无机材料学报 ›› 2020, Vol. 35 ›› Issue (2): 243-249.DOI: 10.15541/jim20190118
吴思1,2,梅雷2,胡孔球2,柴之芳2,3,聂长明1( ),石伟群2(
),石伟群2( )
)
收稿日期:2019-03-21
修回日期:2019-04-27
出版日期:2020-02-20
网络出版日期:2019-09-20
作者简介:吴 思(1993-), 女, 硕士研究生. E-mail: wusi@ihep.ac.cn
WU Si1,2,MEI Lei2,HU Kong-Qiu2,CHAI Zhi-Fang2,3,NIE Chang-Ming1( ),SHI Wei-Qun2(
),SHI Wei-Qun2( )
)
Received:2019-03-21
Revised:2019-04-27
Published:2020-02-20
Online:2019-09-20
Supported by:摘要:
本工作报道了一种含新型八核铀酰(U8)团簇单元([(UO2)8O4(μ3-OH)2(μ2-OH)2] 4+)的草酸铀酰配合物, 该化合物中, U型有机配体链可以增强铀酰之间的交联度, 具有稳定多核铀酰团簇的作用。通过与另外两种含单核和双核的铀酰配位化合物比较, 发现八核铀酰团簇单元的形成是一个pH调控的过程。理化性质分析显示, 荧光、红外、拉曼的信号峰都出现了不同程度的重叠和宽化, 表明八个铀酰离子具有较高的相似度, 这与此多核铀酰团簇的近平面分子构型密切相关。
中图分类号:
吴思,梅雷,胡孔球,柴之芳,聂长明,石伟群. pH调控合成U型配体介导的八核铀酰草酸网络[J]. 无机材料学报, 2020, 35(2): 243-249.
WU Si,MEI Lei,HU Kong-Qiu,CHAI Zhi-Fang,NIE Chang-Ming,SHI Wei-Qun. pH-dependent Synthesis of Octa-nuclear Uranyl-oxalate Network Mediated by U-shaped Linkers[J]. Journal of Inorganic Materials, 2020, 35(2): 243-249.
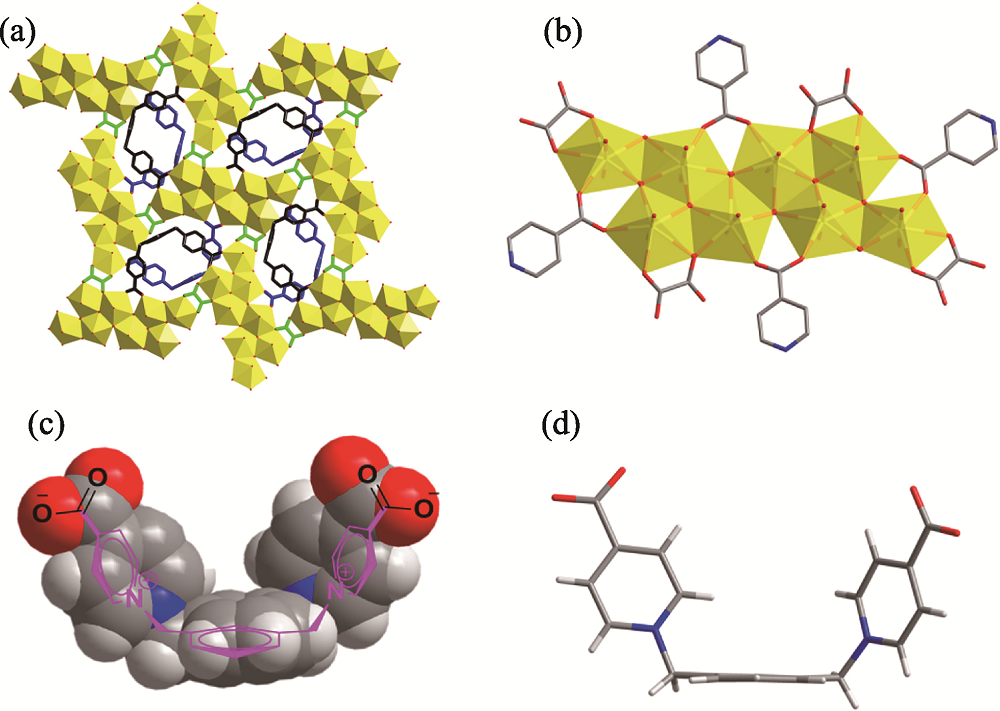
Fig. 1 Octa-nuclear uranyl-oxalate network reinforced by U-shaped zwitterionic dicarboxylate linkers (a) two-dimensional coordination network; (b) octa-nuclear uranyl (U8) motif, [(UO2)8O4(μ3-OH)2(μ2-OH)2]4+; (c) U-shaped linker in a space- filling mode overlapped with its molecular structure; (d) U-shaped linker in a stick mode Color codes: uranyl polyhedra in yellow; U-shaped linkers in dark or blue
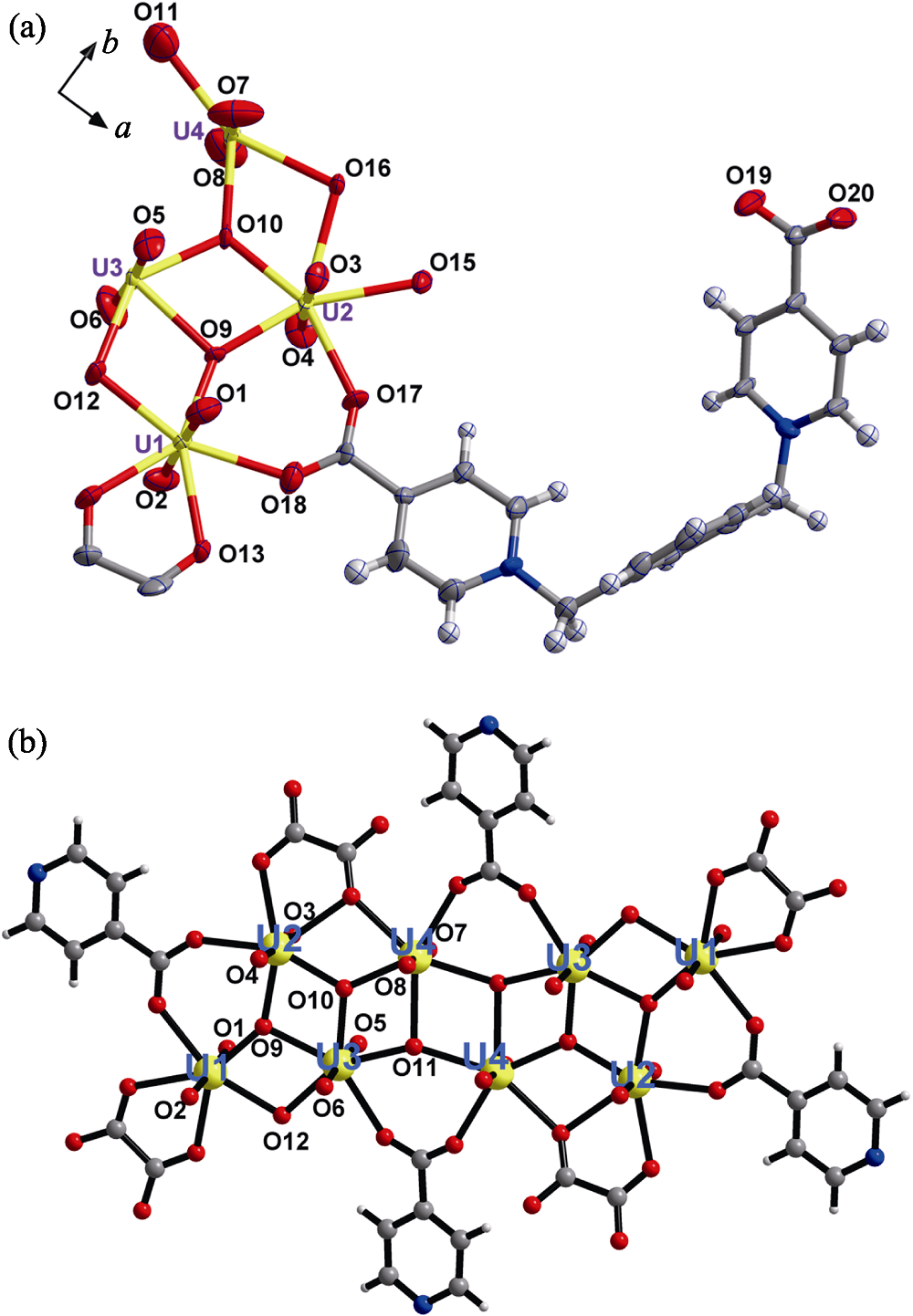
Fig. 2 Crystal structure of compound 1 (a) ORTEP view of compound 1 with the 30% probability level for thermal ellipsoids; (b) octa-nuclear uranyl (U8) motif in compound 1 showing detailed coordination spheres of all uranyl centers Color codes: uranium atoms in yellow; oxygen atoms in red; carbon atoms in dark gray; nitrogen atoms in blue; hydrogen atoms pale gray
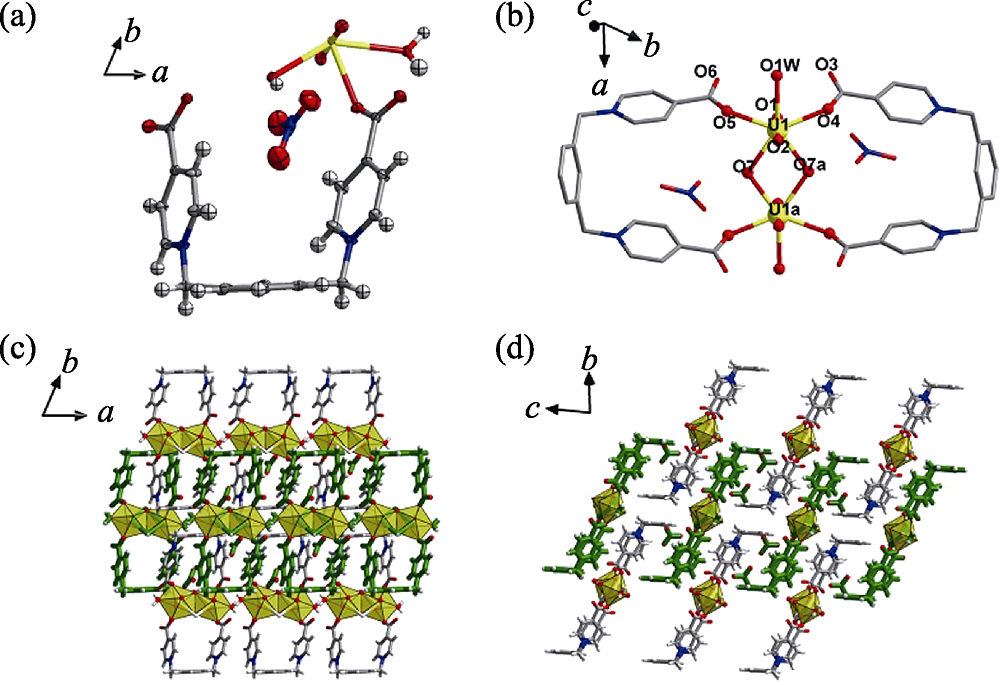
Fig. 3 Crystal structure of compound 2 (a) ORTEP view of compound 2 with the 30% probability level for thermal ellipsoids; (b) coordination environment of each uranyl center for dimeric uranyl motif; (c-d) crystal lattice stacking for compound 2 viewed for c axis (c) and a axis (d) Color codes: uranium atoms in yellow; oxygen atoms in red; carbon atoms in dark gray; nitrogen atoms in blue; hydrogen atoms pale gray; the U-shaped linkers in green
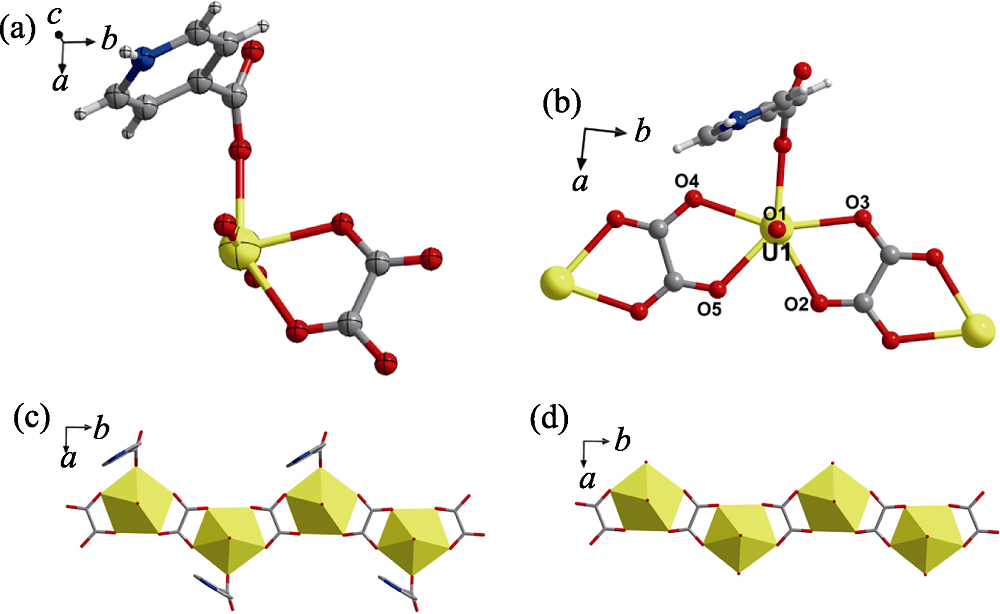
