无机材料学报 ›› 2020, Vol. 35 ›› Issue (3): 390-398.DOI: 10.15541/jim20190314
所属专题: 2020年环境材料论文精选(二)重金属元素去除
• 研究论文 • 上一篇
董丽佳1,吴思颖1,李生波1,魏作富2,杨国1,胡保卫1
收稿日期:2019-06-26
修回日期:2019-08-28
出版日期:2020-03-20
网络出版日期:2019-09-12
作者简介:董丽佳(1984–), 女, 博士, 讲师. E-mail: Donglijia@126.com
DONG Lijia1,WU Siying1,LI Shengbo1,WEI Zuofu2,YANG Guo1,HU Baowei1
Received:2019-06-26
Revised:2019-08-28
Published:2020-03-20
Online:2019-09-12
About author:DONG Lijia (1984–), female, PhD, lecturer. E-mail: Donglijia@126.com
Supported by:摘要:
以农业残留物为原料制备的生物炭被广泛应用于去除重金属, 这对于环境保护具有双重意义。本研究以稻草为原料制备了生物炭, 通过系列静态实验和光谱技术研究其对重金属铕(Eu)的吸附行为及机理。研究发现溶液pH显著影响生物炭对Eu(III)的吸附量, 但不改变吸附反应时间; 腐殖酸/富里酸(HA/FA)在pH<7.0的溶液中能促进生物炭对Eu(III)的吸附, 而在pH>7.0的溶液中则抑制Eu(III)的吸附; 吸附过程主要涉及共沉淀或内表面络合机制; 该吸附属于化学吸附, 且吸附速率受内颗粒扩散过程的限制。此外, Freundlich模型对该吸附拟合最好, Langmuir模型显示稻草生物炭对Eu(III)的最大吸附量为40.717 mg/kg, 这可能与生物炭的层状结构和丰富的官能团有关; 热力学分析表明该吸附是自发的吸热过程。这些发现有利于评估稻草生物炭在去除水中重金属方面潜在的应用价值。
中图分类号:
董丽佳, 吴思颖, 李生波, 魏作富, 杨国, 胡保卫. 稻草生物炭对铕的吸附行为及机理研究[J]. 无机材料学报, 2020, 35(3): 390-398.
DONG Lijia, WU Siying, LI Shengbo, WEI Zuofu, YANG Guo, HU Baowei. Sorption Behaviors and Mechanisms of Eu(III) on Rice Straw-derived Biochar[J]. Journal of Inorganic Materials, 2020, 35(3): 390-398.

Fig. 2 Influence of ionic strength on Eu(III) sorption on biochar as a function of pH(a), and the relative distribution of Eu(III) species in solutions as a function of pH(b) T=(298±2) K, CEu(III)initial=10.0 mg/L, m/V=0.45 g/L
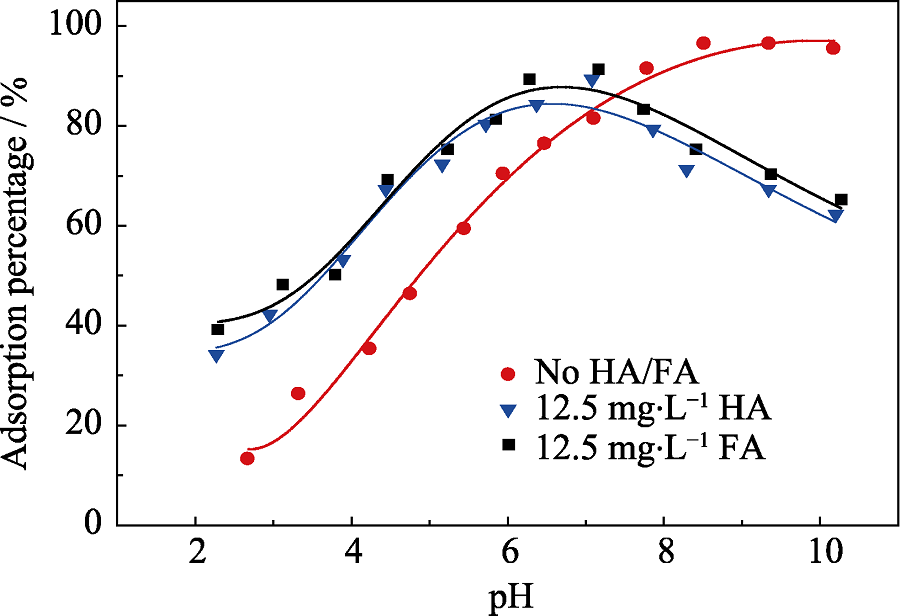
Fig. 3 Effect of HA/FA addition on Eu(III) sorption onto biochar under the conditions of T=(298±2) K, CEu(III)initial= 10.0 mg/L, m/V=0.45 g/L, CNaClO4=0.01 mol/L
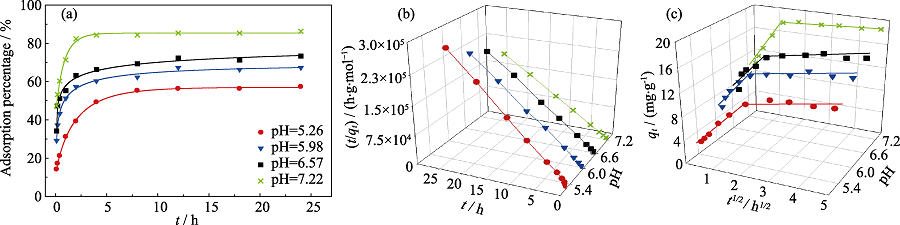
Fig. 4 Eu(III) sorption percentage on biochar as a function of contact time and pH (a), 3D plots of t/qt vs. t for pseudo-second-order model simulation (b), and qt vs. t1/2 for intra-particle diffusion model simulation at 4 pH (c) T=(298±2) K, CEu(III)initial=10.0 mg/L, m/V=0.45 g/L, CNaClO4=0.01 mol/L
| pH | Pseudo-second-order model | Intra-particle diffusion model | |||
|---|---|---|---|---|---|
| qe/(mol∙g-1) | K/(g∙mol-1∙h-1) | r2 | kint/(mg∙g-1∙h-1/2) | r2 | |
| 5.26 | 1.265 × 10-4 | 5.361 × 104 | 1.000 | 2.147 | 0.829 |
| 5.98 | 1.073 × 10-4 | 3.503 × 104 | 1.000 | 1.589 | 0.764 |
