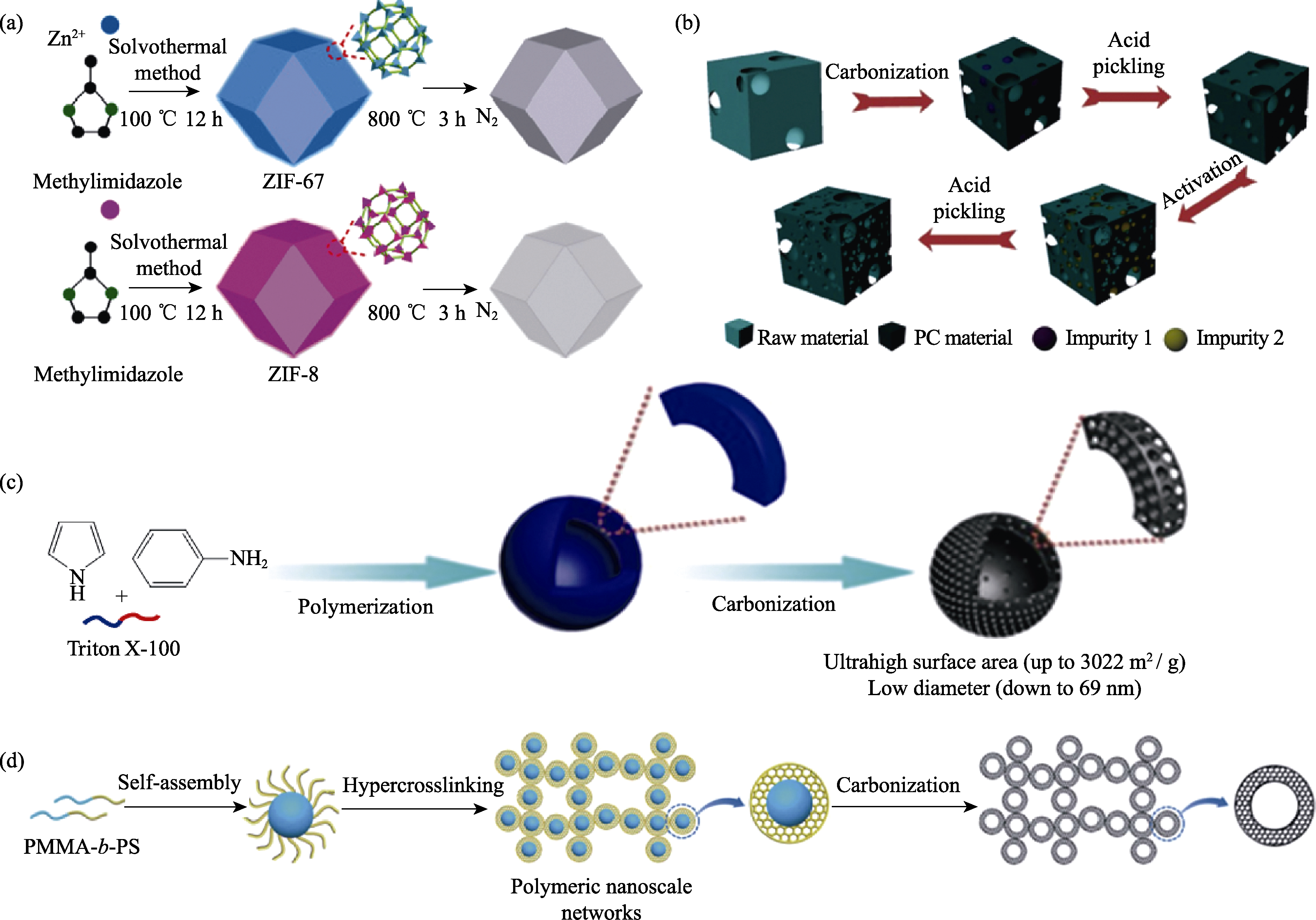
图2 直接热解法制备(a)金属有机骨架热解成碳[52], (b)植物组织制备分级多孔碳[57], (c)苯胺-吡咯共聚物热解成碳[60], (d)嵌段共聚物聚甲基丙烯酸甲酯-苯乙烯热解成碳[61]
Fig. 2 Schematic illustration of direct pyrolysis methods for synthesis of porous carbon nanomaterials (PCN) (a) Preparation of MOF and porous carbon (PC)[58]; (b) Preparation of PC from plant tissue[59]; (c) Preparation of PC from PAN-co-PPy[60]; (d) Preparation of PC from PMMA-co-PS[61]
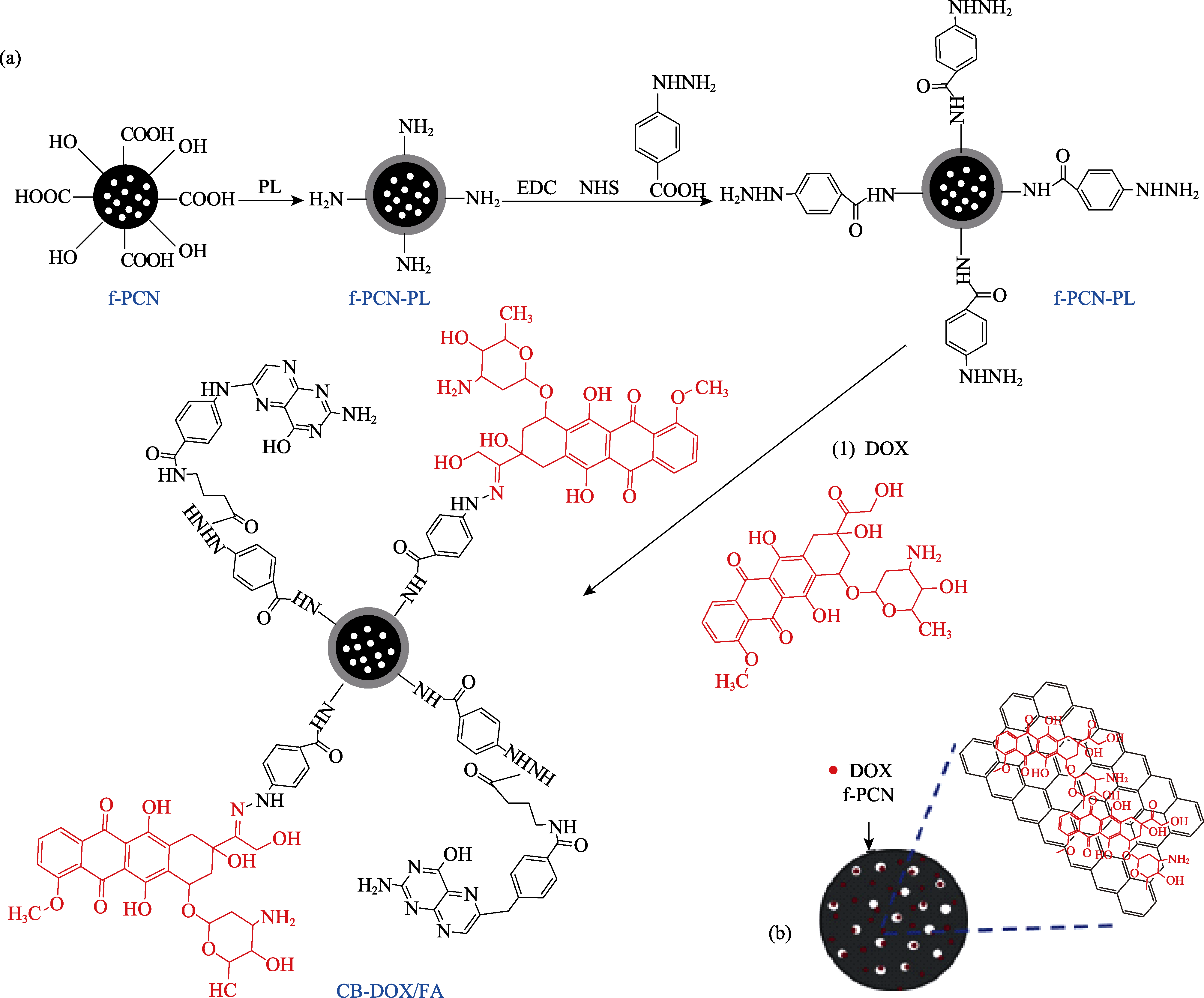
图3 (a)羧基化的多孔碳球与阿霉素和叶酸偶联的制备, (b)阿霉素在孔道和表面的吸附形式[71]
Fig. 3 (a) Schematic illustration of the preparation of DOX and FA conjugate with f-PCN to form CB-DOX/FA, and (b) the loading of DOX inside the pores and at the surface of functionalized PCN (f-PCN)[71]
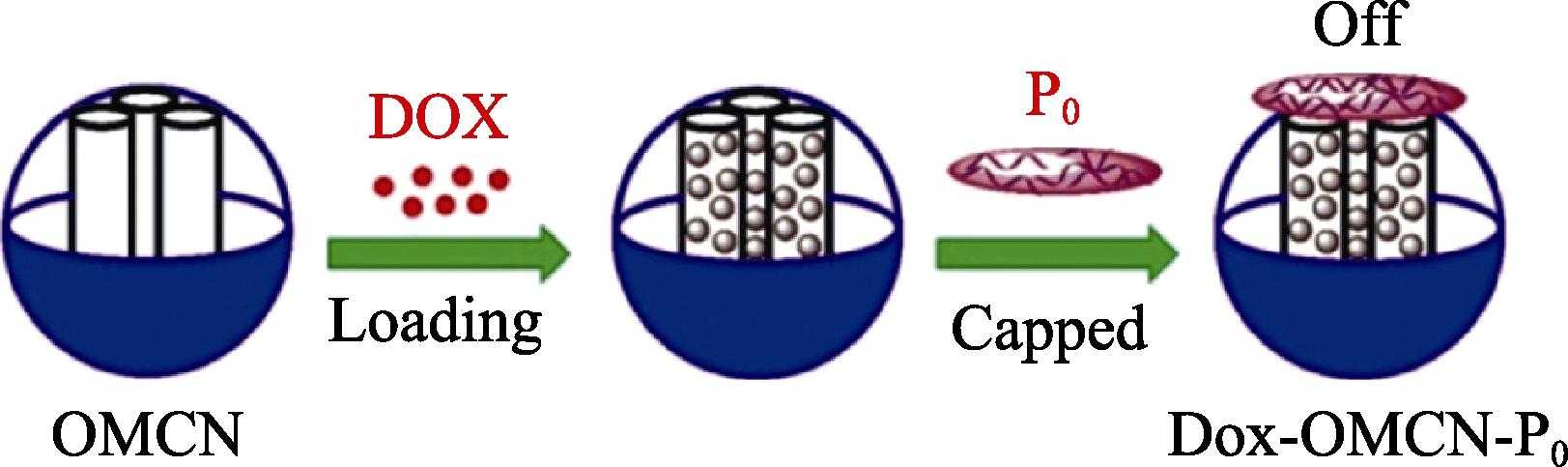
图4 多孔碳球装载阿霉素并基于π-π作用进行通道封装[75]
Fig. 4 Schematic preparation of DOX loaded on oxide mesoporous carbon nanospheres (OMCN) and its channel capped by the interaction of π-π[75]
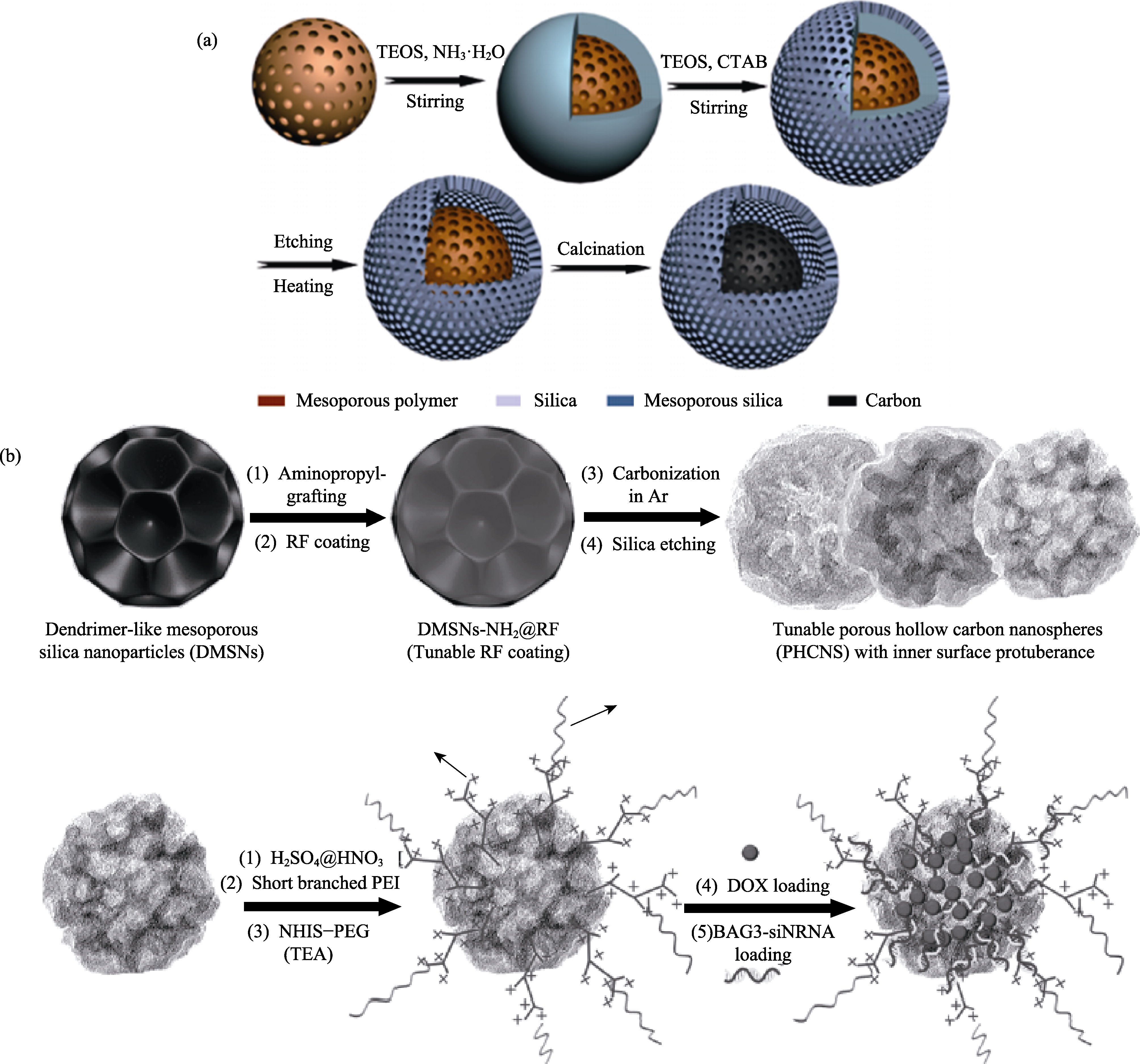
图5 (a) 双孔核壳介孔碳@二氧化硅[81]和(b)分级孔道多孔中空碳球装载阿霉素及小干扰RNA[82]联合给药载体制备方法
Fig. 5 Schematic illustration of preparing combined administration of (a) hierarchical hybrid dual-pore core-shell mesoporous carbon@silica[81] and (b) PHCNs-PEI-PEG for drug and gene co-loading[82] DOX: doxorubicin; PEI; polyethyleneimine; PEG: poly (ethylene glycol); RF: resorcinlo-formaldehyde
| Structure | Response modes | Drugs | >啊. |
|---|---|---|---|
| Porous carbon sphere | pH | Mitoxantrone HCl | [87] |
| Porous carbon sphere | pH | Doxorubicin | [26] |
| Porous carbon sphere | Specific enzyme | Doxorubicin | [91] |
| Porous carbon derived from ZIF | Specific enzyme | Methylene blue | [35] |
| Porous carbon sphere | Redox potential | Doxorubicin | [93] |
| Hollow porous carbon sphere | Near infrared | Doxorubicin | [82] |
| Porous carbon sphere | Near infrared | Doxorubicin | [98] |
| Porous carbon sphere coated with Fe3O4 | Magnetic | Doxorubicin | [102] |
| Mesoporous carbon sphere | pH-redox potential | Doxorubicin | [104] [105] |
| Porous carbon sphere | pH-magnetic | Doxorubicin | [106] |
| Ordered mesoporous carbon sphere | pH-magnetic | Doxorubicin | [107] |
| Ordered mesoporous carbon spheres coated with Fe3O4 | Magnetic-near infrared | Doxorubicin | [108] |
| Porous carbon spheres coated with Au and Fe3O4 | Magnetic-near infrared | Doxorubicin | [109] |
| Hollow porous carbon sphere | pH-redox potential-near infrared | Doxorubicin | [110] |
表2 多孔碳纳米材料构建抗肿瘤药物靶向传递系统
Table 2 Construction of tumor targeting drug delivery system based on porous carbon nanomaterials
| Structure | Response modes | Drugs | >啊. |
|---|---|---|---|
| Porous carbon sphere | pH | Mitoxantrone HCl | [87] |
| Porous carbon sphere | pH | Doxorubicin | [26] |
| Porous carbon sphere | Specific enzyme | Doxorubicin | [91] |
| Porous carbon derived from ZIF | Specific enzyme | Methylene blue | [35] |
| Porous carbon sphere | Redox potential | Doxorubicin | [93] |
| Hollow porous carbon sphere | Near infrared | Doxorubicin | [82] |
| Porous carbon sphere | Near infrared | Doxorubicin | [98] |
| Porous carbon sphere coated with Fe3O4 | Magnetic | Doxorubicin | [102] |
| Mesoporous carbon sphere | pH-redox potential | Doxorubicin | [104] [105] |
| Porous carbon sphere | pH-magnetic | Doxorubicin | [106] |
| Ordered mesoporous carbon sphere | pH-magnetic | Doxorubicin | [107] |
| Ordered mesoporous carbon spheres coated with Fe3O4 | Magnetic-near infrared | Doxorubicin | [108] |
| Porous carbon spheres coated with Au and Fe3O4 | Magnetic-near infrared | Doxorubicin | [109] |
