无机材料学报 ›› 2020, Vol. 35 ›› Issue (4): 439-446.DOI: 10.15541/jim20190260
所属专题: 功能陶瓷论文精选(一):发光材料; 【虚拟专辑】LED发光材料
陈婷1,2,徐彦乔1,江伟辉1,2,谢志翔1,王连军2,3,江莞2,3
收稿日期:2019-05-30
修回日期:2019-06-28
出版日期:2020-04-20
网络出版日期:2019-09-04
作者简介:陈 婷(1984-), 女, 博士, 副教授. E-mail: chenting@jci.edu.cn
基金资助:CHEN Ting1,2,XU Yanqiao1,JIANG Weihui1,2,XIE Zhixiang1,WANG Lianjun2,3,JIANG Wan2,3
Received:2019-05-30
Revised:2019-06-28
Published:2020-04-20
Online:2019-09-04
Supported by:摘要:
Cu-In-Zn-S(CIZS)量子点具有毒性低、发射谱覆盖范围广、Stokes位移大等特点, 在照明领域具有广阔的应用前景。通过离子液体辅助微波法水相合成CIZS量子点, 系统研究了反应时间、配体添加量和前驱体溶液pH对样品的物相组成、显微形貌以及荧光性能的影响。结果表明, 与未添加离子液体制备的样品相比, 离子液体的引入提高了反应速率, 可有效地将反应时间由180 min缩短至30 min; 随着反应时间的延长, 量子点的粒径增大, 其发射峰位由609.2 nm红移至634.6 nm。随着nGSH(谷胱甘肽)/n(CuInZn)的增大, 量子点的粒径逐渐增大, 导致其发射峰位由622.6 nm红移至631.6 nm, 同时量子点的发光强度逐渐增强; 当该比值为15时, 量子点的荧光强度最高。此外, 随着pH的增大, 去质子化的-SH和-NH2与量子点的作用逐渐增强, 有效地钝化了量子点的表面态, 使其荧光强度逐渐上升, 当pH为8.5时, 样品的荧光性能最佳, 同时量子点的平均水合粒径由99 nm增大至241 nm; 量子点溶液的Zeta电位为-27.7~-41.1 mV, 说明量子点溶液具有优异的稳定性。通过ZnS表面修饰可有效提高量子点的荧光强度。将CIZS/ZnS量子点与蓝光芯片结合, 获得了显色指数为85.6、发光效率为34.8 lm/W的白光LED器件, 为水相制备的多元量子点在白光LED中的应用提供了参考。
中图分类号:
陈婷, 徐彦乔, 江伟辉, 谢志翔, 王连军, 江莞. 离子液体辅助微波法水相合成Cu-In-Zn-S/ZnS量子点及其在白光LED中的应用[J]. 无机材料学报, 2020, 35(4): 439-446.
CHEN Ting, XU Yanqiao, JIANG Weihui, XIE Zhixiang, WANG Lianjun, JIANG Wan. Ionic Liquid Assisted Microwave Synthesis of Cu-In-Zn-S/ZnS Quantum Dots and Their Application in White LED[J]. Journal of Inorganic Materials, 2020, 35(4): 439-446.
