无机材料学报 ›› 2020, Vol. 35 ›› Issue (4): 505-510.DOI: 10.15541/jim20190125
所属专题: 2020年能源材料论文精选(三) :太阳能电池、热电材料及其他
• 研究快报 • 上一篇
黄冲1,2,赵伟1( ),王东1,卜克军1,2,王思顺1,黄富强1,3(
),王东1,卜克军1,2,王思顺1,黄富强1,3( )
)
收稿日期:2019-03-26
修回日期:2019-04-30
出版日期:2020-04-20
网络出版日期:2019-06-17
作者简介:黄 冲(1994-), 男, 硕士研究生. E-mail: huangchong@student.sic.ac.cn
HUANG Chong1,2,ZHAO Wei1( ),WANG Dong1,BU Kejun1,2,WANG Sishun1,HUANG Fuqiang1,3(
),WANG Dong1,BU Kejun1,2,WANG Sishun1,HUANG Fuqiang1,3( )
)
Received:2019-03-26
Revised:2019-04-30
Published:2020-04-20
Online:2019-06-17
Supported by:摘要:
通过固相反应法合成一系列插层化合物PdxNbSe2 (x=0~0.17)。它们与2H-NbSe2相同, 属于六方晶格, 空间群为P63/mmc。Pd占据NbSe2层间的八面体空位。随着Pd含量的增加, 晶格常数c线性增大, 而a几乎不变。X射线单晶衍射结果表明, Pd0.17NbSe2的晶格常数为a=b=0.34611(2) nm, c=1.27004(11) nm。每个Pd原子与六个Se原子键合形成[PdSe6]八面体来连接相邻的Nb-Se层, 使晶体结构变得更加稳定, 从而提高化合物的热稳定性。电学测试表明, 随着Pd含量的增加, PdxNbSe2的剩余电阻比减小。此外, 超导转变温度也随着Pd含量的增加而下降, 说明Pd的引入不利于NbSe2的超导态。
中图分类号:
黄冲,赵伟,王东,卜克军,王思顺,黄富强. Pd插层NbSe2化合物的制备、晶体结构和电学性质研究[J]. 无机材料学报, 2020, 35(4): 505-510.
HUANG Chong,ZHAO Wei,WANG Dong,BU Kejun,WANG Sishun,HUANG Fuqiang. Synthesis, Crystal Structure, and Electrical Conductivity of Pd-intercalated NbSe2[J]. Journal of Inorganic Materials, 2020, 35(4): 505-510.

Fig. 1 Crystal structure of Pd0.17NbSe2 along (a) the bc-plane and (b) the ab-plane, (c) [NbSe6] triangular prism in Pd0.17NbSe2, (d) [PdSe6] octahedron in Pd0.17NbSe2
| Chemical formula | Pd0.17NbSe2 |
|---|---|
| Mr/(g∙mol-1) | 269.03 |
| Crystal system | Hexagonal, P63/mmc |
| a, c/nm | 0.34611(2), 1.27004(11) |
| V/nm3 | 0.13176(2) |
| Z | 2 |
| Radiation type | Mo Kα, λ=0.071073 nm |
| Crystal color | Black |
| ρc/(g∙cm-3) | 6.781 |
| µ/mm-1 | 32.93 |
| Crystal size/mm3 | 0.01×0.01×0.003 |
| Diffractometer | Bruker D8 QUEST |
| Tmin, Tmax | 0.494, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 1480, 121, 103 |
