无机材料学报 ›› 2020, Vol. 35 ›› Issue (5): 532-540.DOI: 10.15541/jim20190237
所属专题: 封面文章; 2020年能源材料论文精选(一) :金属离子电池&燃料电池
收稿日期:2019-05-20
修回日期:2019-06-04
出版日期:2020-05-20
网络出版日期:2019-06-17
作者简介:王佳宁(1991-), 女, 博士研究生. E-mail: wangjn@shanghaitech.edu.cn<br/>WANG Jianing(1991-), female, PhD candidate. E-mail: wangjn@shanghaitech.edu.cn
基金资助:
WANG Jianing1,2,JIN Jun1,WEN Zhaoyin1( )
)
Received:2019-05-20
Revised:2019-06-04
Published:2020-05-20
Online:2019-06-17
Supported by:摘要:
采用自组装及热处理方法合成α-MoC1-x纳米晶富集的纳米碳球(α-MoC1-x/CNS), 并将其涂覆在商用聚丙烯隔膜上, 对隔膜实现了界面修饰。电化学性能显示, 与普通的聚丙烯隔膜相比, 采用修饰的α-MoC1-x/CNS-PP隔膜组装的锂硫电池的循环稳定性和倍率性能均得到明显提升, 在0.5C条件下, 电池首周放电比容量提升至1129.7 mAh/g, 经过100周充放电循环后, 电池仍具有855.5 mAh/g的放电比容量, 且在此循环过程中, 库伦效率始终大于98%。在自放电测试中, 电池经过48 h静置后的容量损失率仅为7.7%。结合α-MoC1-x/CNS的微观形貌及XPS分析可知, 在锂硫电池充放电过程中, α-MoC1-x/CNS修饰层有效地阻挡了多硫化锂向负极侧的扩散迁移, 且当α-MoC1-x与多硫离子接触时能产生Mo-S键、硫代和连多硫酸根产物, 进一步巩固了活性物质被约束的程度, 从而使电池性能得到提升。