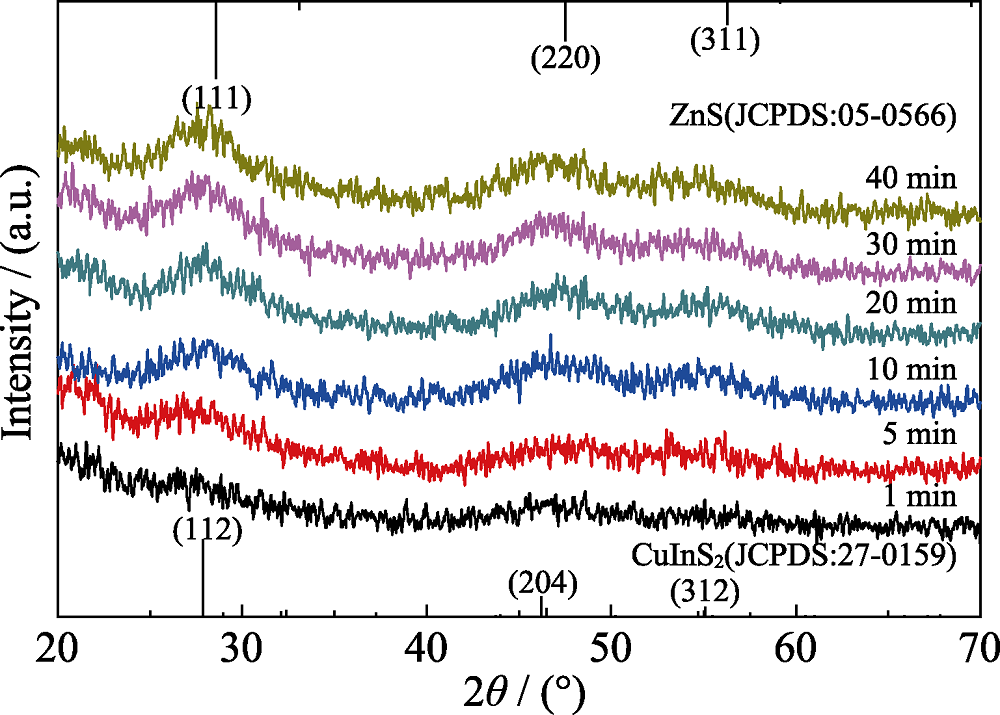
图1 添加离子液体, 在不同反应时间制备的CIZS量子点的XRD图谱(nGSH/n(CuInZn)=15, pH=8.5)
Fig. 1 XRD patterns of CIZS QDs prepared with different reaction time (with adding ionic liquid, nGSH/n(CuInZn)=15, pH=8.5)
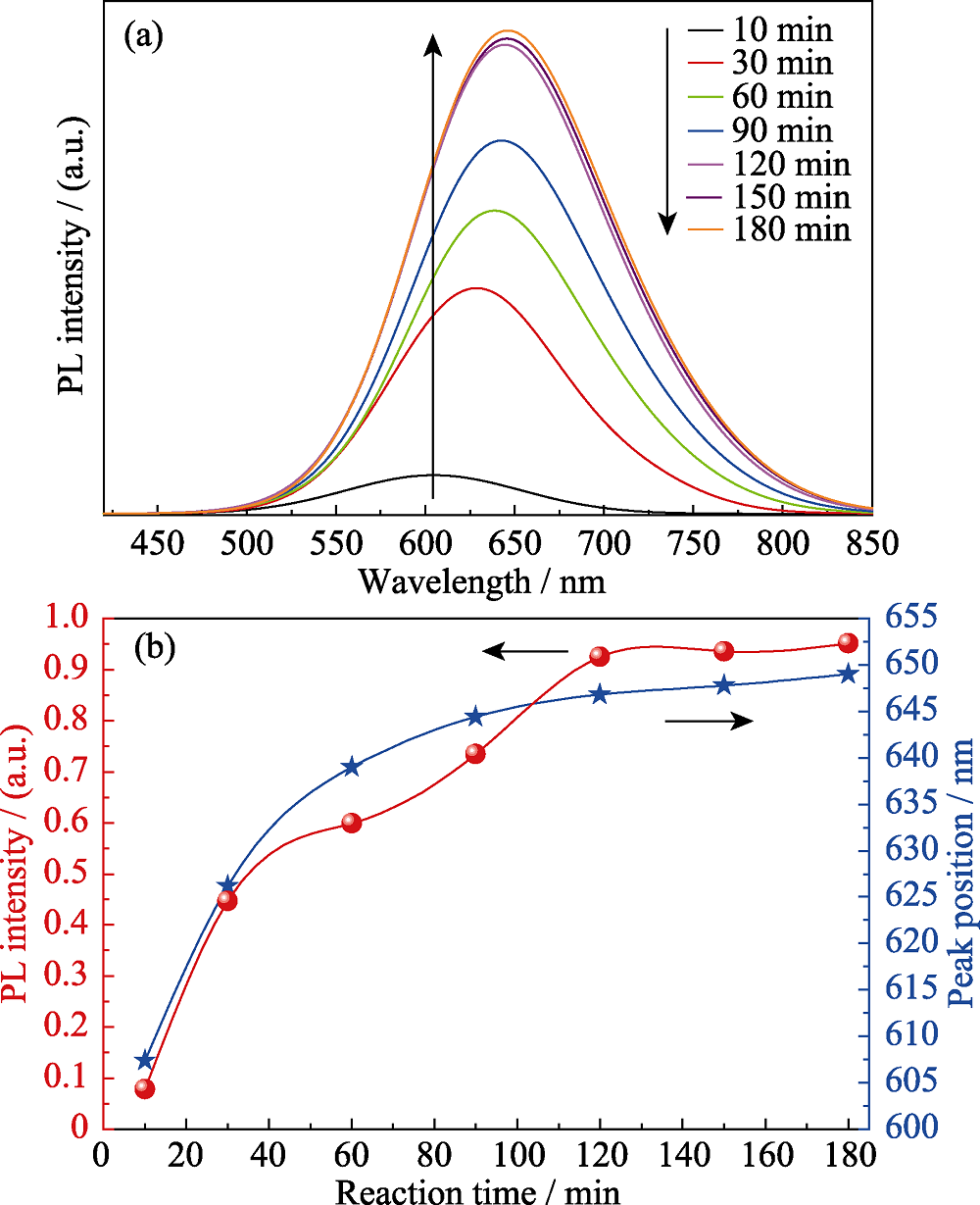
图2 未添加离子液体, 在不同反应时间制备的CIZS量子点的(a)荧光光谱和(b)发射强度-峰位的变化关系(nGSH/n(CuInZn)=15, pH=8.5)
Fig. 2 (a) PL spectra and (b) evolution of the emission intensity and peak position of CIZS QDs prepared with different reaction time (without ionic liquid, nGSH/n(CuInZn)=15, pH=8.5)
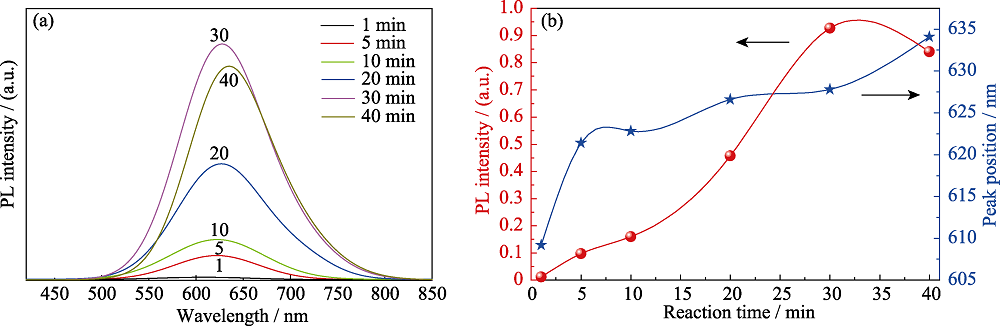
图3 添加离子液体, 在不同反应时间制备CIZS量子点(a)荧光光谱和(b)发射强度-峰位的变化关系(nGSH/n(CuInZn)=15, pH=8.5)
Fig. 3 (a) PL spectra and (b) evolution of the emission intensity and peak position of CIZS QDs prepared with different reaction time (with ionic liquid, nGSH/n(CuInZn)=15, pH=8.5)
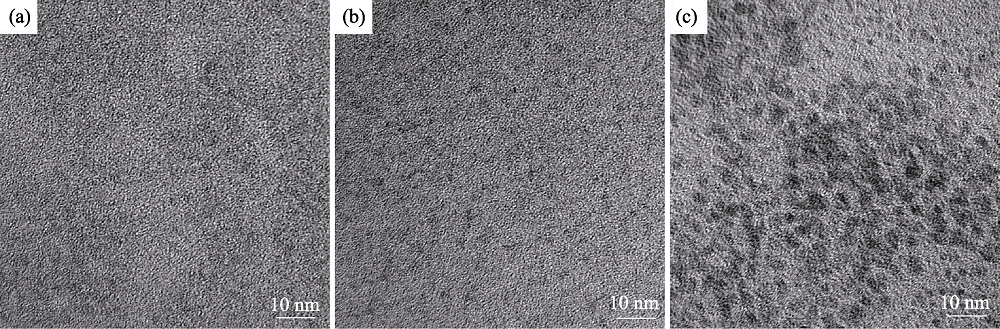
图4 添加离子液体, 在不同反应时间制备CIZS量子点的TEM照片(nGSH/n(CuInZn)=15, pH=8.5)
Fig. 4 TEM images of CIZS QDs prepared with ionic liquid for different reaction time (nGSH/n(CuInZn)=15, pH=8.5) (a) 10 min; (b) 20 min; (c) 30 min
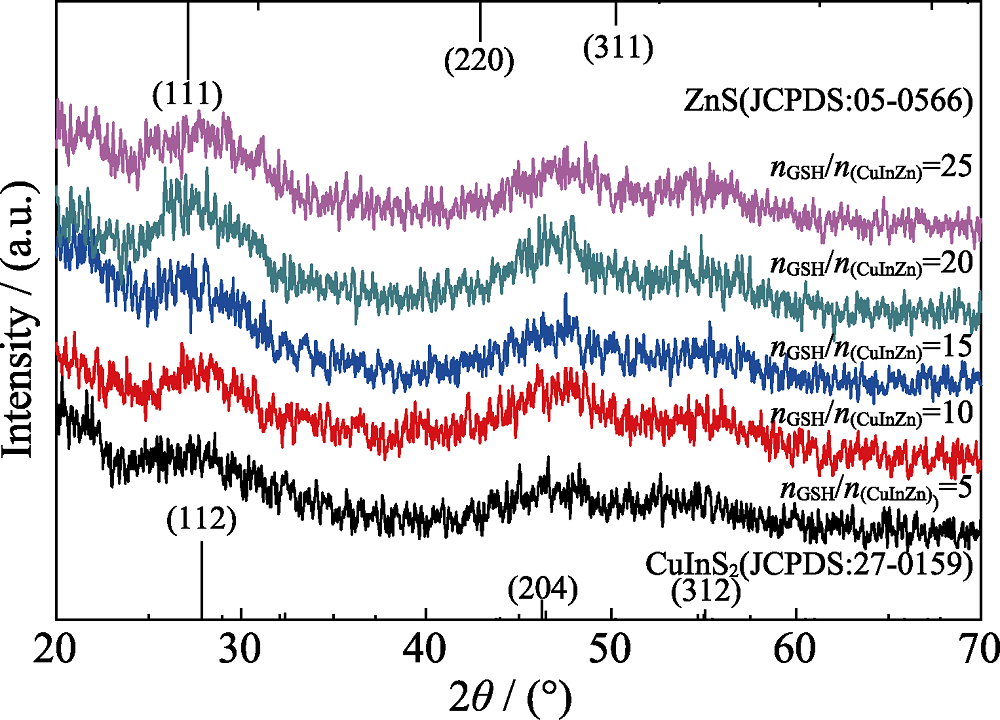
图5 添加离子液体, 不同nGSH/n(CuInZn)制备的CIZS量子点的XRD图谱(反应时间为30 min, pH=8.5)
Fig. 5 XRD patterns of CIZS QDs prepared with different nGSH/n(CuInZn) ratios (with ionic liquid for 30 min, pH=8.5)
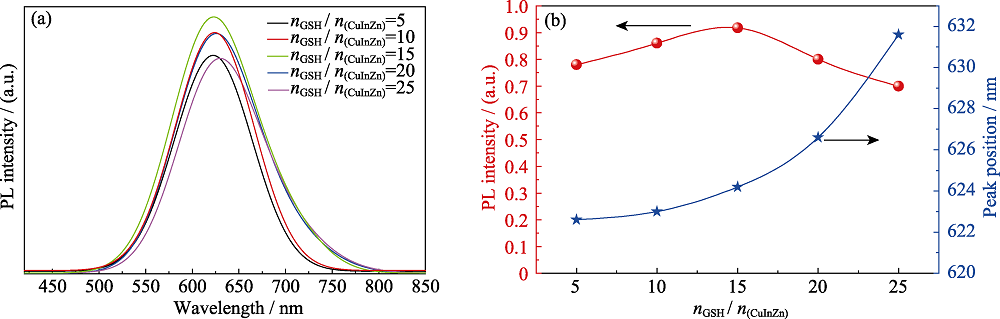
图6 添加离子液体, 不同nGSH/n(CuInZn)制备的CIZS量子点的(a)PL谱和(b)发射强度与峰位的变化关系(反应时间为30 min, pH=8.5)
Fig. 6 (a) PL spectra and (b) evolution of the emission intensity and peak position of CIZS QDs with different nGSH/n(CuInZn) ratios (with ionic liquid for 30 min, pH=8.5)