Fig. 4 Crystal structure of compound 3 (a) ORTEP view of compound 3 with the 30% probability level for thermal ellipsoids; (b) coordination environments of uranyl center; (c-d) the extended structure based on one-dimensional oxalate-bridging monomeric uranyl chain with (c) or without (d) terminal isonicotinate ligands Color codes: uranium atoms or polyhedras in yellow; oxygen atoms in red; carbon atoms in dark gray; nitrogen atoms in blue; hydrogen atoms in pale gray
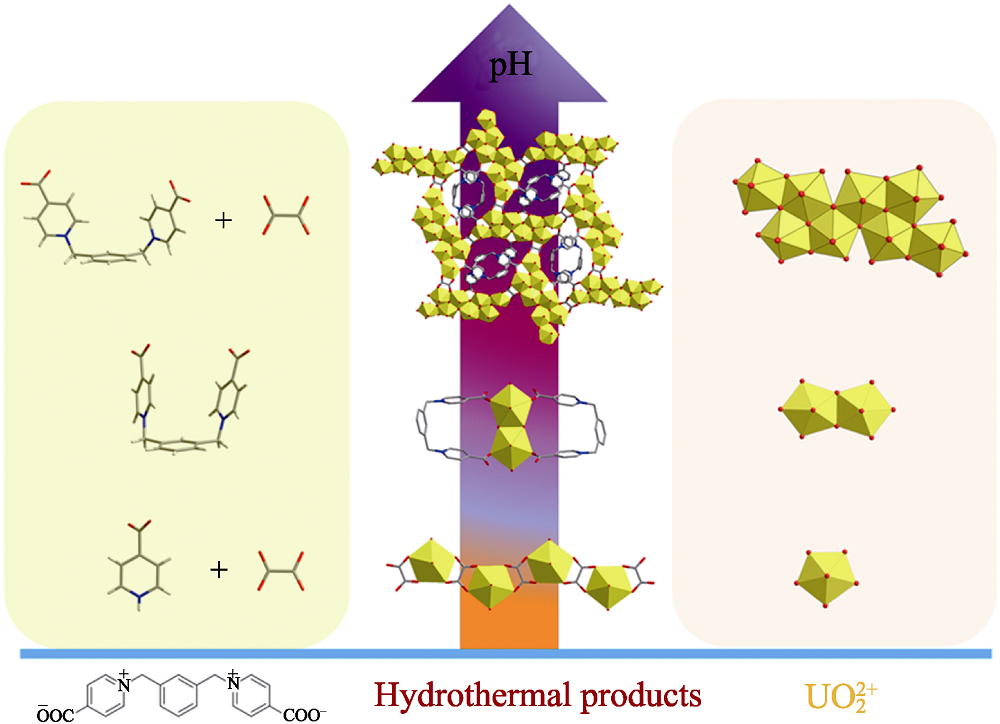
Fig. 5 pH-dependent regulation of hydrothermal reactions of m-Xyl-BPy4CA linkers and uranyl Color codes: uranium polyhedras in yellow; oxygen atoms in red; carbon atoms in gray; nitrogen atoms in blue
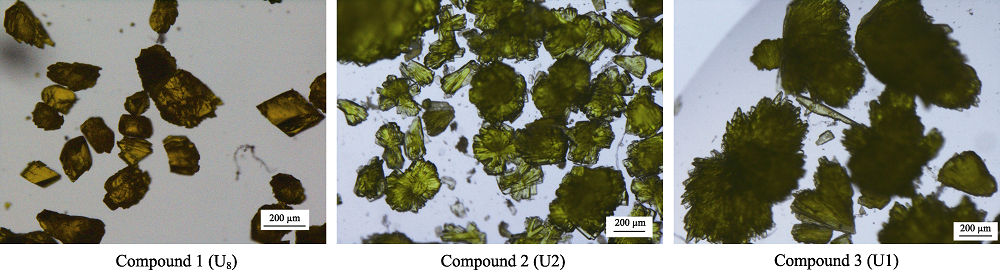
Fig. S1 Different optical morphologies of 1 with octa-nuclear uranyl (U8) motifs, 2 with binuclear uranyl (U2) motifs and 3 with monomeric uranyl (U1) motifs
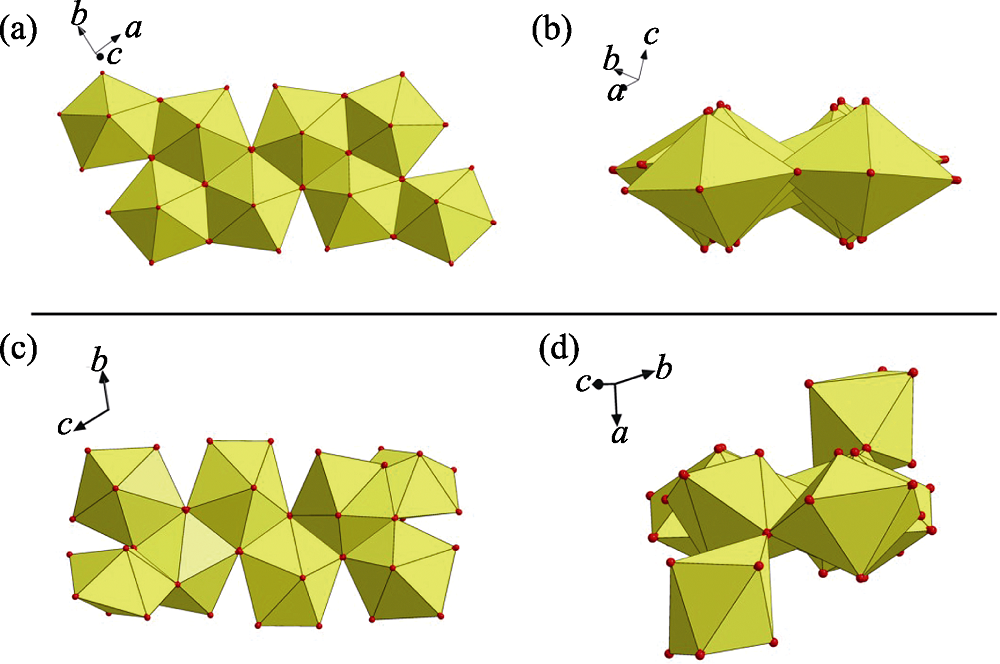
Fig. S5 (a) A nearly planar geometry of U8 motif found in this work; (b) a non-planar U8 motif with cation-cation interactions (CCIs) reported by Loiseau, et al[1]
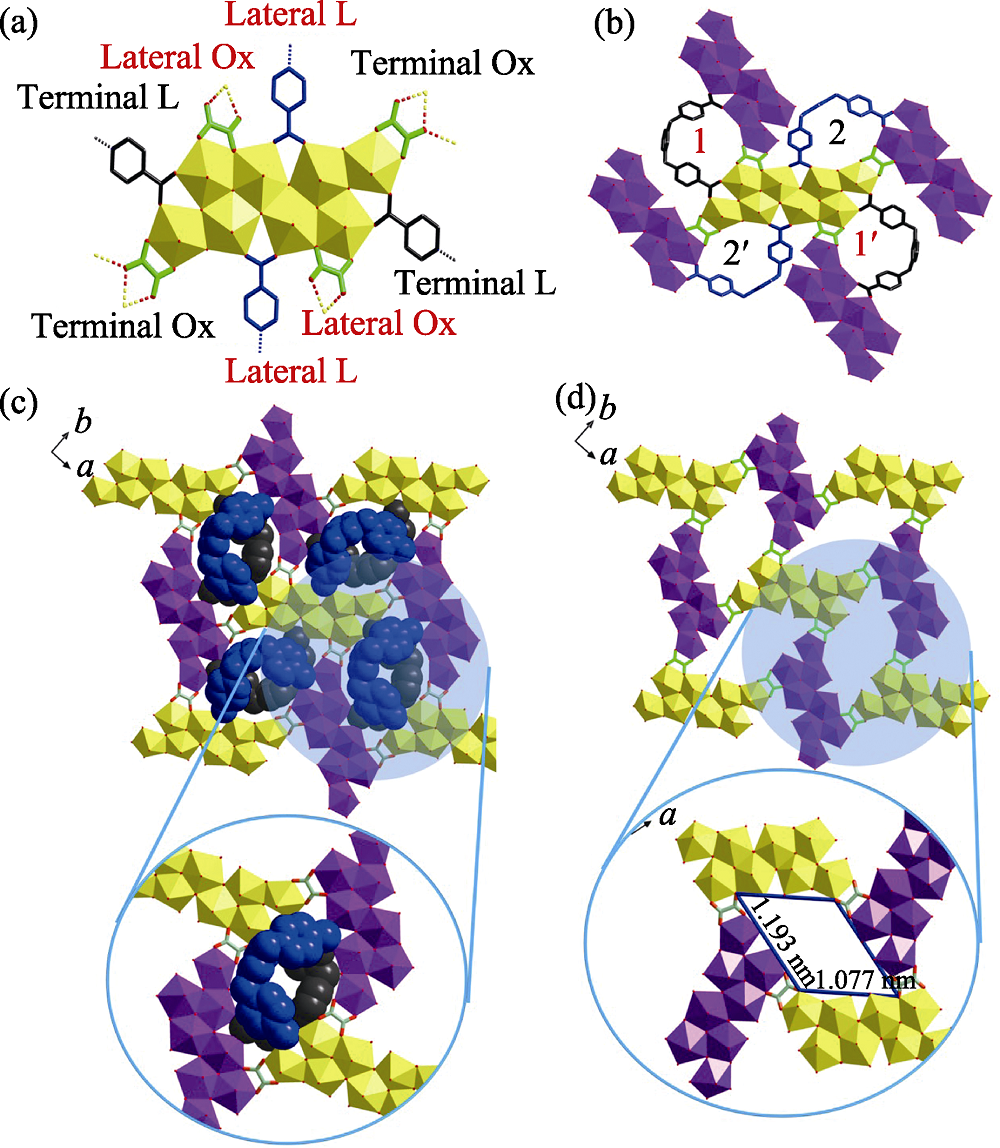
Fig. S6 (a-b) Eight-connected U8 motif with four oxalate (Ox) and four m-Xyl-BPy4CA (L) moieties extends from four directions through oxalate ligands (a), which thus connecting four adjacent ones with each oxalate ligand going together with a U-shaped bidentate m-Xyl-BPy4CA linker (b); (c) U8-based uranyl-oxalate 2D network (enlarged diagram: a minimum rhombic loop); (d) U8-based uranyl-oxalate 2D network with all the cross-linking m-Xyl-BPy4CA linkers omitted for clarity (enlarged diagram: a minimum rhombic loop in size of 1.193 nm× 1.077 nm)
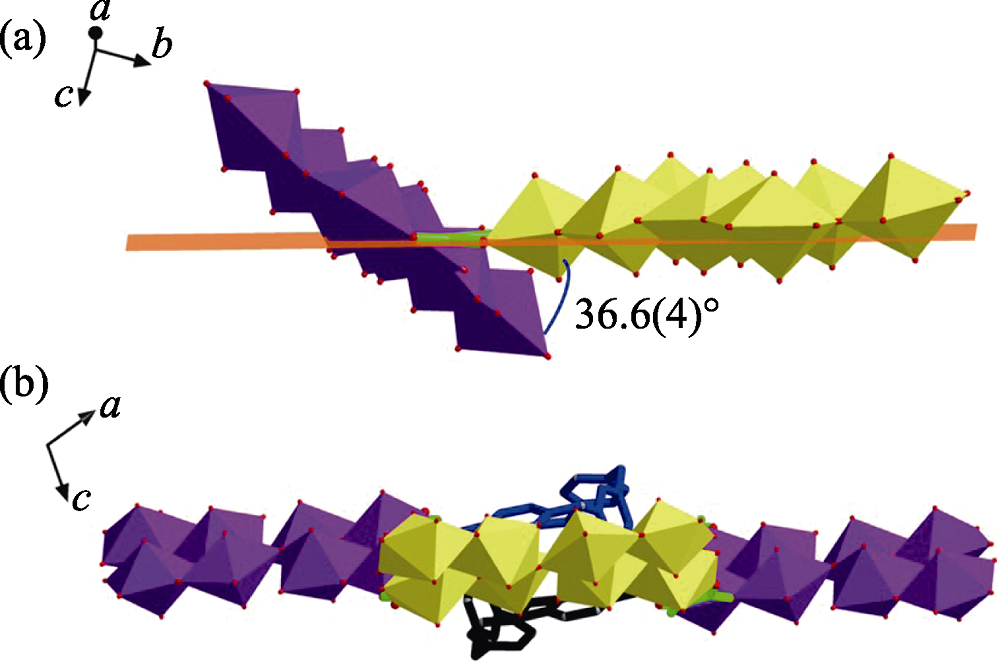
Fig. S7 Each U8 motif displays a different overall orientation from that of its adjacent U8 with an angle of inclination of 36.6(4)° (a), resulting in a distortion of the rhombic loop (b)
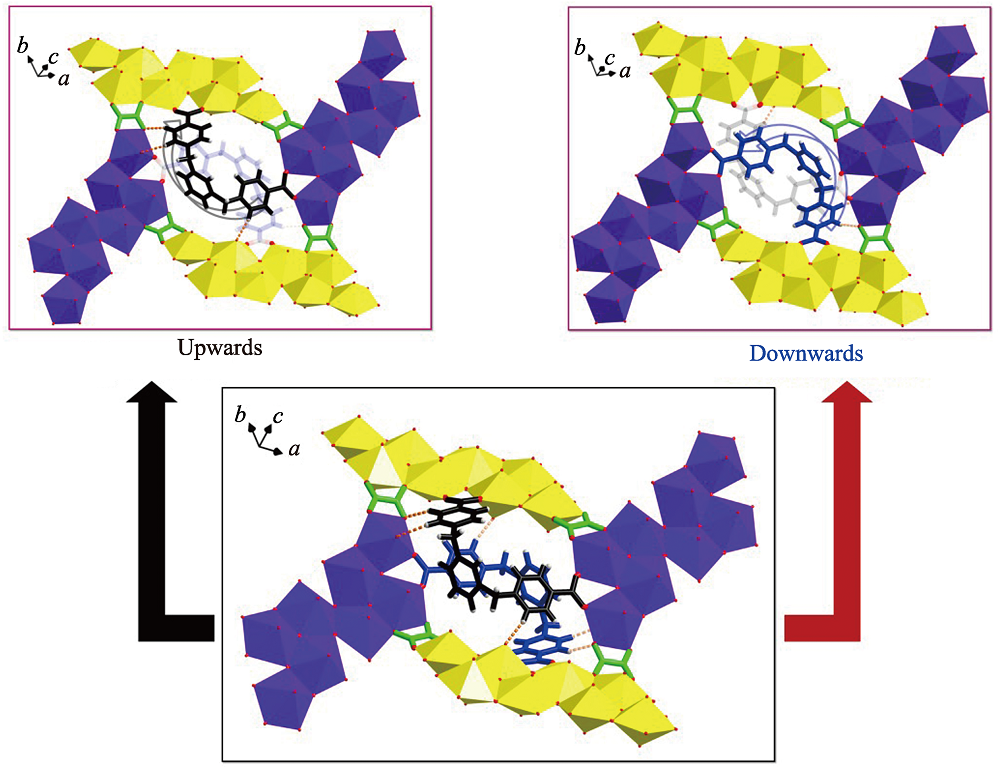
Fig. S9 Two ‘U’-shaped bidentate m-Xyl-BPy4CA ligands located in the cavity of rhombic loop crosslink all the four U8 motifs through coordination bonds and hydrogen bonds (bottom) where one m-Xyl-BPy4CA ligand points upwards (top left) and the other points downwards from the opposite direction (top right)
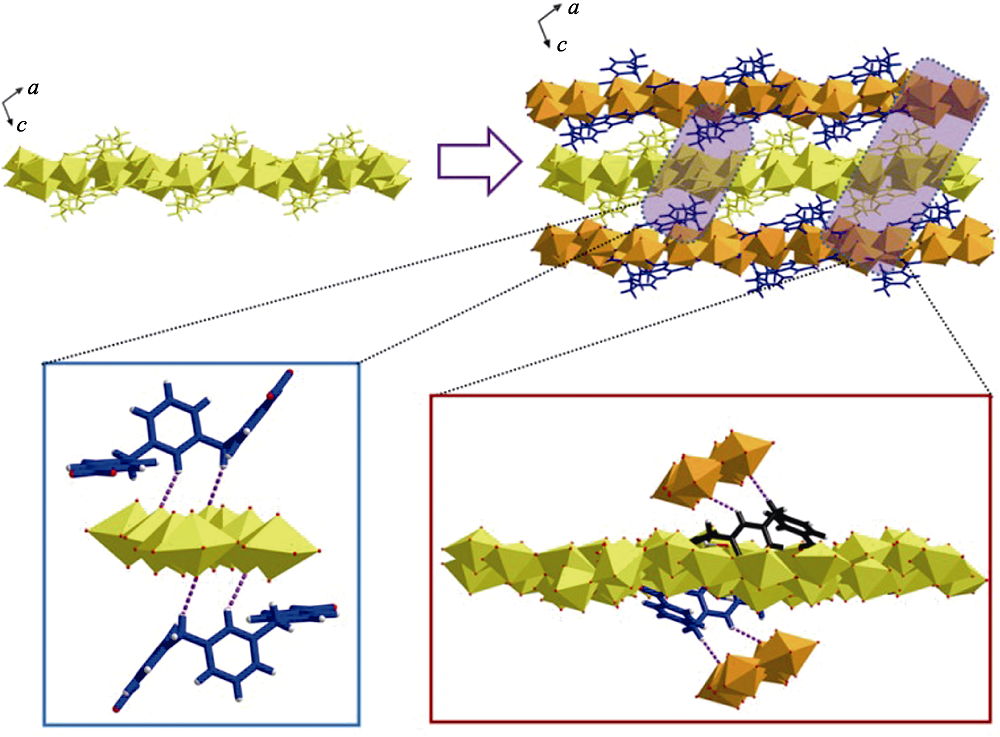