| Hollow porous carbon sphere | pH-redox potential-near infrared | Doxorubicin | [110] |
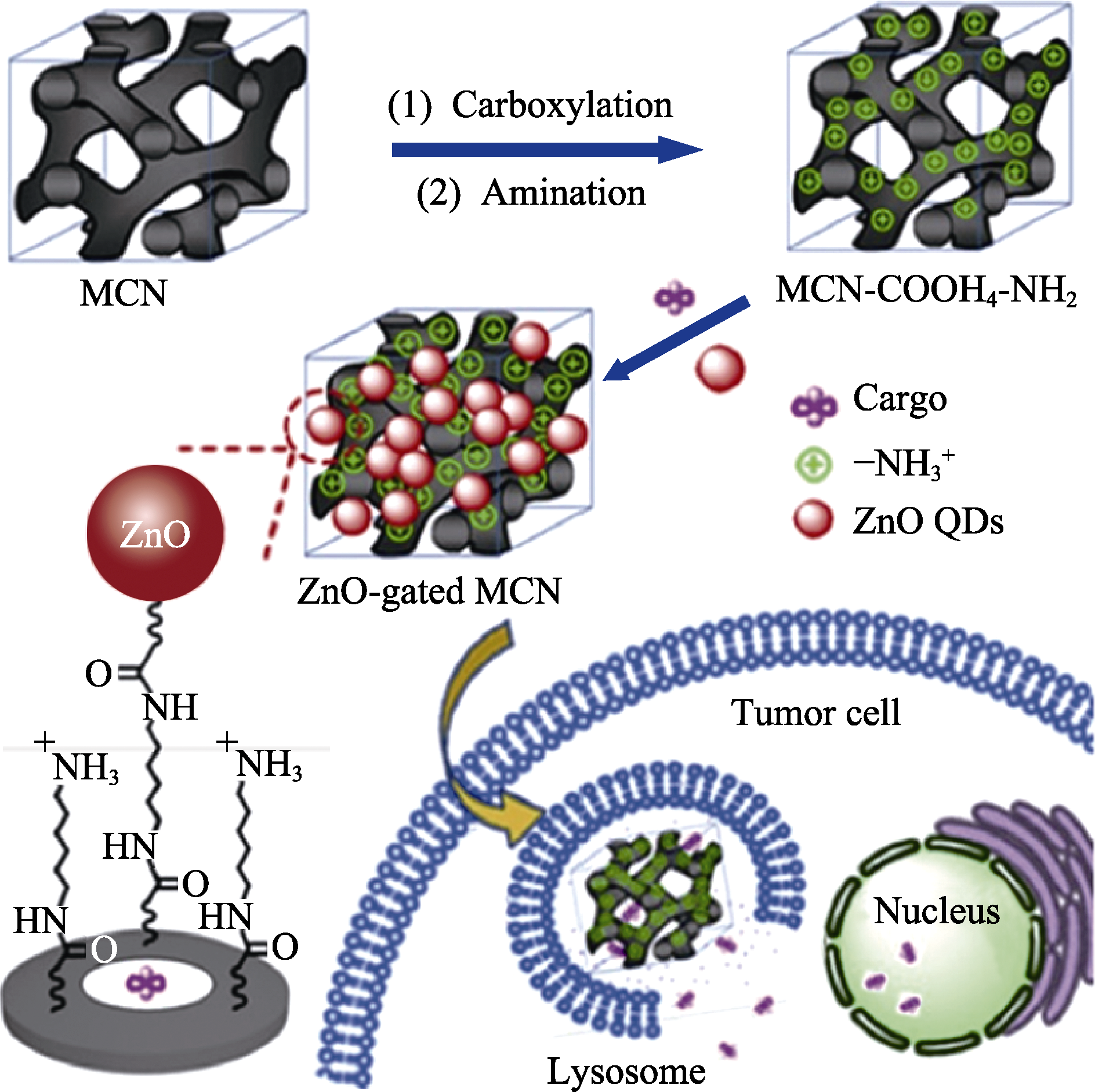
图6 ZnO门控多孔碳球的pH响应性控制释放示意图[87]
Fig. 6 Schematic illustration of pH-responsive controlled release of ZnO-gated MCNs[87] MCN: mesoporous carbon nanoparticles
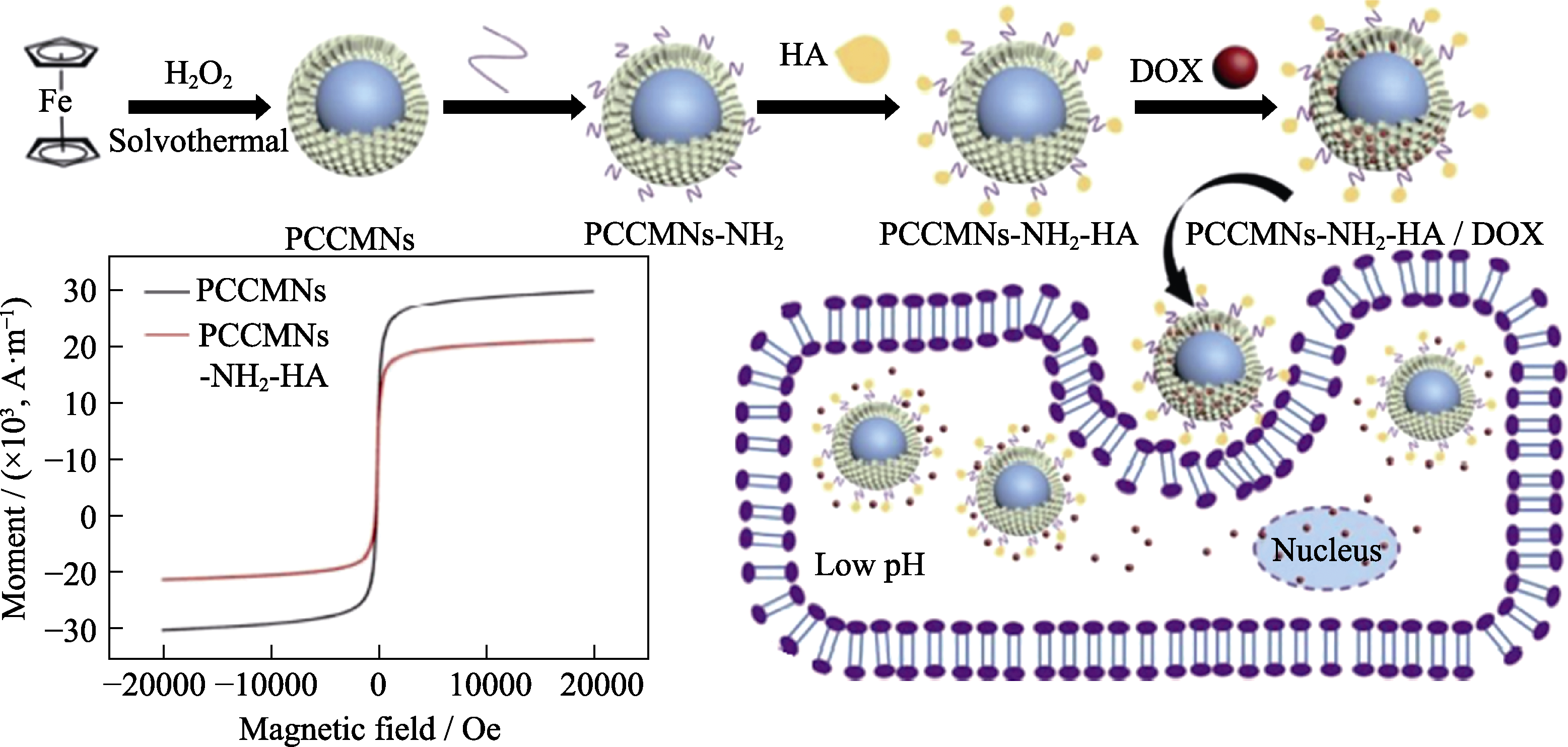
图9 多孔碳包覆Fe3O4纳米粒子并负载阿霉素的制备[102]
Fig. 9 Schematic illustration of MCNs coated Fe3O4 nanoparticles and DOX loaded[102] PCCMNs: porous carbon coated magnetic nanoparticles; DOX: doxorubicin; 1 Oe≈79.62 A/m
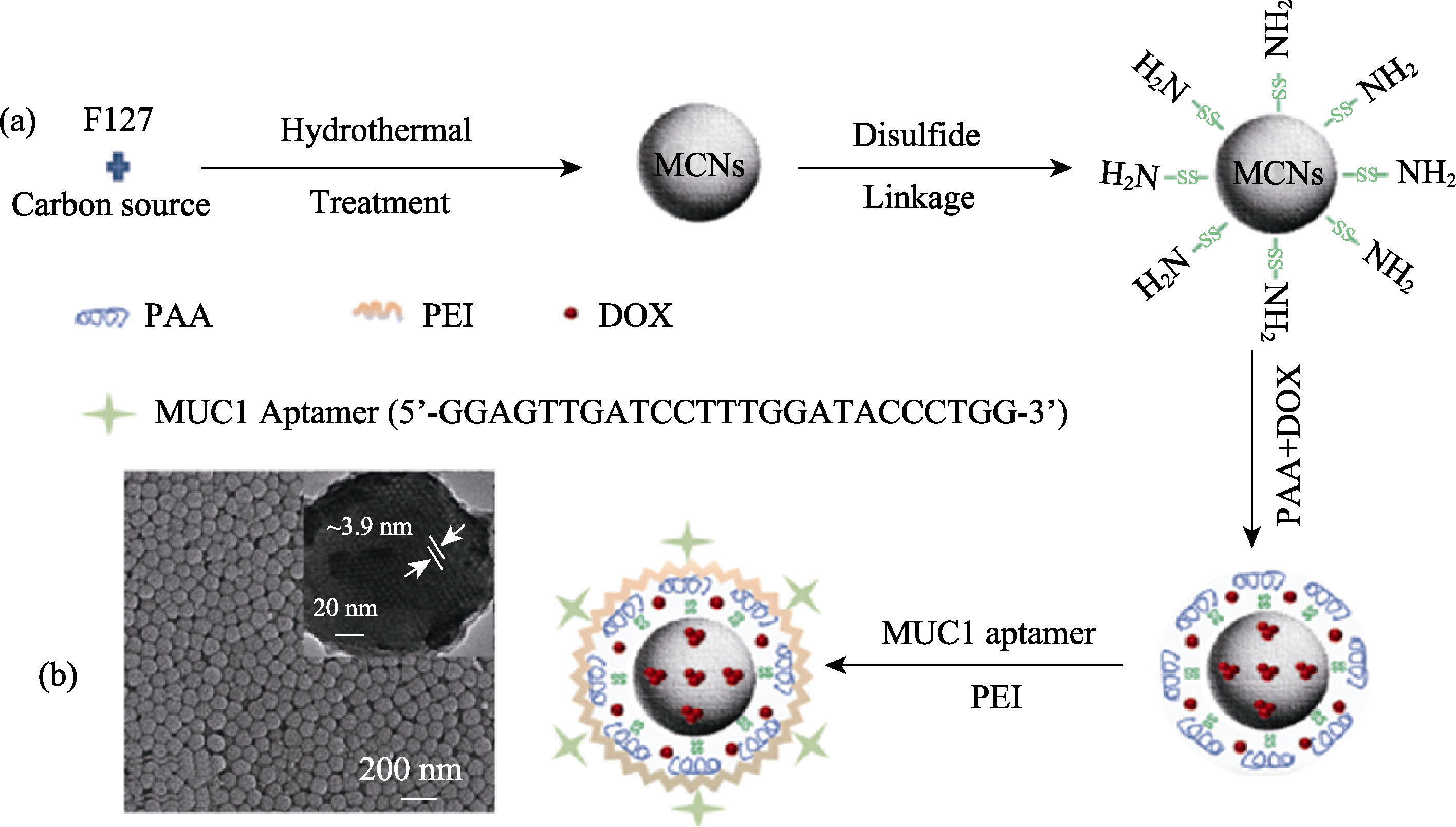
图10 制备pH和谷胱甘肽双敏感的多孔碳球示意图[105]
Fig. 10 Schematic preparation of MCN responsive controlled release by pH and glutathione[105] PAA: polyacrylic acid; PEI: polyc (ethyllene glycol); DOX: doxorubicin
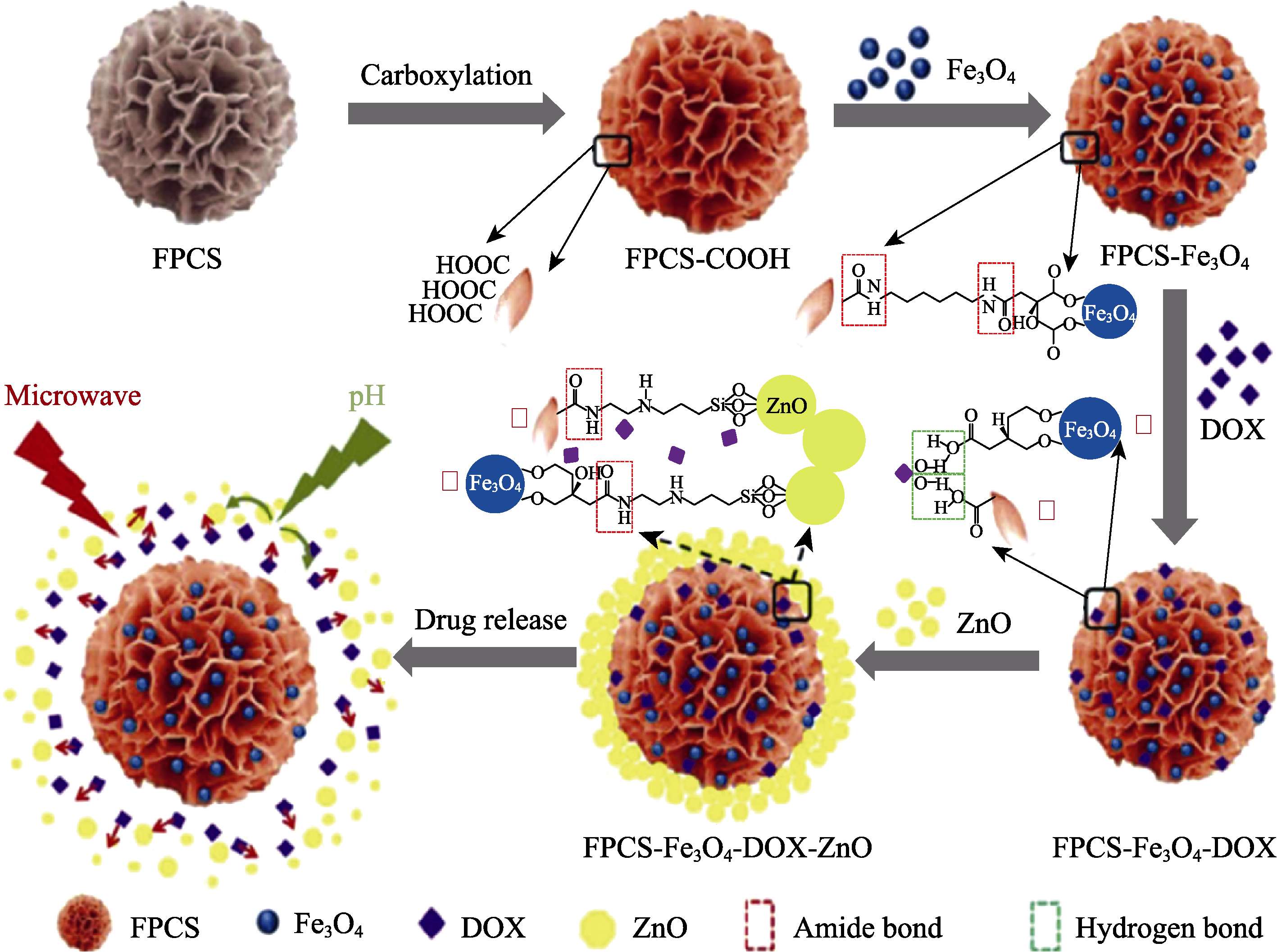
图11 利用花状多孔碳球构建pH和磁复合敏感材料[106]
Fig. 11 Schematic illustration of construction of pH and magnetic composite sensitive materials by flower-like porous carbon composite (FPCS)[106] DOX: doxorubicin
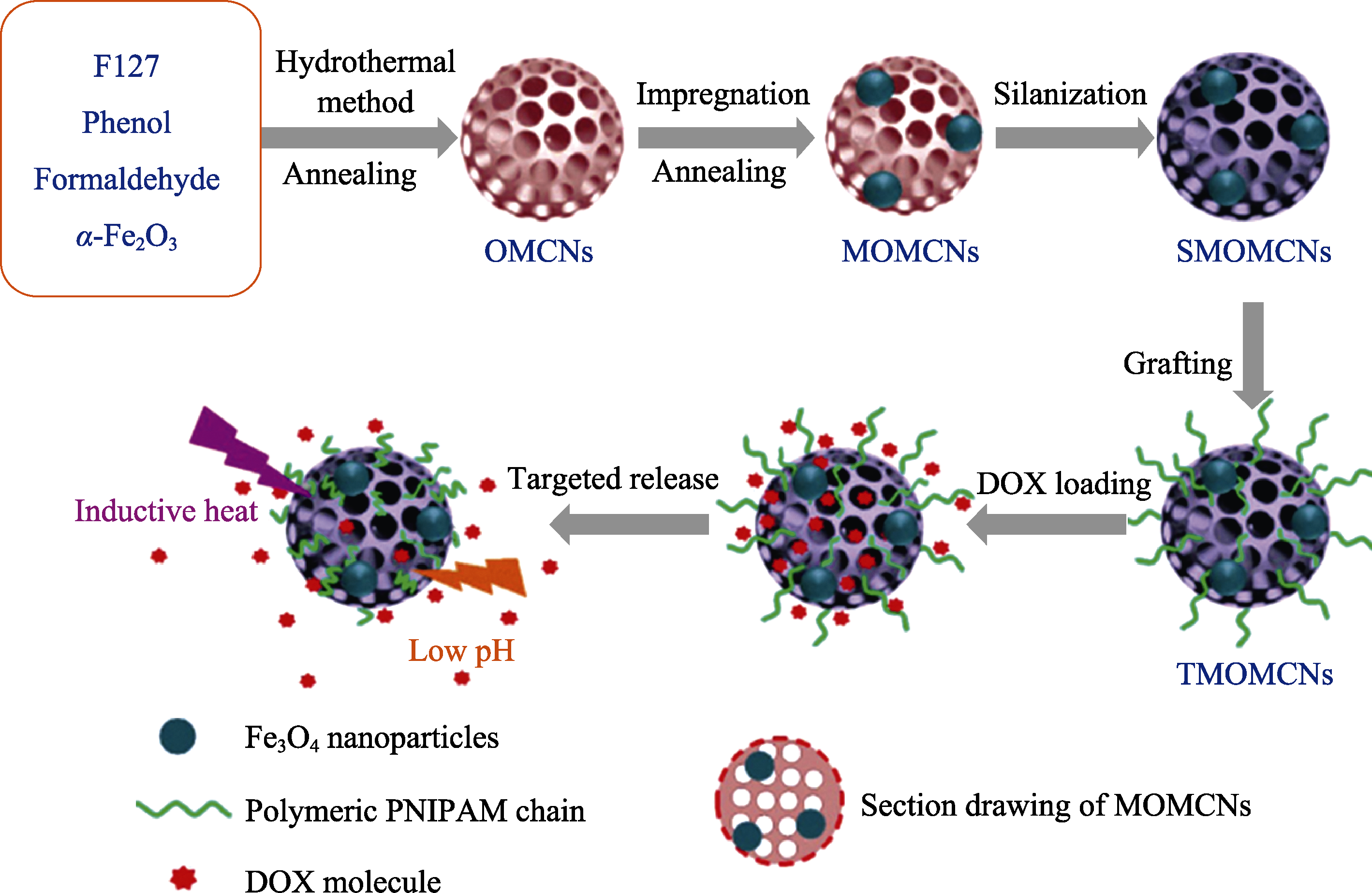
图12 磁热、光热能双敏感释放药物传递载体构建示意图[108]
Fig. 12 Schematic illustration of construction drug delivery by pH and magnetic composite sensitive[108] OMCNs: ordered mesoporous carbon nanospheres; MOMCNs: magnetically OMCNs; TMOMCNs: thermo-sensitiuely MOMCNs; SMOMCNs: silane modified MOMCNs; DOX: doxorubicin
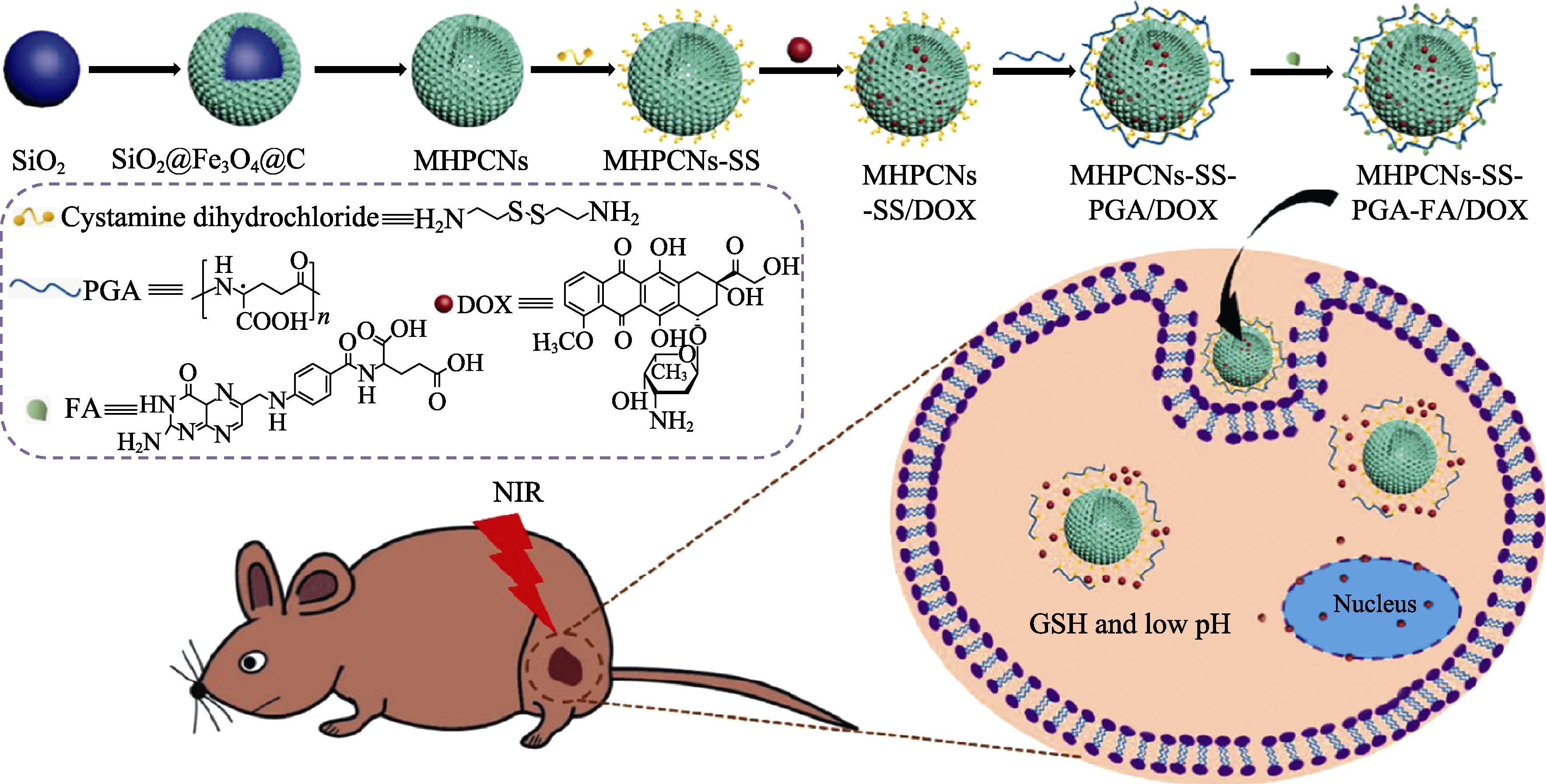
图13 肿瘤化疗pH、谷胱甘肽和光热化疗协同刺激反应药物传递系统的合成示意图[110]
Fig. 13 Schematic preparation of stimuli-responsive MHPCNs based drug delivery system for synergistic pH, glutathione and photothermal of tumor chemotherapy[110] MHPCNs: magnetic hollow porous carbon nanoparticles
| [1] | BRAY F, FERLAY J, SOERJOMATARAM I , et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 2018,68(6):394-424. |
| [2] | CHEN J G, CHEN H Z, ZHU J , et al. Cancer survival in patients from a hospital-based cancer registry, China. Journal of Cancer, 2018,9(5):851-860. |
| [3] | FAN W, LU N, HUANG P , et al. Glucose-responsive sequential generation of hydrogenperoxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angewandte Chemie, International Edition in English, 2017,56(5):1229-1233. |
| [4] | LINDNER O C, PHILLIPS B, MCCABE M G , et al. A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology, 2014,28(5):726-740. |
| [5] | MILLER K D, NOGUEIRA L, MARIOTTO A B , et al. Cancer treatment and survivorship statistics, 2019. CA: A Cancer Journal for Clinicians, 2019,69(5):363-385. |
| [6] | ZHAO X, TIAN K, ZHOU T , et al. PEGylated multi-walled carbon nanotubes as versatile vector for tumor-specific intracellular triggered release with enhanced anti-cancer efficiency: Optimization of length and PEGylation degree. Colloids and Surfaces B: Biointerfaces, 2018,168(1):43-49. |
| [7] | ZHOU S, SHA H, LIU B , et al. Integration of simultaneous and cascade release of two drugs into smart single nanovehicles based on DNA-gated mesoporous silica nanoparticles. Chemical Science (Royal Society of Chemistry: 2010), 2014,5(11):4424-4433. |
| [8] | GUO T, ZHU J, YANG Y , et al. Progress in the construction and application of nano drug delivery systems based on Bletilla striata polysaccharides. Chinese Journal of Pharmaceuticals, 2019,50(9):958-967. |
| [9] | SHI J, WANG B, WANG L , et al. Fullerene (C60)-based tumor-targeting nanoparticles with "off-on" state for enhanced treatment of cancer. Journal of Controlled Release, 2016,235(10):245-258. |