| 6.57 | 9.911 × 10-5 | 3.253 × 104 | 1.000 | 1.513 | 0.749 |
| 7.22 | 8.591 × 10-5 | 1.756 × 104 | 0.999 | 1.630 | 0.650 |
Table 1 Parameters of Eu(III) sorption kinetic on rice straw-derived biochar with 4 pH of solutions
| pH | Pseudo-second-order model | Intra-particle diffusion model | |||
|---|---|---|---|---|---|
| qe/(mol∙g-1) | K/(g∙mol-1∙h-1) | r2 | kint/(mg∙g-1∙h-1/2) | r2 | |
| 5.26 | 1.265 × 10-4 | 5.361 × 104 | 1.000 | 2.147 | 0.829 |
| 5.98 | 1.073 × 10-4 | 3.503 × 104 | 1.000 | 1.589 | 0.764 |
| 6.57 | 9.911 × 10-5 | 3.253 × 104 | 1.000 | 1.513 | 0.749 |
| 7.22 | 8.591 × 10-5 | 1.756 × 104 | 0.999 | 1.630 | 0.650 |
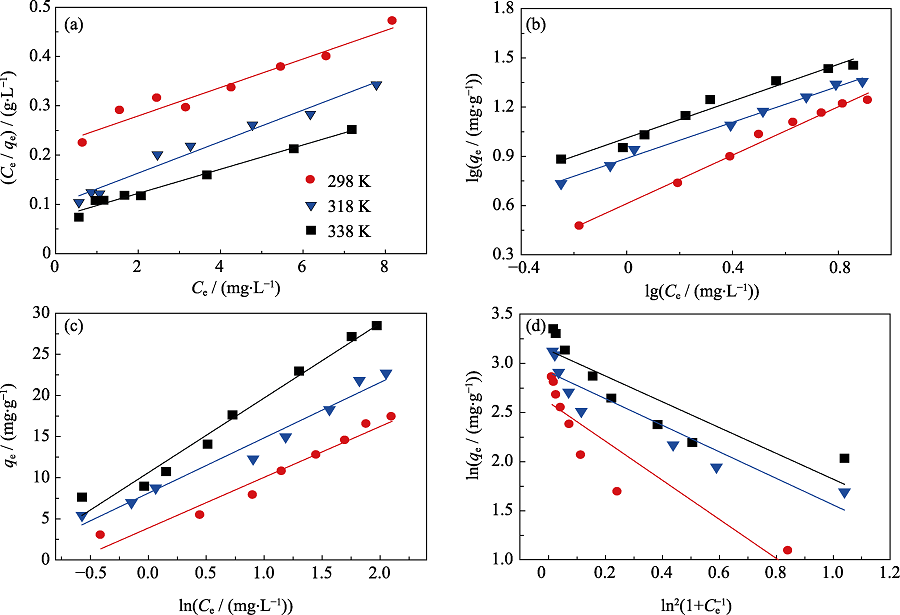
Fig. 6 Linearized Langmuir isotherm (a), Freundlich isotherm(b), Temkin isotherm (c), Dubinin-Radushkevich isotherm (d) of Eu(III) sorption on biochar at different temperatures (pH=5.5±0.2, CNaClO4=0.01 mol/L, m/V=0.45 g/L)
| T/K | Langmuir model | ||
|---|---|---|---|
| qmax/(mg∙g-1) | KL/(L∙mg-1) | r2 | |
| 298 | 34.626 | 0.130 | 0.950 |
| 318 | 31.289 | 0.322 | 0.974 |
| 338 | 40.717 | 0.336 | 0.984 |
| Freundlich model | |||
| KF/(mg1-n∙Ln∙g-1) | n | r2 | |
| 298 | 4.091 | 0.738 | 0.988 |
| 318 | 7.723 | 0.548 | 0.992 |
| 338 | 10.280 | 0.561 | 0.974 |
| Temkin model | |||
| A | b | r2 | |
| 298 | 1.875 | 401.551 | 0.957 |
| 318 | 3.342 | 374.482 | 0.970 |
| 338 | 3.217 | 304.874 | 0.978 |
| Dubinin-Radushkevich model | |||
| qmax/(mg∙g-1) | E/(J∙g-1) | r2 | |
| 298 | 13.518 | 0.502 | 0.824 |
| 318 | 18.320 | 0.609 | 0.873 |
| 338 | 22.920 | 0.617 | 0.801 |
Table 2 Parameters for Eu(III) sorption isotherms onto rice straw-derived biochar at different temperatures
| T/K | Langmuir model | ||
|---|---|---|---|
| qmax/(mg∙g-1) | KL/(L∙mg-1) | r2 | |
| 298 | 34.626 | 0.130 | 0.950 |
| 318 | 31.289 | 0.322 | 0.974 |
| 338 | 40.717 | 0.336 | 0.984 |
| Freundlich model | |||
| KF/(mg1-n∙Ln∙g-1) | n | r2 | |
| 298 | 4.091 | 0.738 | 0.988 |
| 318 | 7.723 | 0.548 | 0.992 |
| 338 | 10.280 | 0.561 | 0.974 |
| Temkin model | |||
| A | b | r2 | |
| 298 | 1.875 | 401.551 | 0.957 |
| 318 | 3.342 | 374.482 | 0.970 |
| 338 | 3.217 | 304.874 | 0.978 |
| Dubinin-Radushkevich model | |||
| qmax/(mg∙g-1) | E/(J∙g-1) | r2 | |
| 298 | 13.518 | 0.502 | 0.824 |
| 318 | 18.320 | 0.609 | 0.873 |
| 338 | 22.920 | 0.617 | 0.801 |