Fig. S10 Hydrogen bonds between adjacent layers of 2D sheets through U8 motifs that interact with neighboured m-Xyl-BPy4CA from another sheet or m-Xyl-BPy4CA interacting with neighboured uranyl group from another sheet
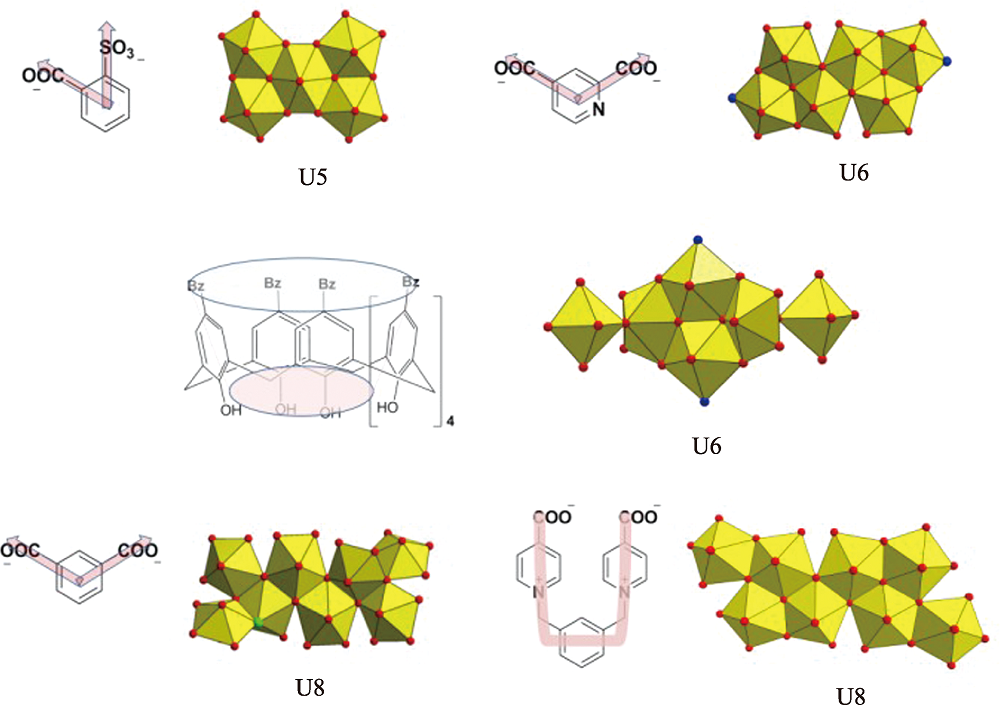
Fig. S11 Some examples of high-nuclear uranyl motif based on nonlinear multi-topic organic ligands, as suggested by the cases of pentanuclear (U5), hexanuclear (U6) and octanuclear (U8) uranyl motifs derived from sulfobenzoate precursors[2], ortho-position or meta-position aromatic/heteroaromatic dicarboxylate[3,4], calixarene ligand[3] and U-shaped linkers used in this work
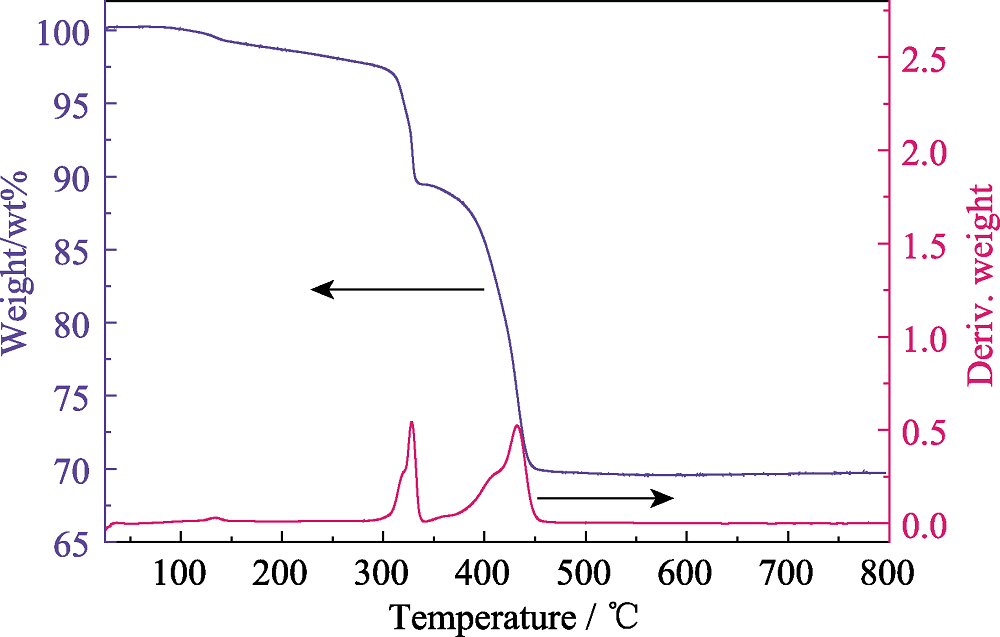
Fig. S13 Thermogravimetric analysis (TGA) of compounds 1, where 1 starts to decompose at ~295 ℃, and finally transforms to U3O8 with residual weight of 69.31% (theoretical value: 70.25%)
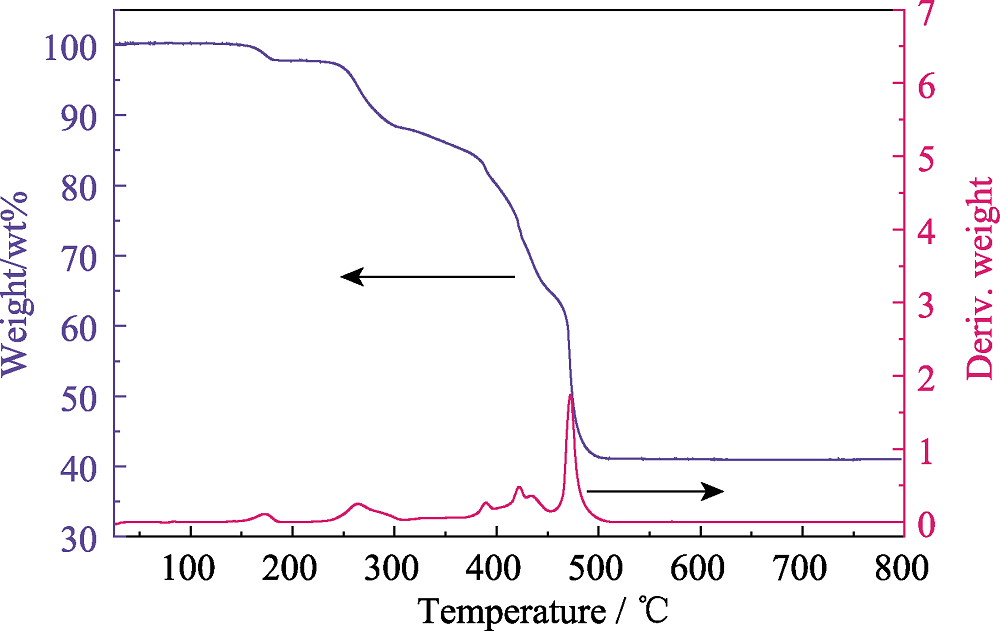
Fig. S14 Thermogravimetric analysis (TGA) of compounds 2, where 2 starts to decompose at ~233 ℃, and finally transforms to U3O8 with residual weight of 40.95% (theoretical value: 40.20%)
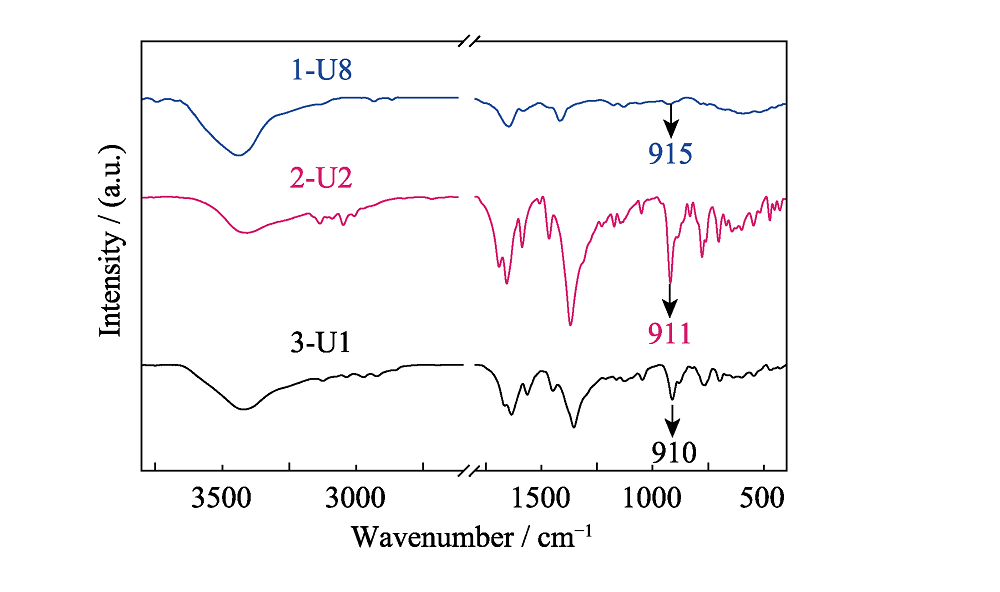
Fig. S15 Fourier transform infrared (IR) spectra of compounds 1 (U8 motif, blue line), 2 (U2 motif, red line) and 3 (U1 motif, black line) with characteristic symmetric ν1vibrations at 915, 911 and 910 nm, respectively
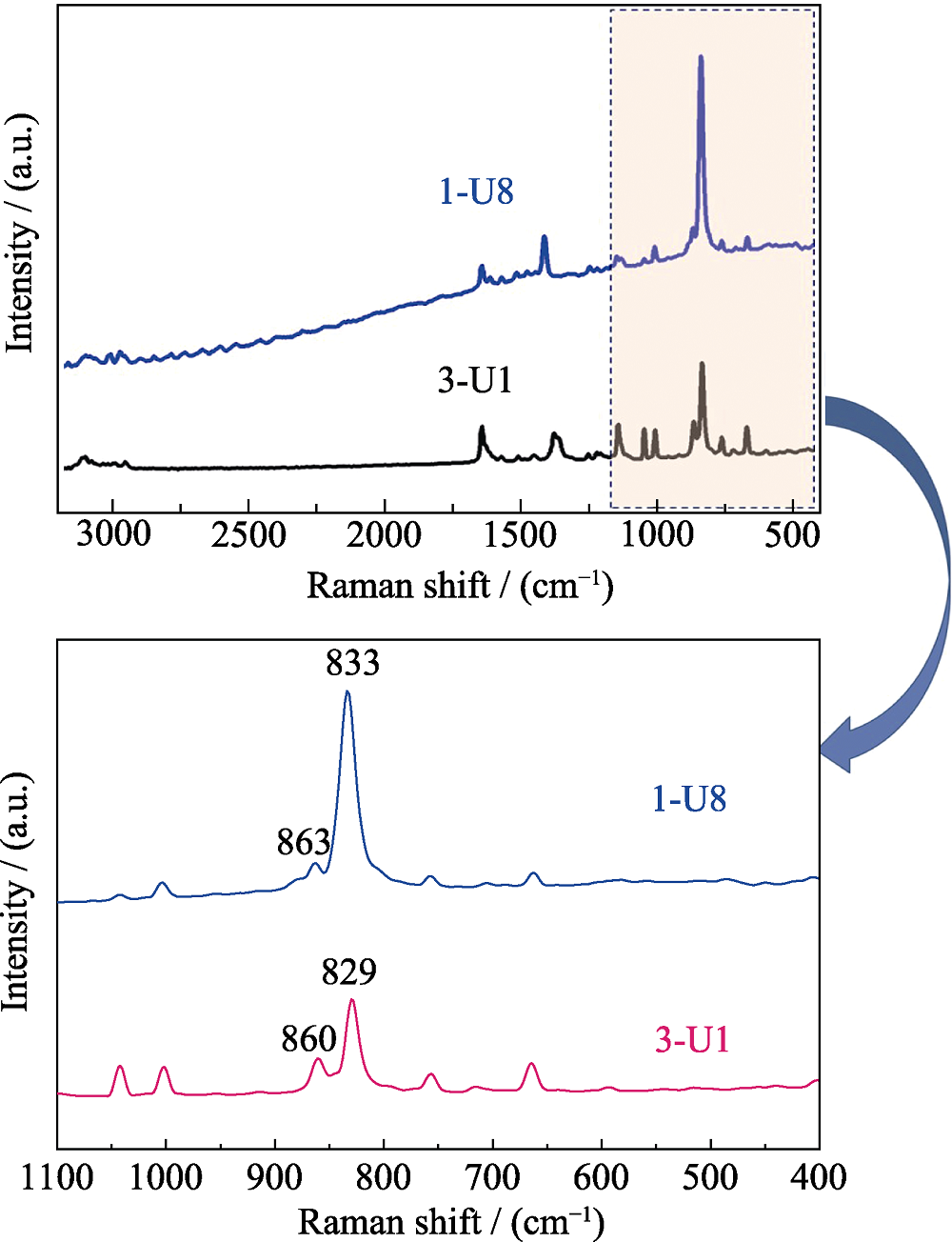
Fig. S16 The Raman spectra of compounds s 1 (U8 motif) and 3 (U1 motif) with characteristic asymmetric ν3 vibrations (1: 833 and 863 cm-1; 3: 829 and 860 cm-1)
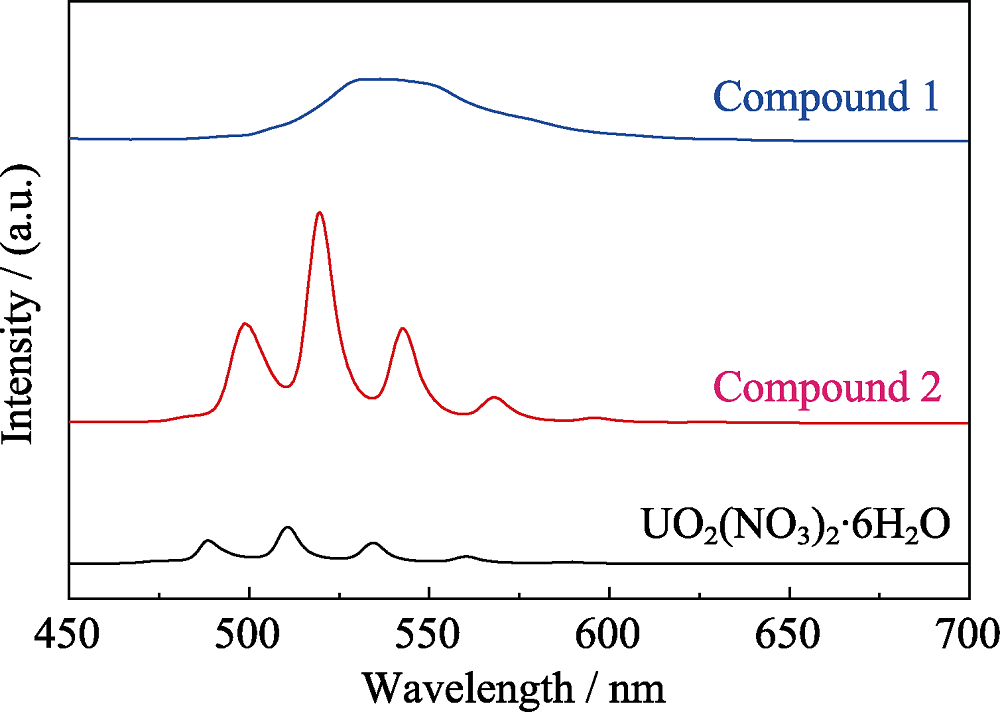
Fig. S17 Solid-state fluorescence spectra of compound 1 and 2 as compared to that of uranyl nitrate (UO2(NO3)2): 1, a broad peak ranging from 530 to 550 nm; 2, five main emission bands located at 499, 520, 543, 568 and 596 nm; UO2(NO3)2, 488, 511, 534, 561 and 589 nm
| Compound 1 | |||
|---|---|---|---|
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1748(17) | U(2)-O(3) | 0.1752(15) |
| U(1)-O(2) | 0.1770(2) | U(2)-O(4) | 0.1751(15) |
| U(1)-O(9) | 0.2208(13) | U(2)-O(9) | 0.2275(12) |
| U(1)-O(12) | 0.2327(15) | U(2)-O(10) | 0.2193(14) |
| U(1)-O(13) | 0.2506(14) | U(2)-O(15) | 0.2466(14) |
| U(1)-O(14) | 0.2440(18) | U(2)-O(16) | 0.2578(14) |
| U(1)-O(18) | 0.2426(16) | U(2)-O(17) | 0.2380(17) |
| U(3)-O(5) | 0.1746(17) | U(4)-O(7) | 0.165(3) |
| U(3)-O(6) | 0.178(2) | U(4)-O(8) | 0.171(2) |
| U(3)-O(9) | 0.2344(14) | U(4)-O(10) | 0.2200(14) |