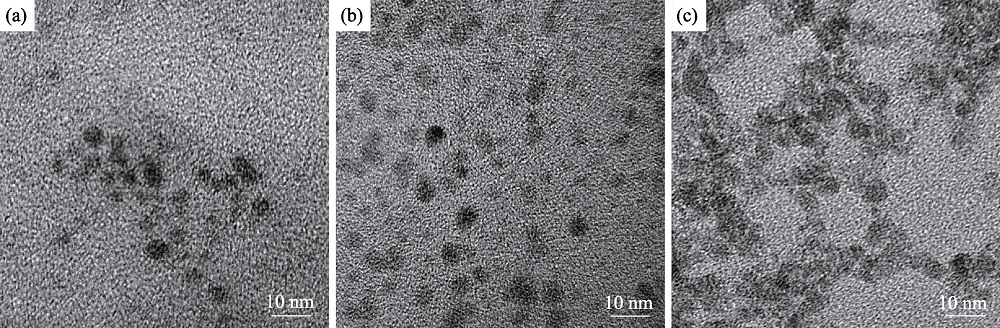
图7 添加离子液体, 不同nGSH/n(CuInZn)配比制备的CIZS量子点的TEM照片(反应时间为30 min, pH=8.5)
Fig. 7 TEM images of CIZS QDs prepared with nGSH/n(CuInZn) ratios (with ionic liquid for 30 min, pH=8.5) at (a) 5, (b) 15, and (c) 25
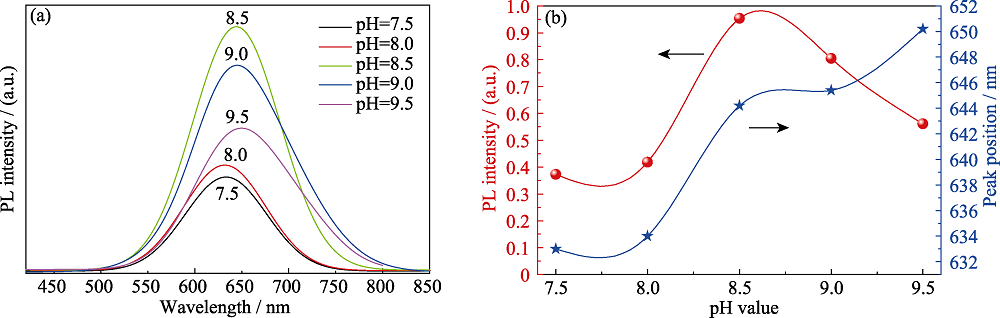
图8 添加离子液体, 不同pH条件下制备的CIZS量子点的(a)PL谱和(b)发射强度-峰位的变化关系(反应时间为30 min, nGSH/n(CuInZn)=15)
Fig. 8 (a) PL spectra and (b) evolution of the emission intensity and peak position of CIZS QDs prepared with different pH (with ionic liquid for 30 min, nGSH/n(CuInZn)=15)
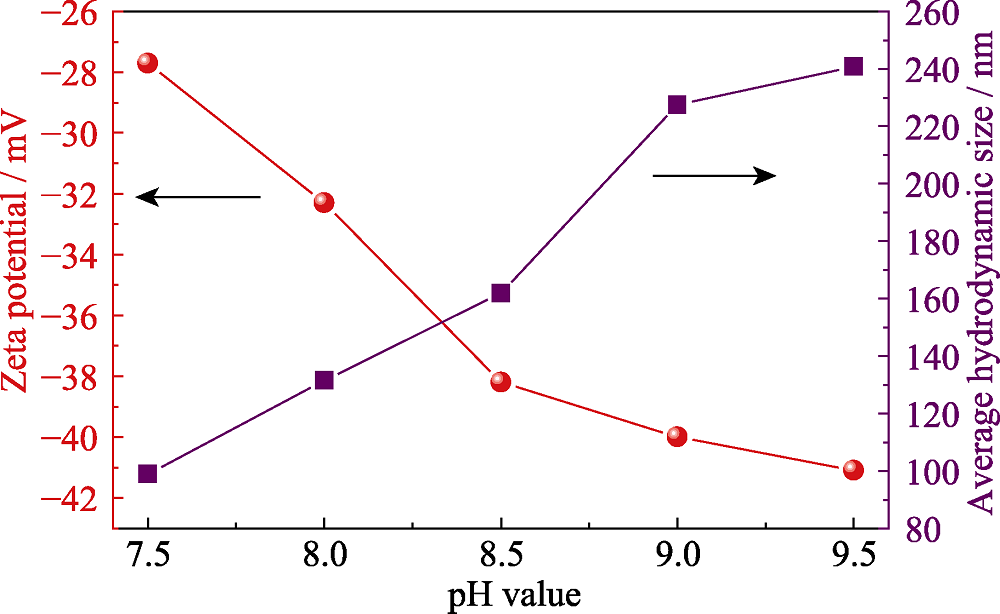
图9 添加离子液体, 不同pH制备的CIZS量子点的Zeta电位与平均水合粒径的关系(反应时间为30 min, nGSH/n(CuInZn)=15)
Fig. 9 Zeta potential and average hydrodynamic size of CIZS QDs with different pH (with ionic liquid for 30 min, nGSH/n(CuInZn)=15)
| [1] | YE S, XIAO F, PAN Y X , et al. Phosphors in phosphor-converted white light-emitting diodes: recent advances in materials, techniques and properties. Mater. Sci. Eng. R, 2010,71(1):1-34. |
| [2] | HOERDER G J, SEIBALD M, BAUMANN D , et al. Sr[Li2Al2O2N2]:Eu2+- a high performance red phosphor to brighten the future. Nat. Commun., 2019,10:1824. |
| [3] | WANG L, XIE R J, SUEHIRO T , et al. Down-conversion nitride materials for solid state lighting: recent advances and perspectives. Chem. Rev., 2018,118(4):1951-2009. |
| [4] | PIETRYGA J M, PARK Y S, LIM J , et al. Spectroscopic and device aspects of nanocrystal quantum dots. Chem. Rev., 2016,116(18):10513-10622. |