| [10] | MEHER J G, KESHARWANI P, CHAURASIA M , et al. Carbon nanotubes (CNTs): A novel drug delivery tool in brain tumor treatment. London: Academic Press, 2018: 375-396. |
| [11] | XIANWU HUA, YAN-WEN BAO, WU F-G . Fluorescent carbon quantum dots with intrinsic nucleolus-targeting capability for nucleolus iImaging and enhanced cytosolic and nuclear drug delivery. ACS Appl. Mater. Interfaces, 2018,10(13):10664-10677. |
| [12] | CHEN Y, TAN C, ZHANG H , et al. Two-dimensional graphene analogues for biomedical applications. Chemical Society Reviews, 2015,44(9):2681-2701. |
| [13] | WEI Y, ZHOU F, ZHANG D , et al. A graphene oxide based smart drug delivery system for tumor mitochondria-targeting photodynamic therapy. Nanoscale, 2016,8(6):3530-3538. |
| [14] | HE B, SHI Y, LIANG Y , et al. Single-walled carbon-nanohorns improve biocompatibility over nanotubes by triggering less protein-initiated pyroptosis and apoptosis in macrophages. Nature Communications, 2018,9(1):2393-2399. |
| [15] | SAHA D, HELDT C L, GENCOGLU M F , et al. A study on the cytotoxicity of carbon-based materials. Materials Science & Engineering. C: Materials for Biological Applications, 2016,68(1):101-108. |
| [16] | SONG M, YUAN S, YIN J , et al. Size-dependent toxicity of nano-C60 aggregates: more sensitive indication by apoptosis-related Bax translocation in cultured human cells. Environmental Science & Technology, 2012,46(6):3457-3464. |
| [17] | ALLEGRI M, PERIVOLIOTIS D K, BIANCHI M G , et al. Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicology Reports, 2016,3:230-243. |
| [18] | KIM T W, CHUNG P W , SLOWING, II, et al. Structurally ordered mesoporous carbon nanoparticles as transmembrane delivery vehicle in human cancer cells. Nano Letters, 2008,8(11):3724-3727. |
| [19] | GOBBO O L, SJAASTAD K, RADOMSKI M W , et al. Magnetic nanoparticles in cancer theranostics. Theranostics, 2015,5(11):1249-1263. |
| [20] | ZHAO Q, LIN Y, HAN N , et al. Mesoporous carbon nanomaterials in drug delivery and biomedical application. Drug Delivery, 2017,24(2):94-107. |
| [21] | SHRESTHA R G, MAJI S, SHRESTHA L K , et al. Nanoarchitectonics of nanoporous carbon materials in supercapacitors applications. Nanomaterials, 2020,10(4): 639-1-27. |
| [22] | CHENG Y J, LUO G F, ZHU J Y , et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Applied Materials & Interfaces, 2015,7(17):9078-9087. |
| [23] | CHEN Y, SHI J . Mesoporous carbon biomaterials. Science China Materials, 2015,58(3):241-257. |
| [24] | MENG Y, WANG S, LI C , et al. Photothermal combined gene therapy achieved by polyethyleneimine-grafted oxidized mesoporous carbon nanospheres. Biomaterials, 2016,100:34-142. |
| [25] | PEI F, AN T, ZANG J , et al. From hollow carbon spheres to N-doped hollow porous carbon bowls: rational design of hollow carbon host for Li-S batteries. Advanced Energy Materials, 2016,6(8):1502539. |
| [26] | ZHU J, LIAO L, BIAN X , et al. pH-controlled delivery of doxorubicin to cancer cells, based on small mesoporous carbon nanospheres. Small, 2012,8(17):2715-2720. |
| [27] | WU F, LIU Y, LU X , et al. Controllable preparation of polydopamine modified gold nanoflowers and its application in photothermal therapy. Chemical Journal of Chinese Universities, 2020,41(3):465-472. |
| [28] | TIAN W, ZHANG H, DUAN X , et al. Porous carbons: structure-criented design and versatile applications. Advanced Functional Materials, 2020,30:1909265. |