中图分类号:
王佳宁, 靳俊, 温兆银. α-MoC1-x纳米晶富集碳球修饰隔膜对锂硫电池性能的影响[J]. 无机材料学报, 2020, 35(5): 532-540.
WANG Jianing, JIN Jun, WEN Zhaoyin. Application of Separators Modified by Carbon Nanospheres Enriched with α-MoC1-x Nanocrystalline in Lithium Sulfur Batteries[J]. Journal of Inorganic Materials, 2020, 35(5): 532-540.

图1 (a, d)α-MoC1-x/CNS前驱体和(b~c, e)α-MoC1-x/CNS的SEM照片; (f)α-MoC1-x/CNS的Mo、C、O元素分布图和(g~i)TEM照片
Fig. 1 SEM images of (a, d) the precursor of the α-MoC1-x/CNS composite and (b-c, e) the α-MoC1-x/CNS composite; (f) Mo, C and O element mappings and (g-i) TEM images of the α-MoC1-x/CNS composite
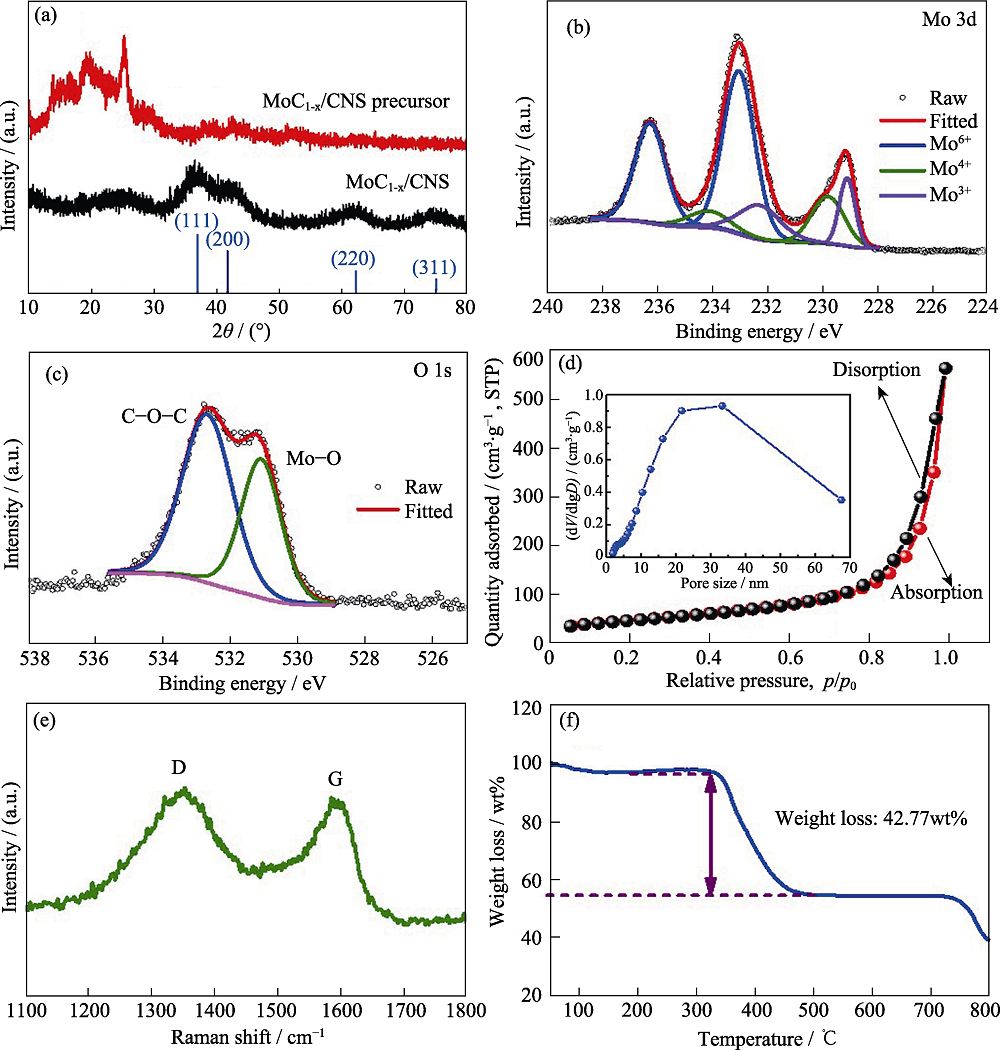
图2 (a)α-MoC1-x/CNS及其前驱体的XRD图谱; α-MoC1-x/CNS的(b)Mo 3d和(c)O 1s XPS图谱, (d)氮气吸附-脱附等温曲线(插图为孔径分布图), (e)Raman光谱和(f)在空气中的热重分析图