| Rint | 0.053 |
| (sinθ/λ)max/nm-1 | 7.55 |
| R1, wR2, Sa | 0.019, 0.035, 1.14 |
| No. of reflections | 121 |
| No. of parameters | 9 |
Table 1 Crystal data and structure refinement of Pd0.17NbSe2
| Chemical formula | Pd0.17NbSe2 |
|---|---|
| Mr/(g∙mol-1) | 269.03 |
| Crystal system | Hexagonal, P63/mmc |
| a, c/nm | 0.34611(2), 1.27004(11) |
| V/nm3 | 0.13176(2) |
| Z | 2 |
| Radiation type | Mo Kα, λ=0.071073 nm |
| Crystal color | Black |
| ρc/(g∙cm-3) | 6.781 |
| µ/mm-1 | 32.93 |
| Crystal size/mm3 | 0.01×0.01×0.003 |
| Diffractometer | Bruker D8 QUEST |
| Tmin, Tmax | 0.494, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 1480, 121, 103 |
| Rint | 0.053 |
| (sinθ/λ)max/nm-1 | 7.55 |
| R1, wR2, Sa | 0.019, 0.035, 1.14 |
| No. of reflections | 121 |
| No. of parameters | 9 |
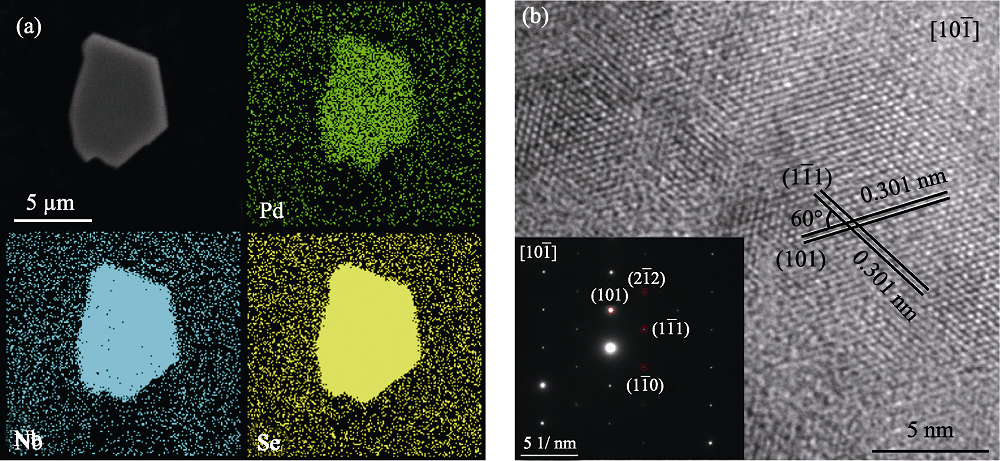
Fig. 2 (a) SEM images of Pd0.17NbSe2 and the corresponding elemental mapping analysis, and (b) HRTEM image of Pd0.17NbSe2 along [101¯] zone axis with inset showing the corresponding SAED pattern
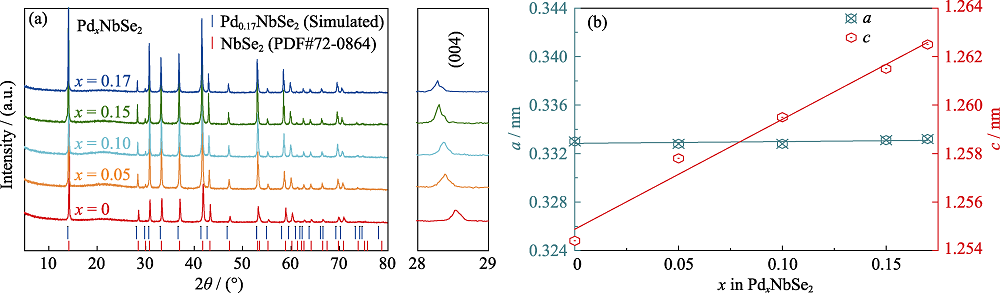
Fig. 4 (a) Powder XRD patterns of PdxNbSe2 (x=0, 0.05, 0.10, 0.15, 0.17), (b) composition dependence of the lattice parameters a and c for PdxNbSe2 (0≤x≤0.17)
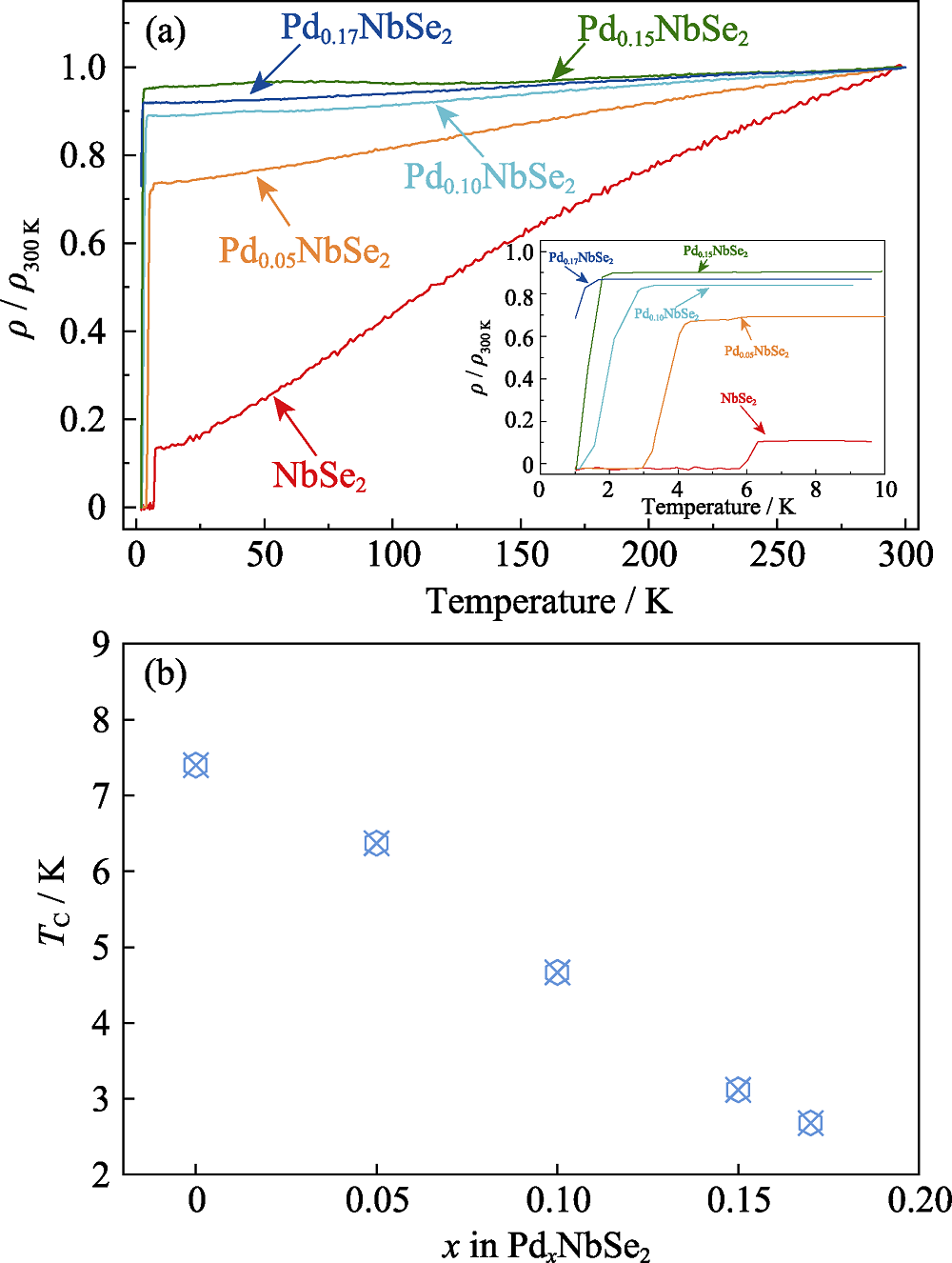
Fig. 6 (a) Temperature dependence of the RRR (ρ/ρ300 K) for PdxNbSe2 (0≤x≤0.17) with inset showing enlarged temperature regionof the superconducting transition, (b) composition dependence of TC
| Atom | Wyck. Site | x | y | z | Uiso* or Ueq/nm2 | Occ. |
|---|---|---|---|---|---|---|
| Nb | 2b | 0 | 1 | 1/4 | 0.000075(2) | 1 |
| Se | 4f | 1/3 | 2/3 | 0.38104(5) | 0.0000736(17) | 1 |
| Pd | 2a | 0 | 1 | 1/2 | 0.000059(11) | 0.171(3) |
Table S1 Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters of Pd0.17NbSe2