| U(3)-O(10) | 0.2237(14) | U(4)-O(11) | 0.242(2) |
| U(3)-O(11c) | 0.248(2) | U(4)-O(11c) | 0.2461(14) |
| U(3)-O(12) | 0.2349(17) | U(4)-O(16) | 0.249(2) |
| U(3)-O(19a) | 0.2439(16) | U(4)-O(20d) | 0.2399(16) |
| Compound 2 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1776(2) | U(1)-O(4) | 0.2364(2) |
| U(1)-O(2) | 0.1784(2) | U(1)-O(5a) | 0.2358(2) |
| U(1)-O(7) | 0.2325(2) | U(1)-O(7a) | 0.2339(2) |
| U(1)-O(1W) | 0.2576(2) | ||
| Compound 3 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.182(3) | U(1)-O(4b) | 0.244(2) |
| U(1)-O(1a) | 0.182(3) | U(1)-O(5) | 0.237(2) |
| U(1)-O(2) | 0.240(2) | U(1)-O(6) | 0.2307(18) |
| U(1)-O(3) | 0.2397(19) | ||
Table S1 Selected bond distances related to uranyl centers in compounds 1, 2 and 3
| Compound 1 | |||
|---|---|---|---|
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1748(17) | U(2)-O(3) | 0.1752(15) |
| U(1)-O(2) | 0.1770(2) | U(2)-O(4) | 0.1751(15) |
| U(1)-O(9) | 0.2208(13) | U(2)-O(9) | 0.2275(12) |
| U(1)-O(12) | 0.2327(15) | U(2)-O(10) | 0.2193(14) |
| U(1)-O(13) | 0.2506(14) | U(2)-O(15) | 0.2466(14) |
| U(1)-O(14) | 0.2440(18) | U(2)-O(16) | 0.2578(14) |
| U(1)-O(18) | 0.2426(16) | U(2)-O(17) | 0.2380(17) |
| U(3)-O(5) | 0.1746(17) | U(4)-O(7) | 0.165(3) |
| U(3)-O(6) | 0.178(2) | U(4)-O(8) | 0.171(2) |
| U(3)-O(9) | 0.2344(14) | U(4)-O(10) | 0.2200(14) |
| U(3)-O(10) | 0.2237(14) | U(4)-O(11) | 0.242(2) |
| U(3)-O(11c) | 0.248(2) | U(4)-O(11c) | 0.2461(14) |
| U(3)-O(12) | 0.2349(17) | U(4)-O(16) | 0.249(2) |
| U(3)-O(19a) | 0.2439(16) | U(4)-O(20d) | 0.2399(16) |
| Compound 2 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1776(2) | U(1)-O(4) | 0.2364(2) |
| U(1)-O(2) | 0.1784(2) | U(1)-O(5a) | 0.2358(2) |
| U(1)-O(7) | 0.2325(2) | U(1)-O(7a) | 0.2339(2) |
| U(1)-O(1W) | 0.2576(2) | ||
| Compound 3 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.182(3) | U(1)-O(4b) | 0.244(2) |
| U(1)-O(1a) | 0.182(3) | U(1)-O(5) | 0.237(2) |
| U(1)-O(2) | 0.240(2) | U(1)-O(6) | 0.2307(18) |
| U(1)-O(3) | 0.2397(19) | ||
| Compound 1 | ||||
|---|---|---|---|---|
| Hydrogen bond | D-H/nm | H··A/nm | D··A/nm | Angle/(°) |
| C6-H6···O6 | 0.093 | 0.215 | 0.305 | 165 |
| C17-H17···O1 | 0.093 | 0.243 | 0.316 | 135 |
| C18-H18···O13 | 0.093 | 0.242 | 0.330 | 159 |
| C15-H15···O5 | 0.093 | 0.245 | 0.322 | 141 |
| C16-H16A···O3 | 0.097 | 0.242 | 0.321 | 138 |
| Compound 2 | ||||