Fig. 2 (a) XRD patterns of the α-MoC1-x/CNS composite and its precursor; (b) Mo 3d and (c) O 1s XPS spectra, (d) N2 adsorption-desorption isotherm (inset: pore size distribution), (e) Raman spectrum, (f) TGA curve (under air flow) of the α-MoC1-x/CNS composite
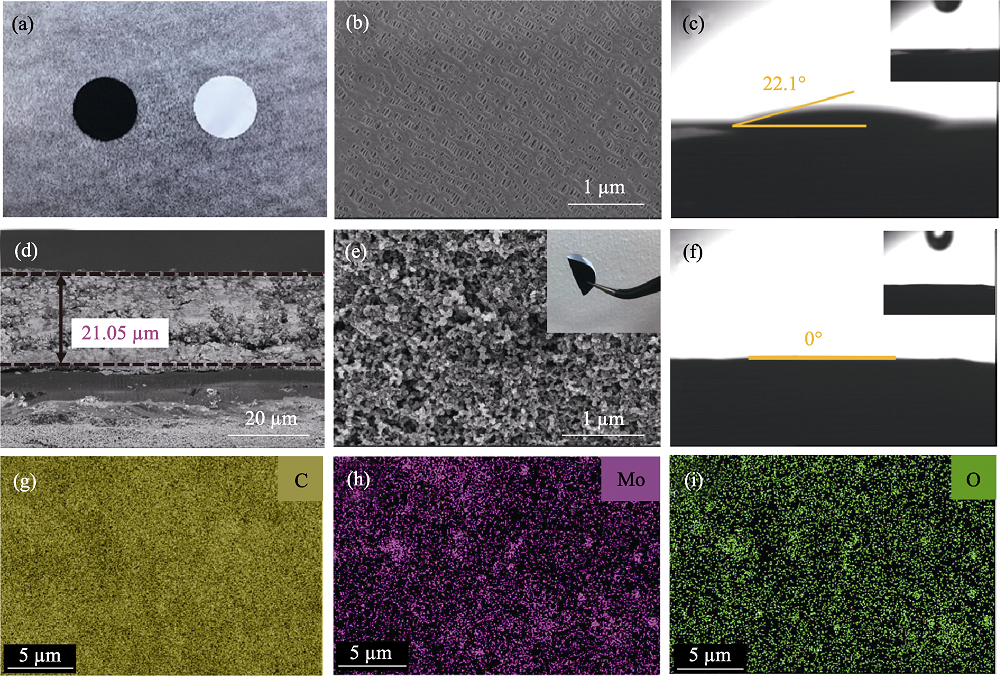
图3 (a)α-MoC1-x/CNS-PP的表面及背面两侧的照片; Celgard 2400的(b)表面SEM照片, (c)电解液接触角测试; α-MoC1-x/CNS-PP的(d)截面和(e)表面SEM照片(插图为α-MoC1-x/CNS-PP折叠后的照片), (f)电解液接触角测试; (g~i)图(e)所对应的C、O、Mo元素分布图
Fig. 3 (a) Photograph of the synthesized α-MoC1-x/CNS-PP separator with positive and negative sides; (b) SEM image and (c) measurement of the electrolyte contact angle for Celgard 2400 separator; (d) Typical cross-sectional, (e) surface SEM images and (f) measurement of the electrolyte contact angle for α-MoC1-x/CNS-PP separator; (g-i) Corresponding elemental mappings of C, O, Mo in (e)
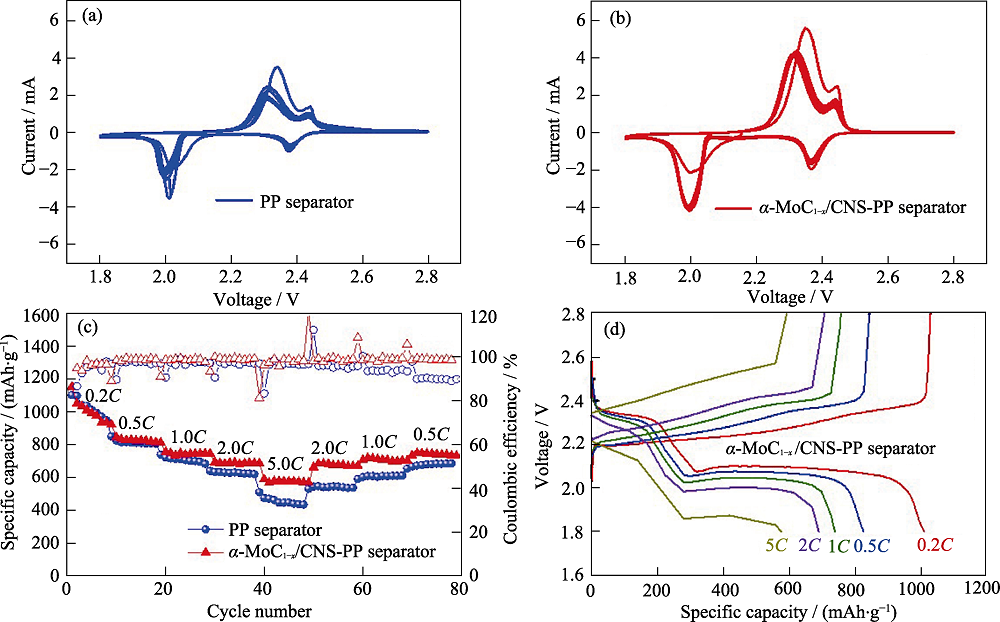