Fig. 7 Linear relationships of lnKd vs. Ce for Eu(III) sorption on biochar at different temperatures (a) and lnKθ vs. 1/T (b). (pH= 5.5±0.2, CNaClO4=0.01 mol/L, m/V=0.45 g/L)
| T/K | ΔGθ/(kJ∙mg-1) | ΔSθ/(J∙mg-1∙K-1) | ΔHθ/(kJ∙mg-1) |
|---|---|---|---|
| 298 | -20.708 | 142.169 | 21.659 |
| 318 | -24.059 | 142.169 | 21.151 |
| 338 | -26.351 | 142.169 | 21.703 |
Table 3 Thermodynamic parameters for Eu(III) sorption on rice straw-derived biochar at different temperatures
| T/K | ΔGθ/(kJ∙mg-1) | ΔSθ/(J∙mg-1∙K-1) | ΔHθ/(kJ∙mg-1) |
|---|---|---|---|
| 298 | -20.708 | 142.169 | 21.659 |
| 318 | -24.059 | 142.169 | 21.151 |
| 338 | -26.351 | 142.169 | 21.703 |
| [1] | HU B, HU Q, LI X , et al. Rapid and highly efficient removal of Eu(III) from aqueous solutions using graphene oxide. J. Mol. Liq., 2017,229(3):6-14. |
| [2] | WANG X X, YU S J, WANG X K . Removal of radionuclides by metal-organic framework-based materials. J. Inorg. Mater., 2019,34(1):17-26. |
| [3] | WANG N, PANG H, YU S , et al. Investigation of adsorption mechanism of layered double hydroxides and their composites on radioactive uranium: a review. Acta Chim. Sinica, 2019,77(2):143-152. |
| [4] | SHENG G, DONG H, SHEN R , et al. Microscopic insights into the temperature-dependent adsorption of Eu(III) onto titanate nanotubes studied by FTIR, XPS, XAFS and batch technique. Chem. Eng. J., 2013,217(2):486-494. |
| [5] | SHENG G, YANG Q, PENG F , et al. Determination of colloidal pyrolusite, Eu(III) and humic substance interaction: a combined batch and EXAFS approach. Chem. Eng. J., 2014,245(6):10-16. |
| [6] | SHENG G, YANG S, LI Y , et al. Retention mechanisms and microstructure of Eu(III) on manganese dioxide studied by batch and high resolution EXAFS technique. Radiochim. Acta, 2014,102(1/2):155-167. |
| [7] | ZHU Y, ZHENG C, WU S , et al. Interaction of Eu(III) on magnetic biochar investigated by batch, spectroscopic and modeling techniques. J. Radioanal. Nucl. Chem., 2018,316(6):1337-1346. |
| [8] | XIE Y, HELVENSTON E M, SHULLER-NICKLES L C , et al. Surface complexation modeling of Eu(III) and U(VI) interactions with graphene oxide. Environ. Sci. Technol., 2016,50(4):1821-1827. |
| [9] | BURNETT J L, CROUDACE I W, WARWICK P E . Pre-concentration of short-lived radionuclides using manganese dioxide precipitation from surface waters. J. Radioanal. Nucl. Chem., 2012,292(1):25-28. |