| [29] | LI S, CHENG C, ZHAO X , et al. Active salt/silica-templated 2D mesoporous FeCo-Nx-carbon as bifunctional oxygen electrodes for zinc-air batteries. Angewandte Chemie, International Edition in English, 2018,57(7):1856-1862. |
| [30] | BENZIGAR M R, JOSEPH S, ILBEYGI H , et al. Highly crystalline mesoporous C60 with ordered pores: a class of nanomaterials for energy applications. Angewandte Chemie, International Edition in English, 2018,57(2):569-573. |
| [31] | ZHANG P, WANG L, YANG S , et al. Solid-state synthesis of ordered mesoporous carbon catalysts via a mechanochemical assembly through coordination cross-linking. Nature Communications, 2017,8:15020. |
| [32] | ZHANG Z, JIA B, LIU L , et al. Hollow multihole carbon bowls: a stress-release structure design for high-stability and high-volumetric-capacity potassium-ion batteries. ACS Nano, 2019,13(10):11363-11371. |
| [33] | PENG L, HUNG C T, WANG S , et al. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. Journal of the American Chemical Society, 2019,141(17):7073-7080. |
| [34] | LI Q, GUO J, ZHU H , et al. Space-confined synthesis of ZIF-67 nanoparticles in hollow carbon nanospheres for CO2 adsorption. Small, 2019,15(8):e1804874. |
| [35] | CAO X, XIA J, MENG X , et al. Stimuli-responsive DNA-gated nanoscale porous carbon derived from ZIF-8. Advanced Functional Materials, 2019,29(34):1902237. |
| [36] | ZHANG F, LIU Y, LEI J , et al. Metal-organic-framework-derived carbon nanostructures for site-specific dual-modality photothermal/ photodynamic thrombus therapy. Advanced Science, 2019,6(17):1901378. |
| [37] | ZHU D, CHENG K, WANG Y , et al. Nitrogen-doped porous carbons with nanofiber-like structure derived from poly (aniline- co-p-phenylenediamine) for supercapacitors. Electrochimica Acta, 2017,224:17-24. |
| [38] | XIA Y, MOKAYA R . Generalized and facile synthesis approach to N-doped highly graphitic mesoporous carbon materials. Chemistry of Materials, 2005,17(6):1553-1560. |
| [39] | PANICKAR R, SOBHAN C B, CHAKRAVORTI S . Chemical vapor deposition synthesis of carbon spheres: Effects of temperature and hydrogen. Vacuum, 2020,172:109108. |
| [40] | XIA Y, MOKAYA R. synthesis of Ordered mesoporous carbon and nitrogen-doped carbor materials with graphitic pore walls viaa simple chemical vapor deposition method. Advanced Materials, 2004,16(17):1553-1558. |
| [41] | LI W, LIU J, ZHAO D . Mesoporous materials for energy conversion and storage devices. Nature Reviews Materials, 2016,1(6):16023. |
| [42] | KNOSSALLA J, JALALPOOR D, SCHüTH F. Hands-on guide to the synthesis of mesoporous hollow graphitic spheres and core-shell materials. Chemistry of Materials, 2017,29(17):2062-7072. |
| [43] | WANGBO, ANG T P, BORGNA A . A rapid hard template method for the synthesis of N-doped mesoporous carbon replicated from TUD-1. Microporous & Mesoporous Materials, 2012,158(8):99-107. |
| [44] | CHUENCHOM L, KRAEHNERT R, SMARSLY B M . Recent progress in soft-templating of porous carbon materials. Soft Matter, 2012,8(42):10801-10813. |
| [45] | LIU J, WICKRAMARATNE N P, QIAO S Z , et al. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater., 2015,14(8):763-774. |
| [46] | PETKOVICH N D, STEIN A . Controlling macro- and mesostructures with hierarchical porosity through combined hard and soft templating. Chemical Society Reviews, 2013,42(9):3721-3739. |
| [47] | WANG S, LV P, SHEN T , et al. Research progress in preparation of biomass-derived mesoporous carbon materials. Modern Chemical Industry, 2018,38(10):23-26. |