| [5] | DAICHO H, IWASAKI T, ENOMOTO K , et al. A novel phosphor for glareless white light-emitting diodes. Nat. Commun., 2012,3:1132. |
| [6] | GUPTA K V K, MULEY A, YADAV P , et al. Combustion synthesis of YAG:Ce and related phosphors. Appl. Phys. B, 2011,105(2):479-484. |
| [7] | KANG X, YANG Y, WANG L , et al. Warm white light emitting diodes with gelatin-coated AgInS2/ZnS core/shell quantum dots. ACS Appl. Mater. Interfaces, 2015,7(50):27713-27719. |
| [8] | CHUANG P H, LIN C C, LIU R S . Emission-tunable CuInS2/ZnS quantum dots: structure, optical properties, and application in white light-emitting diodes with high color rendering index. ACS Appl. Mater. Interfaces, 2014,6(17):15379-15387. |
| [9] | ILAIYARAJA P, MOCHERLA P S V, SRINIVASAN T K , et al. Synthesis of Cu-deficient and Zn-graded Cu-In-Zn-S quantum dots and hybrid inorganic-organic nanophosphor composite for white light emission. ACS Appl. Mater. Interfaces, 2016,8(19):12456-12465. |
| [10] | JO D Y, YANG H . Spectral broadening of Cu-In-Zn-S quantum dot color converters for high color rendering white lighting device. J. Lumin., 2015,166:227-232. |
| [11] | GUGULA K, STEGEMANN L, CYWINSKI P J , et al. Facile surface engineering of CuInS2/ZnS quantum dots for LED down- converters. RSC Adv., 2016,6(12):10086-10093. |
| [12] | CHEN J, LI Y, WANG L , et al. Achieving deep-red-to-near- infrared emissions in Sn-doped Cu-In-S/ZnS quantum dots for red-enhanced white LEDs and near-infrared LEDs. Nanoscale, 2018,10(20):9788-9795. |