| [10] | PRAKASH D, GABANI P, CHANDEL A K , et al. Bioremediation: a genuine technology to remediate radionuclides from the environment. Microb. Biotechnol., 2013,6(4):349-360. |
| [11] | HASSAN K F, SPELLERBERG S, SCHOLTEN B , et al. Development of an ion-exchange method for separation of radioiodine from tellurium and antimony and its application to the production of 124I via the 121Sb (α, n)-process. J. Radioanal. Nucl. Chem., 2014,302(10):689-694. |
| [12] | SHENG G, YANG S, ZHAO D , et al. Adsorption of Eu(III) on titanate nanotubes studied by a combination of batch and EXAFS technique. Sci. China Chem., 2012,55(1):182-194. |
| [13] | LIU X, WU J, ZHANG S W , et al. Amidoxime functionalized hollow carbon spheres for efficient removal of uranium from wastewater. ACS Sustain. Chem. Eng., 2019,7(6):10800-10807. |
| [14] | AMBASHTA R D, SILLANPÄÄ M E . Membrane purification in radioactive waste management: a short review. J. Environ. Radioact, 2012,105(2):76-84. |
| [15] | KIM K W, BAEK Y J, LEE K Y , et al. Treatment of radioactive waste seawater by coagulation-flocculation method using ferric hydroxide and poly acrylamide. J. Nucl. Sci. Technol., 2015,53(3):439-450. |
| [16] | WANG X, CHEN Z, TAN X , et al. Effect of pH, humic acid and addition sequences on Eu(III) sorption onto γ-Al2O3 study by batch and time resolved laser fluorescence spectroscopy. Chem. Eng. J., 2016,287(3):313-320. |
| [17] | LIU X, SUN J, XU X , et al. Adsorption and desorption of U(VI) on different-size graphene oxide. Chem. Eng. J., 2019,360(3):941-950. |
| [18] | WANG X, CHEN Y, WU Y . Diffusion of Eu(III) in compacted bentonite-effect of pH, solution concentration and humic acid. Appl. Radiat. Isotopes, 2004,60(6):963-969. |
| [19] | WANG X, XU D, CHEN L , et al. Sorption and complexation of Eu(III) on alumina: effects of pH, ionic strength, humic acid and chelating resin on kinetic dissociation study. Appl. Radiat. Isotopes, 2006,64(4):414-421. |
| [20] | TAN X L, XU D, CHEN C L , et al. Adsorption and kinetic desorption study of 152+154Eu(III) on multiwall carbon nanotubes from aqueous solution by using chelating resin and XPS methods. Radiochim. Acta, 2008,96(1):23-29. |