图4 采用(a)Celgard 2400和(b)α-MoC1-x/CNS-PP的锂硫电池的循环伏安曲线, (c)普通Celgard 2400和α-MoC1-x/CNS-PP的锂硫电池倍率性能, (d)不同电流密度下α-MoC1-x/CNS-PP的锂硫电池的充放电曲线
Fig. 4 Cyclic voltammograms of the Li-S battery with (a) Celgard 2400 separator and (b) α-MoC1-x/CNS-PP separator; (c) Rate performances with different separators at various current densities; (d) Charge-discharge voltage profiles at various current densities of the Li-S battery with α-MoC1-x/CNS-PP separator
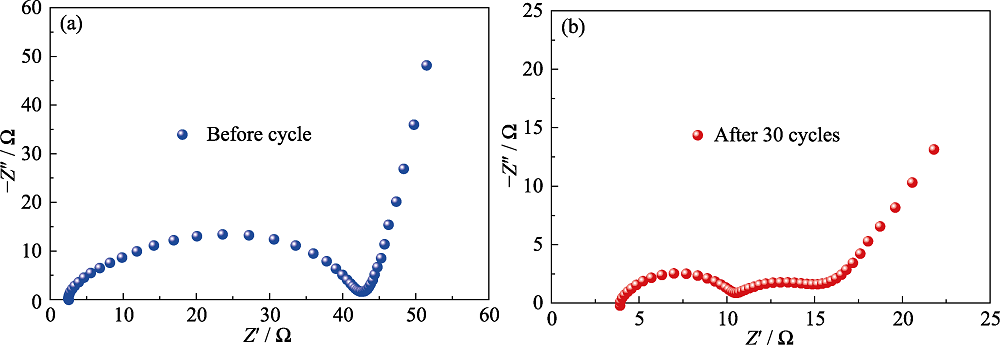
图5 采用α-MoC1-x/CNS-PP的锂硫电池在(a)循环前和(b)循环30周后的阻抗图谱
Fig. 5 Electrochemical impedance plots of Li-S battery with α-MoC1-x/CNS-PP separator (a) before and (b) after 30 cycles
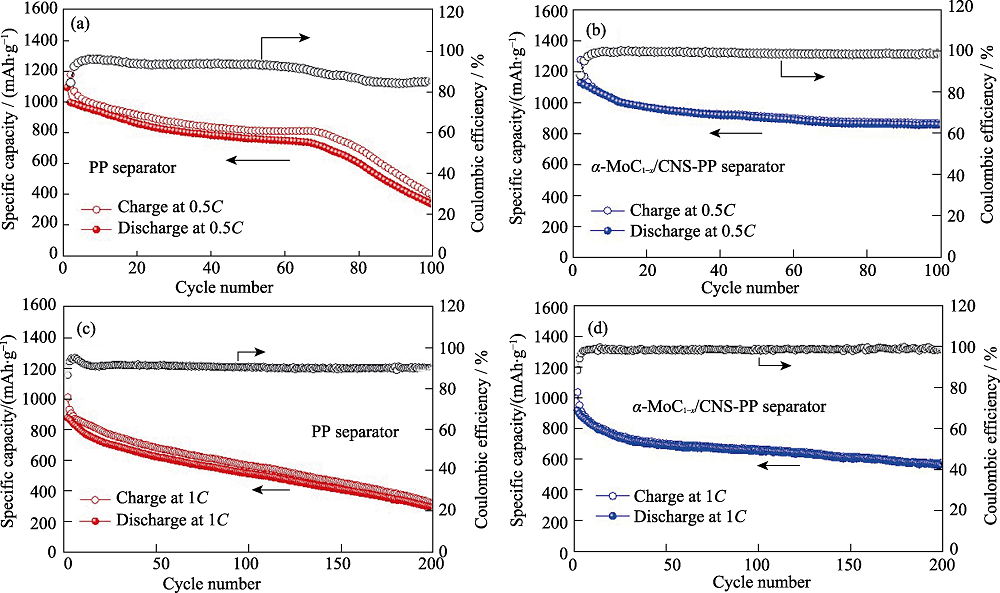
图6 采用(a, c)Celgard 2400和(b, d)α-MoC1-x/CNS-PP的锂硫电池在(a~b)0.5C和(c~d)1C电流密度下的循环性能
Fig. 6 Cycling performance of the Li-S battery with (a, c) Celgard 2400 separator and (b, d) α-MoC1-x/CNS-PP separator at (a-b) 0.5C and (c-d) 1C