| [13] | CHEN F, YAO Y, LIN H , et al. Synthesis of CuInZnS quantum dots for cell labelling applications. Ceram. Int., 2018,44(Suppl.1):S34-S37. |
| [14] | CHEN B, ZHONG H, WANG M , et al. Integration of CuInS2- based nanocrystals for high efficiency and high colour rendering white light-emitting diodes. Nanoscale, 2013,5(8):3514-3519. |
| [15] | XIE R, RUTHERFORD M, PENG X . Formation of high-quality I-III-VI semiconductor nanocrystals by tuning relative reactivity of cationic precursors. J. Am. Chem. Soc., 2009,131(15):5691-5697. |
| [16] | ZHANG W, LOU Q, JI W , et al. Color-tunable highly bright photoluminescence of cadmium-free Cu-doped Zn-In-S nanocrystals and electroluminescence. Chem. Mater., 2014,26(2):1204-1212. |
| [17] | KIM J H, YANG H . High-efficiency Cu-In-S quantum-dot- light-emitting device exceeding 7%. Chem. Mater., 2016,28(17):6329-6335. |
| [18] | WU R, WANG T, WU M , et al. Synthesis of highly stable CuInZnS/ ZnS//ZnS quantum dots with thick shell and its application to quantitative immunoassay. Chem. Eng. J., 2018,348:447-454. |
| [19] | CHEN X, CHEN S, XIA T , et al. Aqueous synthesis of high quality multicolor Cu-Zn-In-S quantum dots. J. Lumin., 2017,188:162-167. |
| [20] | LIU Y, CHEN X, MA Q . An efficient microwave-assisted hydrothermal synthesis of high-quality CuInZnS/ZnS quantum dots. New J. Chem., 2018,42(6):4102-4108. |
| [21] | JIANG T, SONG J, WANG H , et al. Aqueous synthesis of color tunable Cu doped Zn-In-S/ZnS nanoparticles in the whole visible region for cellular imaging. J. Mater. Chem. B, 2015,3(11):2402-2410. |