| [48] | WANG T, WANG H . Research progress on porous carbon materials. Scientia Sinica Chimica, 2019,49(5):729-740. |
| [49] | ZHUO R, ZHANG X, WANG X , et al. Research progress in functional metal-organic frameworks for tumor therapy. Acta Chimica Sinica, 2019,77(11):1156. |
| [50] | WU H B, LOU X W D. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Science Advances, 2017, 3(12): eaap9252. |
| [51] | WANG J, WANG Y, HU H , et al. From metal-organic frameworks to porous carbon materials: recent progress and prospects from energy and environmental perspectives. Nanoscale, 2020,12(7):4238-4268. |
| [52] | TANG J, SALUNKHE R R, LIU J , et al. Thermal conversion of core-shell metal-organic frameworks: a new method for selectively functionalized nanoporous hybrid carbon. Journal of the American Chemical Society, 2015,137(4):1572-1580. |
| [53] | BORCHARDT L, ZHU Q-L, CASCO M E , et al. Toward a molecular design of porous carbon materials. Materials Today, 2017,20(10):592-610. |
| [54] | HU M, REBOUL J, FURUKAWA S , et al. Direct carbonization of Al-based porous coordination polymer for synthesis of nanoporous carbon. Journal of the American Chemical Society, 2012,134(6):2864-2867. |
| [55] | TORAD N L, HU M, KAMACHI Y , et al. Facile synthesis of nanoporous carbons with controlled particle sizes by direct carbonization of monodispersed ZIF-8 crystals. Chemical Communications (Cambridge, England), 2013,49(25):2521-2523. |
| [56] | LIU H, YANG D, WANG X , et al. Metal-organic framework- derived hollow carbon materials for electrochemical energy storage and oxygen reduction reaction. Chinese Journal of Inorganic Chemistry, 2019,35(11):1921-1933. |
| [57] | LI J, GAO Y, HAN K , et al. High performance hierarchical porous carbon derived from distinctive plant tissue for supercapacitor. Scientific Reports, 2019,9(1):17270. |
| [58] | DU J, YU Y, LV H , et al. Cauliflower-derived porous carbon without activation for electrochemical capacitor and CO2 capture applications. Journal of Nanoparticle Research, 2018, 20(1): 15-1-12. |
| [59] | QIAN W, GUO J, YAN F , Design, synthesis and application of poly (ionic liquid)-based functional materials. Polym Bull., 2015,10(10):94-104. |
| [60] | XU F, TANG Z, HUANG S , et al. Facile synthesis of ultrahigh-surface-area hollow carbon nanospheres for enhanced adsorption and energy storage. Nature Communications, 2015,6:7221. |
| [61] | LI Z, WU D, HUANG X , et al. Fabrication of novel polymeric and carbonaceous nanoscale networks by the union of self-assembly and hypercrosslinking. Energy & Environmental Science, 2014,7(9):3006. |
| [62] | WU W, CHENG J, SHANG Y , Progress on preparation and application of porous carbon spheres. New chem. Mat., 2014,42(12):217-219. |
| [63] | O.NYAMORI V, MHLANGA D S, COVILLE J N. The use of organometallic transition metal complexes in the synthesis of shaped carbon nanomaterials. Journal of Organometallic Chemistry, 2008,693(13):2205-2222. |
| [64] | HO B N, PFEFFER C M, SINGH A T K. Update on nanotechnology-based drug delivery systems in cancer treatment. Anticancer Research, 2017,37(11):5975-5981. |
| [65] | CHEN D, DOUGHERTY C A, ZHU K , et al. Theranostic applications of carbon nanomaterials in cancer: Focus on imaging and cargo delivery. Journal of Controlled Release, 2015,210:230-245. |
| [66] | LI H, LIN Q, CHEN J , et al. Research progress of carbon nanomaterials in cancer drug delivery. Chemical Journal of Chinese Universities., 2019,50(1):100-106. |