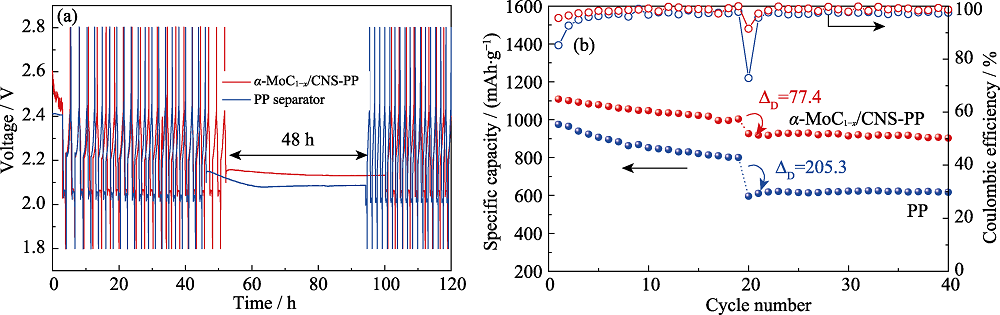
图7 采用Celgard 2400和α-MoC1-x/CNS-PP的电池的自放电测试
Fig. 7 Self-discharge tests for lithium sulfur batteries with Celgard 2400 separator or α-MoC1-x/CNS-PP separator (a) Discharge-charge profiles within 120 h; (b) Corresponding cycling performance
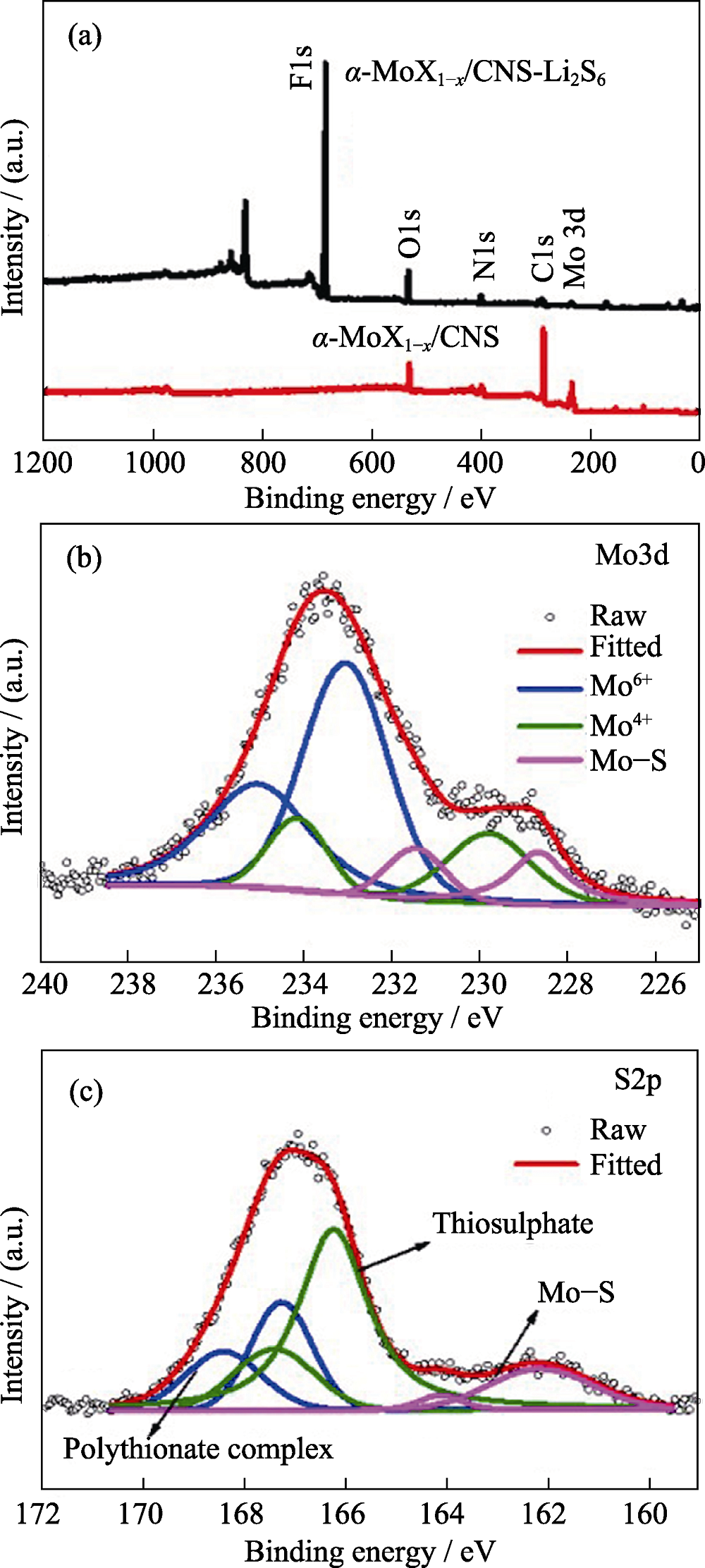
图8 (a)α-MoC1-x/CNS与α-MoC1-x/CNS-Li2S6的XPS图谱; α-MoC1-x/CNS-Li2S6的(b)Mo3d和(c)S2p XPS图谱
Fig. 8 (a) XPS survey spectra of α-MoC1-x/CNS and α-MoC1-x/CNS-Li2S6; (b) Mo3d and (c) S2p XPS core level spectra of α-MoC1-x/CNS-Li2S6
| [1] | BERG E J, VILLEVIEILLE C, STREICH D , et al. Rechargeable batteries: grasping for the limits of chemistry. J. Electrochem. Soc., 2015,162(14):A2468-A2475. |
| [2] |
MANTHIRAM A, FU Y Z, CHUNG S H , et al. Rechargeable lithium- sulfur batteries. Chem. Rev., 2014,114(23):11751-11787.