| [22] | XU Y, CHEN T, HU X , et al. The off-stoichiometry effect on the optical properties of water-soluble copper indium zinc sulfide quantum dots. J. Colloid Interf. Sci., 2017,496:479-486. |
| [23] | 陈婷, 徐彦乔, 王连军 . 一种离子液体辅助微波法合成I-III-VI族多元量子点的制备方法及其制得的产品. 中国, CN201610920307. 2. 2018.08.03. |
| [24] | ZHANG J, XIE R, YANG W . A simple route for highly luminescent quaternary Cu-Zn-In-S nanocrystal emitters. Chem. Mater., 2011,23(14):3357-3361. |
| [25] | CHENG J, LI D, CHENG T , et al. Aqueous synthesis of high- fluorescence CdZnTe alloyed quantum dots. J. Alloys. Compd., 2014,589:539-544. |
| [26] | ZHANG J, SUN W, YIN L , et al. One-pot synthesis of hydrophilic CuInS2 and CuInS2-ZnS colloidal quantum dots. J. Mater. Chem. C, 2014,2(24):4812-4817. |
| [27] | YU Y, XU L, CHEN J , et al. Hydrothermal synthesis of GSH- TGA co-capped CdTe quantum dots and their application in labeling colorectal cancer cells. Colloid. Surface B, 2012,95:247-253. |
| [28] | CHEN Y, LI W, WANG J , et al. Microwave-assisted ionic liquid synthesis of Ti3+ self-doped TiO2 hollow nanocrystals with enhanced visible-light photoactivity. Appl. Catal. B, 2016,191:94-105. |
| [29] | ZHU Y J, CHEN F . Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev., 2014,114(12):6462-6555. |
| [30] | VEMPATI S, ERTAS Y, UYAR T . Sensitive surface states and their passivation mechanism in CdS quantum dots. J. Phys. Chem. C, 2013,117(41):21609-21618. |