| [21] | CHEN Z, HE J, CHEN L , et al. Sorption and desorption properties of Eu(III) on attapulgite. J. Radioanal. Nucl. Chem., 2016,307(2):1093-1104. |
| [22] | LIU X, MA R, WANG X , et al. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: a review. Environ. Pollut., 2019,252(5):62-73. |
| [23] | FRIŠTÁK V, MICHÁLEKOVÁ-RICHVEISOVÁ B, VÍGLAŠOVÁ E , et al. Sorption separation of Eu and As from single-component systems by Fe-modified biochar: kinetic and equilibrium study. J. Iran Chem. Soc., 2017,14(3):521-530. |
| [24] | HUANG Q, SONG S, CHEN Z , et al. Biochar-based materials and their applications in removal of organic contaminants from wastewater: state-of-the-art review. Biochar, 2019,1(1):45-73. |
| [25] | INYANG MI, GAO B, YAO Y , et al. A review of biochar at a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Env. Sci. Tec., 2015,46(2):406-433. |
| [26] | KLOSS S, ZEHETNER F, DELLANTONIO A , et al. Characterization of slow pyrolysis biochars: effects of feedstocks and pyrolysis temperature on biocharproperties. J. Environ. Qual., 2012,41(4):990-1000. |
| [27] | KOŁODYŃSKA D, KRUKOWSKA J, THOMAS P . Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J., 2017,307(1):353-363. |
| [28] | SHENG G, LI J, SHAO D , et al. Adsorption of copper(II) on multiwalled carbon nanotubes in the absence and presence of humic or fulvic acids. J. Hazard. Mater., 2010,178(1/2/3):333-340. |
| [29] | KIM W K, SHIM T, KIM Y S , et al. Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresource Technol., 2013,138(6):266-270. |
| [30] | TONG X J, LI J Y, YUAN J H , et al. Adsorption of Cu(II) by biochars generated from three crop straws. Chem. Eng. J., 2011,172(2/3):828-834. |
| [31] | ZONG P, WANG H, PAN H , et al. Application of NKF-6 zeolite for the removal of U(VI) from aqueous solution. J. Radioanal. Nucl. Chem., 2013,295(3):1969-1979. |
| [32] | DONG L, CHANG K, WANG L , et al. Application of biochar derived from rice straw for the removal of Th(IV) from aqueous solution. Sep. Sci. Technol., 2018,53(10):1511-1521. |
| [33] | YANG S, SHENG G, MONTAVON G , et al. Investigation of Eu(III) immobilization on γ-Al2O3 surfaces by combining batch technique and EXAFS analysis: role of contact time and humic acid. Geochim. Cosmochim. Acta, 2013,121(11):84-104. |