DOI URL PMID |
| [3] | AO X, WU W X, WU T , et al. Operating temperature on cathode material and electrochemical performance of Na-NiCl2 batteries. J. Inorg. Mater., 2017,32(12):1243-1249. |
| [4] |
YIN Y X, XIN S, GUO Y G , et al. Lithium-sulfur batteries: electrochemistry, materials, and prospects. Angew. Chem. Int. Ed., 2013,52(50):13186-13200.
DOI URL PMID |
| [5] | WANG Y H, JIN J, GUO Z S , et al. Direct view for the deformation evolution of sulfur electrode during Li-S battery cycling. J. Inorg. Mater., 2017,32(3):247-251. |
| [6] |
HASSOUN J, SCROSATI B . A high-performance polymer tin sulfur lithium ion battery. Angew. Chem. Int. Ed., 2010,49(13):2371-2374.
DOI URL PMID |
| [7] |
LIN Z, LIU Z C, DUDNEY N J , et al. Lithium superionic sulfide cathode for all-solid lithium-sulfur batteries. ACS Nano, 2013,7(3):2829-2833.
DOI URL PMID |
| [8] |
KIM J W, OCON J D, PARK D W , et al. Functionalized graphene- based cathode for highly reversible lithium-sulfur batteries. ChemSusChem, 2014,7(5):1265-1273.
DOI URL PMID |
| [9] | DIAO Y, XIE K, HONG X B , et al. Analysis of the sulfur cathode capacity fading mechanism and review of the latest development for Li-S battery. Acta Chim. Sin., 2013,71(4):508-518. |
| [10] |
MIKHAYLIK Y V, AKRIDGE J R . Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc., 2004,151(11):A1969-A1976.
DOI URL PMID |
| [11] | JI X L, NAZAR L F . Advances in Li-S batteries. J. Mater. Chem., 2010,20(44):9821-9826. |
| [12] | XU G Y, DING B, PAN J , et al. High performance lithium-sulfur batteries: advances and challenges. J. Mater. Chem. A, 2014,2(32):12662-12676. |
| [13] |
SU Y S, MANTHIRAM A . Lithium-sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun., 2012,3:1166.
DOI URL PMID |
| [14] |
ZU C X, SU Y S, FU Y Z , et al. Improved lithium-sulfur cells with a treated carbon paper interlayer. Phys. Chem. Chem. Phys., 2013,15(7):2291-2297.
DOI URL PMID |
| [15] |
XIAO Z B, YANG Z, WANG L , et al. A lightweight TiO2/graphene interlayer, applied as a highly effective polysulfide absorbent for fast, long-life lithium-sulfur batteries. Adv. Mater., 2015,27(18):2891-2898.
DOI URL PMID |
| [16] |
ZHOU W D, XIAO X C, CAI M , et al. Polydopamine-coated, nitrogen- doped, hollow carbon sulfur double-layered core-shell structure for improving lithium sulfur batteries. Nano Lett., 2014,14(9):5250-5256.