| [31] | LENG Z, HUANG L, SHAO F , et al. Facile synthesis of Cu-In-Zn-S alloyed nanocrystals with temperature-dependent photoluminescence spectra. Mater. Lett., 2014,119:100-103. |
| [32] | DING Y, SHEN S Z, SUN H , et al. Synthesis of L-glutathione- capped-ZnSe quantum dots for the sensitive and selective determination of copper ion in aqueous. Sensor. Actuat. B, 2014,203:35-43. |
| [33] | WANG Y, LIANG X, MA X , et al. Simple and greener synthesis of highly photoluminescence Mn2+-doped ZnS quantum dots and its surface passivation mechanism. Appl. Surf. Sci., 2014,316:54-61. |
| [34] | LUO Z, ZHANG H, HUANG J , et al. One-step synthesis of water- soluble AgInS2 and ZnS-AgInS2 composite nanocrystals and their photocatalytic activities. J. Colloid Interf. Sci., 2012,377(1):27-33. |
| [35] | ZHANG J, LI J, ZHANG J , et al. Aqueous synthesis of ZnSe nanocrystals by using glutathione as ligand: the pH-mediated coordination of Zn2+ with glutathione. J. Phys. Chem. C, 2010,114(25):11087-11091. |
| [36] | REGULACIO M D, WIN K Y, LO S L , et al. Aqueous synthesis of highly luminescent AgInS2-ZnS quantum dots and their biological applications. Nanoscale, 2013,5(6):2322-2327. |
| [37] | KO M, YOON H C, YOO H , et al. Highly efficient green Zn-Ag- In-S/Zn-In-S/ZnS QDs by a strong exothermic reaction for down- converted green and tripackage white LEDs. Adv. Funct. Mater., 2017,27(4):1602638. |
| [38] | LIU Z, TANG A, WANG M , et al. Heating-up synthesis of cadimum-free and color-tunable quaternary and five-component Cu-In-Zn-S-based semiconductor nanocrystals. J. Mater. Chem. C, 2015,3(39):10114-10120. |