| [67] | ZHU S, XU G . Single-walled carbon nanohorns and their applications. Nanoscale, 2010,2(12):2538-2549. |
| [68] | LAI L, BARNARD A S . Functionalized nanodiamonds for biological and medical applications. Journal of Nanoscience & Nanotechnology, 2015,15(2):989-999. |
| [69] | NARDECCHIA S, CARRIAZO D, FERRER M L , et al. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: synthesis and applications. Chemical Society Reviews, 2013,42(2):794-830. |
| [70] | CHENG L, WANG C, FENG L , et al. Functional nanomaterials for phototherapies of cancer. Chemical Reviews, 2014,114(21):10869-10939. |
| [71] | KAPRI S, MAITI S, BHATTACHARYYA S . Lemon grass derived porous carbon nanospheres functionalized for controlled and targeted drug delivery. Carbon, 2016,100:223-235. |
| [72] | KAPRI S, MAJEE R, BHATTACHARYYA S . Chemical modifications of porous carbon nanospheres obtained from ubiquitous precursors for targeted drug delivery and live cell imaging. Acs Sustainable Chemistry & Engineering, 2018,6(7):8503-8514. |
| [73] | XING Y, DAI L . Nanodiamonds for nanomedicine. Nanomedicine, 2009,4(2):207-218. |
| [74] | LI C, MENG Y, WANG S , et al. Mesoporous carbon nanospheres featured fluorescent aptasensor for multiplediagnosis of cancer in vitro and in vivo. ACS Nano, 2015,9(12):12096-12103. |
| [75] | LI C, QIAN M, WANG S , et al. Aptavalve-gated mesoporous carbon nanospheres image cellular mucin and provide on-demand targeted drug delivery. Theranostics, 2017,7(13):3319-3325. |
| [76] | CHEN C, GENG J, PU F , et al. Polyvalent nucleic acid/mesoporous silica nanoparticle conjugates: dual stimuli-responsive vehicles for intracellular drug delivery. Angewandte Chemie, International Edition in English, 2011,50(4):882-886. |
| [77] | CHOI Y, KIM S, CHOI M-H , et al. Highly biocompatible carbon nanodots for simultaneous bioimaging and targeted photodynamic therapy in vitro and in vivo. Advanced Functional Materials, 2014,24(37):5781-5789. |
| [78] | KONG Q, ZHANG L, LIU J , et al. Facile synthesis of hydrophilic multi-colour and upconversion photoluminescent mesoporous carbon nanoparticles for bioapplications. Chemical Communications (Cambridge, England), 2014,50(99):15772-15775. |
| [79] | LI X, YAN Y, LIN Y , et al. Hollow mesoporous carbon as a near-infrared absorbing carrier compared with mesoporous carbon nanoparticles for chemo-photothermal therapy. Journal of Colloid and Interface Science, 2017,494:159-169. |
| [80] | CHEN Y, XU P, WU M , et al. Colloidal RBC-shaped, hydrophilic, and hollow mesoporous carbon nanocapsules for highly efficient biomedical engineering. Advanced Materials, 2014,26(25):4294-4301. |
| [81] | FANG Y, ZHENG G, YANG J , et al. Dual-pore mesoporous carbon@silica composite core-shell nanospheres for multidrug delivery. Angewandte Chemie, International Edition in English, 2014,53(21):5366-5370. |
| [82] | DU X, ZHAO C, ZHOU M , et al. Hollow carbon nanospheres with tunable hierarchical pores for drug, gene, and photothermal synergistic treatment. Small, 2017,13(6):1602592. |
| [83] | SINGHAL R, ORYNBAYEVA Z, KALYANA SUNDARAM R V, et al. Multifunctional carbon-nanotube cellular endoscopes. Nat. Nanotechnol., 2011,6(1):57-64. |
| [84] | CHENG R, MENG F, DENG C , et al. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials, 2013,34(14):3647-3657. |
| [85] | VEISEH O, GUNN J W, ZHANG M . Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Advanced Drug Delivery Reviews, 2010,62(3):284-304. |