DOI URL PMID |
| [17] | FU Y Z, MANTHIRAM A . Orthorhombic bipyramidal sulfur coated with polypyrrole nanolayers as a cathode material for lithium- sulfur batteries. J. Phys. Chem. C, 2012,116(16):8910-8915. |
| [18] | MA G Q, WEN Z Y, JIN J , et al. Enhanced cycle performance of Li-S battery with a polypyrrole functional interlayer. J. Power Sources, 2014,267:542-546. |
| [19] |
XING Y, YANG Y, CHEN R J , et al. Strongly coupled carbon nanosheets/molybdenum carbide nanocluster hollow nanospheres for high-performance aprotic Li-O2 battery. Small, 2018,14(19):1704366
DOI URL PMID |
| [20] | WANG C L, SUN L S, ZHANG F F , et al. Formation of Mo-polydopamine hollow spheres and their conversions to MoO2/C and Mo2C/C for efficient electrochemical energy storage and catalyst. Small, 2017,13(32):1701246. |
| [21] | ZHANG S P, WANG G, JIN J , et al. Self-catalyzed decomposition of discharge products on the oxygen vacancy sites of MoO3 nanosheets for low-overpotential Li-O2 batteries. Nano Energy, 2017,36:186-196. |
| [22] |
ZHOU F, LI Z, LUO X , et al. Low cost metal carbide nanocrystals as binding and electrocatalytic sites for high performance Li-S batteries. Nano Lett., 2018,18(2):1035-1043.
DOI URL PMID |
| [23] |
NI L B, ZHAO G J, YANG G , et al. Dual core-shell-structured S@C@MnO2 nanocomposite for highly stable lithium-sulfur batteries. ACS Appl. Mater. Interfaces, 2017,9(40):34793-34803.
DOI URL PMID |
| [1] | 李高然, 李红阳, 曾海波. 硼基材料在锂硫电池中的研究进展[J]. 无机材料学报, 2022, 37(2): 152-162. |
| [2] | 李婷婷, 张阳, 陈加航, 闵宇霖, 王久林. 锂硫电池S@pPAN正极用柔性黏结剂研究[J]. 无机材料学报, 2022, 37(2): 182-188. |
| [3] | 金高尧, 何海传, 吴杰, 张梦源, 李亚娟, 刘又年. 锂硫电池正极用钴掺杂空心多孔碳载体材料[J]. 无机材料学报, 2021, 36(2): 203-209. |
| [4] | 汤嘉伟, 王永邦, 马成, 杨海潇, 王际童, 乔文明, 凌立成. 甲基萘沥青基有序中孔炭的制备及电化学性能[J]. 无机材料学报, 2021, 36(10): 1031-1038. |
| [5] | 蒋浩,吴淏,侯成义,李耀刚,肖茹,张青红,王宏志. 切割方向对桦木衍生的取向微通道生物质炭锂硫电池隔膜性能的影响[J]. 无机材料学报, 2020, 35(6): 717-723. |
| [6] | 李亚东, 李伟平, 王琴, 郑道光, 王建新. 碳纤维支撑柔性碳硫复合电极的制备、物性及电池性能研究[J]. 无机材料学报, 2019, 34(4): 373-378. |
| [7] | 王宇晖, 靳 俊, 郭战胜, 温兆银. 锂硫电池循环过程中变形演化的直接观测[J]. 无机材料学报, 2017, 32(3): 247-251. |
| [8] | 柴二亚, 潘俊安, 袁国龙, 程豪, 安峰, 谢淑红. 聚苯胺包覆蛋白石页岩/硫复合材料的制备及其电化学性能[J]. 无机材料学报, 2017, 32(11): 1165-1170. |
| [9] | 杨书廷, 闫 崇, 曹朝霞, 史梦姣, 李艳蕾, 尹艳红. 以荷叶制备多级孔碳硫复合正极材料及性能研究[J]. 无机材料学报, 2016, 31(2): 135-140. |
| [10] | 马国强, 温兆银, 王清松, 靳 俊, 吴相伟, 张敬超. CeO2纳米晶的添加对锂硫电池电化学性能的影响[J]. 无机材料学报, 2015, 30(9): 913-918. |
| [11] | 陈飞彪, 王英男, 吴伯荣, 熊云奎, 廖维林, 吴 锋, 孙 喆. 锂硫电池石墨烯/硫复合正极材料的制备及其电化学性能[J]. 无机材料学报, 2014, 29(6): 627-632. |
| [12] | 胡菁菁, 李国然, 高学平. 锂/硫电池的研究现状、问题及挑战[J]. 无机材料学报, 2013, 28(11): 1181-1186. |
| [13] | 陈 龙, 刘景东, 张诗群. 负载ZnS的介孔炭复合硫正极材料的制备及性能研究[J]. 无机材料学报, 2013, 28(10): 1127-1131. |
| [14] | 靳广洲,樊秀菊,孙桂大,高俊斌,朱建华. 碳化钼的制备与表征[J]. 无机材料学报, 2007, 22(3): 504-508. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||