| [1] | 刘文龙, 赵瑾, 刘娟, 毛小建, 章健, 王士维. 微波干燥自发凝固成型氧化铝湿坯[J]. 无机材料学报, 2023, 38(4): 461-468. |
| [2] | 陈勇强, 王怡雪, 张帆, 李红霞, 董宾宾, 闵志宇, 张锐. 微波加热制备特种陶瓷材料研究进展[J]. 无机材料学报, 2022, 37(8): 841-852. |
| [3] | 张笑宇, 刘永盛, 李然, 李耀刚, 张青红, 侯成义, 李克睿, 王宏志. 基于Cu3(HHTP)2薄膜的离子液体电致变色电极[J]. 无机材料学报, 2022, 37(8): 883-890. |
| [4] | 姚晓刚, 彭海益, 顾忠元, 何飞, 赵相毓, 林慧兴. 聚苯醚/钙镧钛微波复合基板[J]. 无机材料学报, 2022, 37(5): 493-498. |
| [5] | 曹志军, 李在均. 钌-生物质碳人工酶的制备及在比色检测杀虫剂毒死蜱残留中的应用[J]. 无机材料学报, 2022, 37(5): 554-560. |
| [6] | 张枫娟, 韩博宁, 曾海波. 钙钛矿量子点光伏与荧光聚光电池: 现状与挑战[J]. 无机材料学报, 2022, 37(2): 117-128. |
| [7] | 付宇坤, 曾敏, 饶先发, 钟盛文, 张慧娟, 姚文俐. 锂离子电池高镍LiNi0.8Mn0.2O2正极材料的微波合成及其Co、Al共改性[J]. 无机材料学报, 2021, 36(7): 718-724. |
| [8] | 田建建, 马霞, 王敏, 姚鹤良, 华子乐, 张玲霞. 锡量子点制备及其电催化还原二氧化碳产甲酸性能[J]. 无机材料学报, 2021, 36(12): 1337-1342. |
| [9] | 牟庭海, 许文涛, 凌军荣, 董天文, 秦梓轩, 周有福. 微波烧结制备ZrO2-AlN复合陶瓷的微观结构与性能研究[J]. 无机材料学报, 2021, 36(11): 1231-1236. |
| [10] | 舒孟洋, 陆嘉琳, 张志洁, 沈涛, 徐家跃. CsPbBr3钙钛矿量子点/C3N4超薄纳米片0D/2D复合材料: 增强的稳定性和光催化活性[J]. 无机材料学报, 2021, 36(11): 1217-1222. |
| [11] | 朱正旺,冯锐,柳扬,张扬,谢文翰,董丽杰. 类鱼骨结构CoFe2O4纳米纤维的制备与性能[J]. 无机材料学报, 2020, 35(9): 1011-1016. |
| [12] | 李孟夏, 陆越, 王利斌, 胡先罗. Mn3O4@ZnO核壳结构纳米片阵列的可控合成及其在水系锌离子电池中的应用[J]. 无机材料学报, 2020, 35(1): 86-92. |
| [13] | 李盛菘, 郑永超, 孟澍临, 吴骊珠, 钟近艺, 赵冲林. 核壳型量子点-纳米金颗粒组装体高效检测神经性毒剂模拟剂[J]. 无机材料学报, 2019, 34(8): 893-898. |
| [14] | 刘旸, 于姗, 郑凯文, 陈维维, 董兴安, 董帆, 周莹. N-Bi2O2CO3/CdSe量子点光催化氧化NO及原位红外光谱研究[J]. 无机材料学报, 2019, 34(4): 425-432. |
| [15] | 杨英,潘德群,张政,陈甜,韩晓敏,张力松,郭学益. Ag2Se量子点共敏化固态染料敏化太阳能电池光电性能研究[J]. 无机材料学报, 2019, 34(2): 137-144. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||