| Hydrogen bond | D-H/nm | H···A/nm | D···A/nm | Angle/(°) |
| O7-H7···O10 | 0.073 | 0.216 | 0.285 | 161 |
| C16-H16···O10 | 0.093 | 0.258 | 0.324 | 128 |
| C15-H16···O9 | 0.093 | 0.298 | 0.358 | 123 |
Table S2 Distances and angles for hydrogen bonds observed in compounds 1 and 2
| Compound 1 | ||||
|---|---|---|---|---|
| Hydrogen bond | D-H/nm | H··A/nm | D··A/nm | Angle/(°) |
| C6-H6···O6 | 0.093 | 0.215 | 0.305 | 165 |
| C17-H17···O1 | 0.093 | 0.243 | 0.316 | 135 |
| C18-H18···O13 | 0.093 | 0.242 | 0.330 | 159 |
| C15-H15···O5 | 0.093 | 0.245 | 0.322 | 141 |
| C16-H16A···O3 | 0.097 | 0.242 | 0.321 | 138 |
| Compound 2 | ||||
| Hydrogen bond | D-H/nm | H···A/nm | D···A/nm | Angle/(°) |
| O7-H7···O10 | 0.073 | 0.216 | 0.285 | 161 |
| C16-H16···O10 | 0.093 | 0.258 | 0.324 | 128 |
| C15-H16···O9 | 0.093 | 0.298 | 0.358 | 123 |
| Compound 1 | Compound 2 | Compound 3 | |
|---|---|---|---|
| Formula | C22H16N2O20U4 | C40H38N6O22U2 | C8H5NO8U |
| Formula weight | 1580.49 | 1430.82 | 481.16 |
| Crystal system | monoclinic | triclinic | orthorhombic |
| Space group | P21/c | P-1 | Ibam |
| a/nm | 1.15944(14) | 0.98277(3) | 2.6039(4) |
| b/nm | 1.9854(3) | 1.05830(4) | 1.17462(13) |
| c/nm | 1.5002(2) | 1.15097(4) | 0.91646(17) |
| α/(º) | 90 | 82.951(2) | 90 |
| β/(º) | 105.390(3) | 88.168(2) | 90 |
| γ/(º) | 90 | 66.735(2) | 90 |
| V/nm3 | 3.3296(8) | 1.09126(7) | 2.8031(7) |
| Z | 4 | 1 | 8 |
| T/K | 296 | 297 | 293 |
| F(000) | 2760 | 680 | 1728 |
| Dc/(g·cm-3) | 3.153 | 2.177 | 2.280 |
| μ/mm-1 | a 19.480 | b 7.507 | c 32.914 |
| Rint | 0.073 | 0.028 | 0.088 |
| R1, wR2 (all data) | 0.0646, 0.1536 | 0.0227, 0.0491 | 0.0755, 0.2833 |
Table S3 Crystal data and structure refinement for compounds 1, 2 and 3
| Compound 1 | Compound 2 | Compound 3 | |
|---|---|---|---|
| Formula | C22H16N2O20U4 | C40H38N6O22U2 | C8H5NO8U |
| Formula weight | 1580.49 | 1430.82 | 481.16 |
| Crystal system | monoclinic | triclinic | orthorhombic |
| Space group | P21/c | P-1 | Ibam |
| a/nm | 1.15944(14) | 0.98277(3) | 2.6039(4) |
| b/nm | 1.9854(3) | 1.05830(4) | 1.17462(13) |
| c/nm | 1.5002(2) | 1.15097(4) | 0.91646(17) |
| α/(º) | 90 | 82.951(2) | 90 |
| β/(º) | 105.390(3) | 88.168(2) | 90 |
| γ/(º) | 90 | 66.735(2) | 90 |
| V/nm3 | 3.3296(8) | 1.09126(7) | 2.8031(7) |
| Z | 4 | 1 | 8 |
| T/K | 296 | 297 | 293 |
| F(000) | 2760 | 680 | 1728 |
| Dc/(g·cm-3) | 3.153 | 2.177 | 2.280 |