| [86] | CARDONE R A, CASAVOLA V, RESHKIN S J . The role of disturbed pH dynamics and the Na +/H + exchanger in metastasis . Nature Reviews: Cancer, 2005,5(10):786-795. |
| [87] | HUANG X, WU S, DU X . Gated mesoporous carbon nanoparticles as drug delivery system for stimuli-responsive controlled release. Carbon, 2016,101:135-142. |
| [88] | KESSENBROCK K, PLAKS V, WERB Z . Matrix metalloproteinases: regulators of the tumor microenvironment. Cell, 2010,141(1):52-67. |
| [89] | SCHILLING D, GARRIDO C, COMBS S E , et al. The Hsp70 inhibiting peptide aptamer A17 potentiates radiosensitization of tumor cells by Hsp90 inhibition. Cancer Letters, 2017,390:146-152. |
| [90] | BRAYMAN M, THATHIAH A, CARSON D D. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reproductive Biology and Endocrinology, 2004, 2: 4-1-9. |
| [91] | SARKAR S, GULATI K, MISHRA A , et al. Protein nanocomposites: Special inferences to lysozyme based nanomaterials. International Journal of Biological Macromolecules, 2020,151:467-482. |
| [92] | SCHAFER F Q, BUETTNER G R . Redox environment of the cell as viewed through the redox state of the glutathione disulfide/ glutathione couple. Free Radical Biology and Medicine, 2001,30(11):1191-1212. |
| [93] | ZHOU L, DONG K, CHEN Z W , et al. Near-infrared absorbing mesoporous carbon nanoparticle as an intelligent drug carrier for dual-triggered synergistic cancer therapy. Carbon, 2015,82:479-488. |
| [94] | BROADERS K E, GRANDHE S, FRECHET J M . A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. Journal of the American Chemical Society, 2011,133(4):756-758. |
| [95] | CHEN W, BALAKRISHNAN K, KUANG Y , et al. Reactive oxygen species (ROS) inducible DNA cross-linking agents and their effect on cancer cells and normal lymphocytes. Journal of Medicinal Chemistry, 2014,57(11):4498-4510. |
| [96] | WANG L Z, LE L, KANG A , et al. Effects on the dispersion and cytotoxicity of ordered mesoporous carbon nanoparticles modified with PVP or PEG. Journal of Pharmacy Practice, 2016,34(2):158-163. |
| [97] | GUPTA N, RAI D B, JANGID A K , et al. A Review of theranostics applications and toxicities of carbon nanomaterials. Current Drug Metabolism, 2019,20(6):506-532. |
| [98] | XU G, LIU S, NIU H , et al. Functionalized mesoporous carbon nanoparticles for targeted chemo-photothermal therapy of cancer cells under near-infrared irradiation. RSC Advance, 2014,4(64):33986-33997. |
| [99] | XUE C, LONGFEI T, TIANLONG L , et al. Micro-nanomaterials for tumor microwave hyperthermia: design, preparation, and application. Current Drug Delivery, 2017,14:307-322. |
| [100] | SHAH B P, PASQUALE N, DE G , et al. Core-shell nanoparticle- based peptide therapeutics and combined hyperthermia for enhanced cancer cell apoptosis. ACS Nano, 2014,8(9):9379-9387. |
| [101] | WANG Y, GU H . Core-shell-type magnetic mesoporous silica nanocomposites for bioimaging and therapeutic agent delivery. Advanced Materials, 2015,27(3):576-585. |
| [102] | WU F, SUN B, CHU X , et al. Hyaluronic acid-modified porous carbon-coated Fe3O4 nanoparticles for magnetic resonance imaging- guided photothermal/chemotherapy of tumors. Langmuir, 2019,35(40):13135-13144. |
| [103] | HAI W, YULIANG Z, GUANGJUN N . Multifunctional nanoparticle systems for combined chemoand photothermal cancer therapy. Frontiers of Materials Science, 2014,7:118-128. |
| [104] | ZHANG Y, HAN L, HU L-L , et al. Mesoporous carbon nanoparticles capped with polyacrylic acid as drug carrier for bi-trigger continuous drug release. Journal of Materials Chemistry B, 2016,4(30):5178-5184. |