| Atom | Wyck. Site | x | y | z | Uiso* or Ueq/nm2 | Occ. |
|---|---|---|---|---|---|---|
| Nb | 2b | 0 | 1 | 1/4 | 0.000075(2) | 1 |
| Se | 4f | 1/3 | 2/3 | 0.38104(5) | 0.0000736(17) | 1 |
| Pd | 2a | 0 | 1 | 1/2 | 0.000059(11) | 0.171(3) |
| U11/nm2 | U22/nm2 | U33/nm2 | U12/nm2 | U13/nm2 | U23 | |
|---|---|---|---|---|---|---|
| Nb | 0.000074(3) | 0.000074(3) | 0.000075(4) | 0.0000372(13) | 0 | 0 |
| S | 0.000064(2) | 0.000064(2) | 0.000094(3) | 0.0000318(10) | 0 | 0 |
| Pd | 0.000062(13) | 0.000062(13) | 0.000052(19) | 0.000031(7) | 0 | 0 |
Table S2 Atomic displacement parameters of Pd0.17NbSe2
| U11/nm2 | U22/nm2 | U33/nm2 | U12/nm2 | U13/nm2 | U23 | |
|---|---|---|---|---|---|---|
| Nb | 0.000074(3) | 0.000074(3) | 0.000075(4) | 0.0000372(13) | 0 | 0 |
| S | 0.000064(2) | 0.000064(2) | 0.000094(3) | 0.0000318(10) | 0 | 0 |
| Pd | 0.000062(13) | 0.000062(13) | 0.000052(19) | 0.000031(7) | 0 | 0 |
| Bond | Distance/nm | Bond | Distance/nm |
|---|---|---|---|
| Nb1—Se2i | 0.26006(4) | Se2—Pd3viii | 0.25051(4) |
| Nb1—Se2ii | 0.26006(4) | Se2—Nb1viii | 0.26006(4) |
| Nb1—Se2iii | 0.26006(4) | Se2—Nb1vii | 0.26006(4) |
| Nb1—Se2iv | 0.26006(4) | Pd3—Se2ix | 0.25051(4) |
| Nb1—Se2 | 0.26006(4) | Pd3—Se2iv | 0.25051(4) |
| Nb1—Se2v | 0.26006(4) | Pd3—Se2x | 0.25051(4) |
| Nb1—Pd3vi | 0.31751(3) | Pd3—Se2i | 0.25051(4) |
| Nb1—Pd3 | 0.31751(3) | Pd3—Se2xi | 0.25051(4) |
| Se2—Pd3vii | 0.25051(4) | Pd3—Nb1xi | 0.31751(3) |
| Se2—Pd3 | 0.25051(4) | ||
| Bond | Angle/(°) | Bond | Angle/(°) |
| Se2i—Nb1—Se2ii | 134.813 (8) | Pd3viii—Se2—Nb1 | 133.822 (3) |
| Se2i—Nb1—Se2iii | 79.58 (2) | Nb1viii—Se2—Nb1 | 83.434 (16) |
| Se2ii—Nb1—Se2iii | 83.434 (16) | Pd3vii—Se2—Nb1vii | 76.881 (6) |
| Se2i—Nb1—Se2iv | 83.434 (16) | Pd3—Se2—Nb1vii | 133.822 (3) |
| Se2ii—Nb1—Se2iv | 79.58 (2) | Pd3viii—Se2—Nb1vii | 133.822 (3) |