| μ/mm-1 | a 19.480 | b 7.507 | c 32.914 |
| Rint | 0.073 | 0.028 | 0.088 |
| R1, wR2 (all data) | 0.0646, 0.1536 | 0.0227, 0.0491 | 0.0755, 0.2833 |
| [1] | ALTMAIER M, GAONA X, FANGHANEL T , et al. Recent advances in aqueous actinide chemistry and thermodynamics. Chemical Reviews, 2013,113(2):901-943. |
| [2] | JONES M B, GAUNT A J . Recent developments in synthesis and structural chemistry of nonaqueous actinide complexes. Chemical Reviews, 2013,113(2):1137-1198. |
| [3] | WANG K X, CHEN J S . Extended structures and physicochemical properties of uranyl-organic compounds. Accounts of Chemical Research, 2011,44(7):531-540. |
| [4] | ANDREWS M B, CAHILL C L . Uranyl bearing hybrid materials: synthesis, speciation, and solid-state structures. Chemical Reviews, 2013,113(2):1121-1136. |
| [5] | YANG W T, PARKER T G, SUN Z M , et al. Structural chemistry of uranium phosphonates. Coordination Chemistry Reviews, 2015,303(1):86-109. |
| [6] | LOISEAU T, MIHALCEA I, HENRY N , et al. The crystal chemistry of uranium carboxylates. Coordination Chemistry Reviews, 2014,266(35):69-109. |
| [7] | RAI D, FELMY A R, RYAN J L , et al. Uranium (IV) hydrolysis constants and solubility product of UO2·xH2O(am). Inorganic Chemistry, 1990,29(2):260-264. |
| [8] | AHRLAND S . On the complex chemistry of the uranyl ion Ι. The hydrolysis of the 6-valent uranium in aqueous solutions. Acta Chemica Scandinavica, 1949,3(4):374-400. |
| [9] | ZANONATO P, DI BERNARDO P, BISMONDO A , et al. Hydrolysis of uranium (VI) at variable temperatures (10-85 ℃). Journal of the American Chemical Society, 2004,126(17):5515-5522. |
| [10] | SALMON L, THUERY P, EPHRITIKHINE M , et al. Crystal structure of the first octanuclear uranium (IV) complex with compartmental schiff base ligands. Polyhedron, 2004,23(4):623-627. |
| [11] | MIHALCEA I, HENRY N, CLAVIER N , et al. Occurence of an octanuclear motif of uranyl isophthalate with cation-cation interactions through edge-sharing connection mode. Inorganic Chemistry, 2011,50(13):6243-6249. |
| [12] | PASQUALE S, SATTIN S, ESCUDERO-ADAN E C , et al. Giant regular polyhedra from calixarene carboxylates and uranyl. Nature Communications, 2012,3(1):785. |
| [13] | THUERY P . A highly adjustable coordination system: nanotubular and molecular cage species in uranyl ion complexes with kemp's triacid. Crystal Growth & Design, 2014,14(3):901-904. |