| [34] | CHEN C, WANG X, NAGATSU M . Europium adsorption on multiwall carbon nanotube/iron oxide magnetic composite in the presence of polyacrylic acid. Environ. Sci. Technol., 2009,43(7):2362-2367. |
| [35] | TAN X, WANG X, GECKEIS H , et al. Sorption of Eu(III) on humic acid or fulvic acid bound to hydrous alumina studied by SEMEDS, XPS, TRLFS and batch techniques. Environ. Sci. Technol., 2008,42(17):6532-6537. |
| [36] | PAN D, FAN Q, LI P , et al. Sorption of Th(IV) on Na-bentonite: effects of pH, ionic strength, humic substances and temperature. Chem. Eng. J., 2011,172(2/3):898-905. |
| [37] | LI Y, SHENG G, SHENG J . Magnetite decorated graphene oxide for the highly efficient immobilization of Eu(III) from aqueous solution. J. Mol. Liq., 2014,199(11):474-480. |
| [38] | WANG X X, YANG S B, SHI W Q , et al. Different interaction mechanisms of Eu(III) and 243Am(III) with carbon nanotubes studied by batch, spectroscopy technique and theoretical calculation. Environ. Sci. Technol., 2015,49(19):11721-11728. |
| [39] | WANG X, LU S, CHEN L , et al. Efficient removal of Eu(III) from aqueous solutions using super-adsorbent of bentonite-polyacrylamide composites. J. Radioanal. Nucl. Chem., 2015,306(2):497-505. |
| [40] | HO Y S . Review of second-order models for adsorption systems. J. Hazard. Mater., 2006,136(3):681-689. |
| [41] | HO Y S, MCKAY G . Pseudo-second order model for sorption processes. Process Biochem., 1999,34(5):451-465. |
| [42] | IJAGBEMI C O, BAEK M H, KIM D S . Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J. Hazard. Mater., 2009,166(1):538-546. |
| [43] | YANG S, HU J, CHEN C , et al. Mutual effects of Pb(II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions. Environ. Sci. Technol., 2011,45(8):3621-3627. |
| [44] | BISWAS K, SAHA S K, GHOSH U C . Adsorption of fluoride from aqueous solution by a synthetic iron(III)-auminum(III) mixed oxide. Ind. Eng. Chem. Res., 2007,46(16):5346-5356. |
| [45] | LI J, ZHANG S, CHEN C , et al. Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces, 2012,4(9):4991-5000. |
| [1] | 宋环, 王琳, 王宏青, 石伟群. 碱化Ti3C2Tx MXene对Eu(III)高效去除与机理研究[J]. 无机材料学报, 2020, 35(1): 65-72. |
| [2] | 张 俊,王德平,姚爱华,黄文旵. 纳米羟基磷灰石对Ni2+的吸附性能及机理研究[J]. 无机材料学报, 2009, 24(2): 269-274. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||