| Se2iii—Nb1—Se2iv | 134.813 (7) | Nb1viii—Se2—Nb1vii | 83.434 (16) |
| Se2i—Nb1—Se2 | 83.433 (16) | Nb1—Se2—Nb1vii | 83.434 (16) |
| Se2ii—Nb1—Se2 | 134.812 (7) | Se2—Pd3—Se2ix | 92.612 (17) |
| Se2iii—Nb1—Se2 | 134.812 (8) | Se2—Pd3—Se2iv | 87.388 (17) |
| Se2iv—Nb1—Se2 | 83.433 (16) | Se2ix—Pd3—Se2iv | 180.0 |
| Se2i—Nb1—Se2v | 134.812 (8) | Se2—Pd3—Se2x | 92.612 (17) |
| Se2ii—Nb1—Se2v | 83.434 (16) | Se2ix—Pd3—Se2x | 87.388 (17) |
| Se2iii—Nb1—Se2v | 83.434 (16) | Se2iv—Pd3—Se2x | 92.612 (17) |
| Bond | Angle/(°) | Bond | Angle/(°) |
| Se2iv—Nb1—Se2v | 134.812 (8) | Se2—Pd3—Se2i | 87.388 (17) |
| Se2—Nb1—Se2v | 79.58 (2) | Se2ix—Pd3—Se2i | 92.612 (17) |
| Se2i—Nb1—Pd3vi | 129.790 (11) | Se2iv—Pd3—Se2i | 87.388 (17) |
| Se2ii—Nb1—Pd3vi | 50.210 (11) | Se2x—Pd3—Se2i | 180.0 |
| Se2iii—Nb1—Pd3vi | 50.210 (11) | Se2—Pd3—Se2xi | 180.0 |
| Se2iv—Nb1—Pd3vi | 129.790 (11) | Se2ix—Pd3—Se2xi | 87.388 (17) |
| Se2—Nb1—Pd3vi | 129.790 (11) | Se2iv—Pd3—Se2xi | 92.612 (17) |
| Se2v—Nb1—Pd3vi | 50.210 (11) | Se2x—Pd3—Se2xi | 87.388 (17) |
| Se2i—Nb1—Pd3 | 50.210 (11) | Se2i—Pd3—Se2xi | 92.612 (17) |
| Se2ii—Nb1—Pd3 | 129.790 (11) | Se2—Pd3—Nb1 | 52.909 (12) |
| Se2iii—Nb1—Pd3 | 129.790 (11) | Se2ix—Pd3—Nb1 | 127.092 (12) |
| Se2iv—Nb1—Pd3 | 50.210 (11) | Se2iv—Pd3—Nb1 | 52.908 (12) |
| Se2—Nb1—Pd3 | 50.210 (11) | Se2x—Pd3—Nb1 | 127.092 (12) |
| Se2v—Nb1—Pd3 | 129.790 (11) | Se2i—Pd3—Nb1 | 52.908 (12) |
| Pd3vi—Nb1—Pd3 | 180.0 | Se2xi—Pd3—Nb1 | 127.091 (12) |
| Pd3vii—Se2—Pd3 | 87.388 (17) | Se2—Pd3—Nb1xi | 127.091 (12) |
| Pd3vii—Se2—Pd3viii | 87.388 (17) | Se2ix—Pd3—Nb1xi | 52.908 (12) |
| Pd3—Se2—Pd3viii | 87.388 (17) | Se2iv—Pd3—Nb1xi | 127.092 (12) |
| Pd3vii—Se2—Nb1viii | 133.822 (2) | Se2x—Pd3—Nb1xi | 52.908 (12) |
| Pd3—Se2—Nb1viii | 133.822 (3) | Se2i—Pd3—Nb1xi | 127.092 (12) |