| [14] | WANG L H, SHANG R, ZHENG Z , et al. Two systems of [DabcoH2]2+/[PipH2]2+-uranyl-oxalate showing reversible crystal-to- crystal transformations controlled by the diammonium/uranyl/oxalate ratios in aqueous solutions ([DabcoH2]2+=1,4-diazabicyclo- [2.2.2]-octaneH2 and [PipH2]2+ = PiperazineH2). Crystal Growth & Design, 2013,13(6):2597-2606. |
| [15] | CHAPELET-ARAB B, NOWOGROCKI G, ABRAHAM E , et al. Crystal structure of new uranyl oxalates (NH4)2[UO2(C2O4)·2H2O] and (NH4)2-x(N2H5)x[UO2(C2O4)3]·3H2O (x=0 and x=1). Comparison with other uranyl oxalates. Radiochimica Acta, 2005,93(5):279-285. |
| [16] | GIESTING P A, PORTER N J, BURNS P C , et al. A series of sheet-structured alkali metal uranyl oxalate hydrates: structures and IR spectra. Zeitschrift für Kristallographie, 2006,221(8):589-599. |
| [17] | GIESTING P A, PORTER N J, BURNS P C , et al. Uranyl oxalate hydrates: structures and IR spectra. Zeitschrift für Kristallographie, 2006,221(4):252-259. |
| [18] | DUVIEUBOURG L, NOWOGROCKI G, ABRAHAM F , et al. Hydrothermal synthesis and crystal structures of new uranyl oxalate hydroxides: α- and β-[(UO2)2 (C2O4)(OH)2(H2O)2] and [(UO2)2((C2O4)(OH)2(H2O)2]·H2O. Journal of Solid State Chemistry, 2005,178(11):3437-3444. |
| [19] | THUERY P . Reaction of uranyl nitrate with carboxylic diacids under hydrothermal conditions. Crystal structure of complexes with L(+)-tartaric and oxalic acids. Polyhedron, 2007,26(1):101-106. |
| [20] | VOLOGZHANINA A V, SEREZHKINA L B, NEKLYUDOVA N A , et al. Synthesis and characterisation of a trinuclear uranyl complex: crystal structure of (CN3H6)5[(UO2)3O(OH)2(CH3COO)(C2O4)3]. Inorganica Chimica Acta, 2009,362(14):4921-4925. |
| [21] | CHUGH C A, SHARMA A, SHARMA A , et al. Kinetics and mechanism of thermal decomposition of uranyl oxalate. Asian Journal of Chemistry, 2011,23(4):1865-1866. |
| [22] | BARTLETT J R, COONEY R P , et al. On the determination of uranium oxygen bond lengths in dioxouranium (VI) compounds by raman-spectroscopy. Journal of Molecular Structure, 1989,193(1):295-300. |
| [23] | BRACHMANN A, GEIPEL G, BERNHARD G , et al. Study of uranyl (VI) malonate complexation by time resolved laser-induced fluorescence spectroscopy (TRLFS). Radiochimica Acta, 2002,90(3):147-153. |