| [105] | ZHANG Y, CHANG Y-Q, HAN L , et al. Aptamer-anchored di-polymer shell-capped mesoporous carbon as a drug carrier for bi-trigger targeted drug delivery. Journal of Materials Chemistry B, 2017,5(33):6882-6889. |
| [106] | YANG Z, WANG L, LIU Y , et al. ZnO capped flower-like porous carbon-Fe3O4 composite as carrier for bi-triggered drug delivery. Materials Science & Engineering. C: Materials for Biological Applications, 2020,107:110256. |
| [107] | CHEN L, ZHENG J, DU J , et al. Folic acid-conjugated magnetic ordered mesoporous carbon nanospheres for doxorubicin targeting delivery. Materials Science & Engineering. C: Materials for Biological Applications, 2019,104:109939. |
| [108] | CHEN L, ZHANG H, ZHENG J , et al. Thermo-sensitively and magnetically ordered mesoporous carbon nanospheres for targeted controlled drug release and hyperthermia application. Materials Science & Engineering. C: Materials for Biological Applications, 2018,84:21-31. |
| [109] | WANG H, CAO G, GAI Z , et al. Magnetic/NIR-responsive drug carrier, multicolor cell imaging, and enhanced photothermal therapy of gold capped magnetite-fluorescent carbon hybrid nanoparticles. Nanoscale, 2015,7(17):7885-7895. |
| [110] | WU F, ZHANG M, LU H , et al. Triple stimuli-responsive magnetic Hollow porous carbon-based nanodrug delivery system for magnetic resonance imaging-guided synergistic photothermal/ chemotherapy of cancer. ACS Appl. Mater. Interfaces, 2018,10(26):21939-21949. |
| [111] | ZHANG D X, ESSER L, VASANI R B , et al. Porous silicon nanomaterials: recent advances in surface engineering for controlled drug-delivery applications. Nanomedicine (Lond), 2019,14(24):3213-3230. |
| [112] | ZHANG J, ZHANG J, LI W , et al. Degradable hollow Mesoporous silicon/carbon nanoparticles for photoacoustic imaging-guided highly effective chemo-thermal tumor therapy in vitro and in vivo. Theranostics, 2017,7(12):3007-3020. |
| [113] | GUO W H, QI Y F, ZHANG Y Q , et al. Biocompatible caramelized carbonaceous nanospheres supported paramagnetic ultrathin manganese oxide nanosheets via self-sacrificing reduction as a MRI contrast agent for liver imaging. Carbon, 2016,110:321-329. |
| [1] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| [2] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [3] | 陈强, 白书欣, 叶益聪. 热管理用高导热碳化硅陶瓷基复合材料研究进展[J]. 无机材料学报, 2023, 38(6): 634-646. |
| [4] | 林俊良, 王占杰. 铁电超晶格的研究进展[J]. 无机材料学报, 2023, 38(6): 606-618. |
| [5] | 牛嘉雪, 孙思, 柳鹏飞, 张晓东, 穆晓宇. 铜基纳米酶的特性及其生物医学应用[J]. 无机材料学报, 2023, 38(5): 489-502. |
| [6] | 苑景坤, 熊书锋, 陈张伟. 聚合物前驱体转化陶瓷增材制造技术研究趋势与挑战[J]. 无机材料学报, 2023, 38(5): 477-488. |
| [7] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [8] | 杨洋, 崔航源, 祝影, 万昌锦, 万青. 柔性神经形态晶体管研究进展[J]. 无机材料学报, 2023, 38(4): 367-377. |
| [9] | 游钧淇, 李策, 杨栋梁, 孙林锋. 氧化物双介质层忆阻器的设计及应用[J]. 无机材料学报, 2023, 38(4): 387-398. |
| [10] | 陈昆峰, 胡乾宇, 刘锋, 薛冬峰. 多尺度晶体材料的原位表征技术与计算模拟研究进展[J]. 无机材料学报, 2023, 38(3): 256-269. |
| [11] | 张超逸, 唐慧丽, 李宪珂, 王庆国, 罗平, 吴锋, 张晨波, 薛艳艳, 徐军, 韩建峰, 逯占文. 新型GaN与ZnO衬底ScAlMgO4晶体的研究进展[J]. 无机材料学报, 2023, 38(3): 228-242. |
| [12] | 齐占国, 刘磊, 王守志, 王国栋, 俞娇仙, 王忠新, 段秀兰, 徐现刚, 张雷. GaN单晶的HVPE生长与掺杂进展[J]. 无机材料学报, 2023, 38(3): 243-255. |
| [13] | 林思琪, 李艾燃, 付晨光, 李荣斌, 金敏. Zintl相Mg3X2(X=Sb, Bi)基晶体生长及热电性能研究进展[J]. 无机材料学报, 2023, 38(3): 270-279. |
| [14] | 刘岩, 张珂颖, 李天宇, 周菠, 刘学建, 黄政仁. 陶瓷材料电场辅助连接技术研究现状及发展趋势[J]. 无机材料学报, 2023, 38(2): 113-124. |
| [15] | 谢兵, 蔡金峡, 王铜铜, 刘智勇, 姜胜林, 张海波. 高储能密度聚合物基多层复合电介质的研究进展[J]. 无机材料学报, 2023, 38(2): 137-147. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||