| [24] | MEI L, WANG C Z, ZHU L Z , et al. Exploring new assembly modes of uranyl terephthalate: templated syntheses and structural regulation of a series of rare 2d→3d polycatenated frameworks. Inorganic Chemistry, 2017,56(14):7694-7706. |
| [25] | NATRAJAN L S . Developments in the photophysics and photochemistry of actinide ions and their coordination compounds. Coordination Chemistry Reviews, 2012,256(15/16):1583-1603. |
| [26] | THUERY P, HARROWFIELD J . Solvent effects in solvo-hydrothermal synthesis of uranyl ion complexes with 1,3-adamantanediacetate. CrystEngComm, 2015,17(21):4006-4018. |
| [27] | THUERY P, HARROWFIELD J . Structural variations in the uranyl/4,4'-biphenyldicarboxylate system. rare examples of 2d→3d polycatenated uranyl-organic networks. Inorganic Chemistry, 2015,54(16):8093-8102. |
| [28] | THUERY P, RIVIERE E, HARROWFIELD J , et al. Uranyl and uranyl-3d block cation complexes with 1,3-adamantanedicarboxylate: crystal structures, luminescence, and magnetic properties. Inorganic Chemistry, 2015,54(6):2838-2850. |
| [1] | 顾薛苏, 殷杰, 王康龙, 崔崇, 梅辉, 陈忠明, 刘学建, 黄政仁. 颗粒级配对黏结剂喷射打印碳化硅陶瓷性能的影响[J]. 无机材料学报, 0, (): 216-. |
| [2] | 陈雨, 林埔安, 蔡冰, 张文华. 钙钛矿太阳能电池无机空穴传输材料的研究进展[J]. 无机材料学报, 0, (): 105-. |
| [3] | 田煜彬, 田超凡, 李森, 赵永鑫, 邢涛, 李智, 陈萧如, 向帅蓉, 代鹏程. 高导电性生物质碳布的制备及其燃料电池气体扩散层性能[J]. 无机材料学报, 0, (): 127-. |
| [4] | 江润璐, 吴鑫, 郭昊骋, 郑琦, 王连军, 江莞. UiO-67基导电复合材料的制备及其热电性能研究[J]. 无机材料学报, 0, (): 197-. |
| [5] | 李海燕, 旷峰华, 吴昊龙, 刘小根, 包亦望, 万德田. 残余拉应力的温度依赖性及其对裂纹扩展行为的影响[J]. 无机材料学报, 0, (): 214-. |
| [6] | 方万丽, 沈黎丽, 李海燕, 陈薪羽, 陈宗琦, 寿春晖, 赵斌, 杨松旺. NiOx介孔层的成膜过程对碳电极钙钛矿太阳能电池性能的影响[J]. 无机材料学报, 0, (): 2-. |
| [7] | 丁统顺, 丰平, 孙学文, 单沪生, 李琪, 宋健. Fmoc-FF-OH钝化钙钛矿薄膜及其太阳能电池性能研究[J]. 无机材料学报, 0, (): 50-. |
| [8] | 徐昊, 钱伟, 花银群, 叶云霞, 戴峰泽, 蔡杰. 皮秒激光加工的微织构对碳化硅润湿性的影响[J]. 无机材料学报, 0, (): 73-. |
| [9] | 邱海洋, 苗广潭, 李辉, 栾奇, 刘国侠, 单福凯. 等离子体处理对突触晶体管长程塑性的影响[J]. 无机材料学报, 2023, 38(4): 406-412. |
| [10] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [11] | 杨洋, 崔航源, 祝影, 万昌锦, 万青. 柔性神经形态晶体管研究进展[J]. 无机材料学报, 2023, 38(4): 367-377. |
| [12] | 吴俊林, 丁继扬, 黄新友, 朱丹阳, 黄东, 代正发, 杨文钦, 蒋兴奋, 周健荣, 孙志嘉, 李江. Gd2O2S:Tb闪烁陶瓷的制备与结构: 水浴合成中H2SO4/Gd2O3摩尔比的影响[J]. 无机材料学报, 2023, 38(4): 452-460. |
| [13] | 陈鑫力, 李岩, 王伟胜, 石智文, 竺立强. 明胶/羧化壳聚糖栅控氧化物神经形态晶体管[J]. 无机材料学报, 2023, 38(4): 421-428. |
| [14] | 游钧淇, 李策, 杨栋梁, 孙林锋. 氧化物双介质层忆阻器的设计及应用[J]. 无机材料学报, 2023, 38(4): 387-398. |
| [15] | 方仁瑞, 任宽, 郭泽钰, 徐晗, 张握瑜, 王菲, 张培文, 李悦, 尚大山. 基于氧化物基电解质栅控晶体管突触的关联学习[J]. 无机材料学报, 2023, 38(4): 399-405. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||