| Pd3viii—Se2—Nb1viii | 76.881 (6) | Se2xi—Pd3—Nb1xi | 52.909 (12) |
| Pd3vii—Se2—Nb1 | 133.822 (2) | Nb1—Pd3—Nb1xi | 180.0 |
| Pd3—Se2—Nb1 | 76.881 (6) |
Table S3 Geometric parameters for Pd0.17NbSe2
| Bond | Distance/nm | Bond | Distance/nm |
|---|---|---|---|
| Nb1—Se2i | 0.26006(4) | Se2—Pd3viii | 0.25051(4) |
| Nb1—Se2ii | 0.26006(4) | Se2—Nb1viii | 0.26006(4) |
| Nb1—Se2iii | 0.26006(4) | Se2—Nb1vii | 0.26006(4) |
| Nb1—Se2iv | 0.26006(4) | Pd3—Se2ix | 0.25051(4) |
| Nb1—Se2 | 0.26006(4) | Pd3—Se2iv | 0.25051(4) |
| Nb1—Se2v | 0.26006(4) | Pd3—Se2x | 0.25051(4) |
| Nb1—Pd3vi | 0.31751(3) | Pd3—Se2i | 0.25051(4) |
| Nb1—Pd3 | 0.31751(3) | Pd3—Se2xi | 0.25051(4) |
| Se2—Pd3vii | 0.25051(4) | Pd3—Nb1xi | 0.31751(3) |
| Se2—Pd3 | 0.25051(4) | ||
| Bond | Angle/(°) | Bond | Angle/(°) |
| Se2i—Nb1—Se2ii | 134.813 (8) | Pd3viii—Se2—Nb1 | 133.822 (3) |
| Se2i—Nb1—Se2iii | 79.58 (2) | Nb1viii—Se2—Nb1 | 83.434 (16) |
| Se2ii—Nb1—Se2iii | 83.434 (16) | Pd3vii—Se2—Nb1vii | 76.881 (6) |
| Se2i—Nb1—Se2iv | 83.434 (16) | Pd3—Se2—Nb1vii | 133.822 (3) |
| Se2ii—Nb1—Se2iv | 79.58 (2) | Pd3viii—Se2—Nb1vii | 133.822 (3) |
| Se2iii—Nb1—Se2iv | 134.813 (7) | Nb1viii—Se2—Nb1vii | 83.434 (16) |
| Se2i—Nb1—Se2 | 83.433 (16) | Nb1—Se2—Nb1vii | 83.434 (16) |
| Se2ii—Nb1—Se2 | 134.812 (7) | Se2—Pd3—Se2ix | 92.612 (17) |
| Se2iii—Nb1—Se2 | 134.812 (8) | Se2—Pd3—Se2iv | 87.388 (17) |
| Se2iv—Nb1—Se2 | 83.433 (16) | Se2ix—Pd3—Se2iv | 180.0 |
| Se2i—Nb1—Se2v | 134.812 (8) | Se2—Pd3—Se2x | 92.612 (17) |
| Se2ii—Nb1—Se2v | 83.434 (16) | Se2ix—Pd3—Se2x | 87.388 (17) |
| Se2iii—Nb1—Se2v | 83.434 (16) | Se2iv—Pd3—Se2x | 92.612 (17) |
| Bond | Angle/(°) | Bond | Angle/(°) |
| Se2iv—Nb1—Se2v | 134.812 (8) | Se2—Pd3—Se2i | 87.388 (17) |
| Se2—Nb1—Se2v | 79.58 (2) | Se2ix—Pd3—Se2i | 92.612 (17) |
| Se2i—Nb1—Pd3vi | 129.790 (11) | Se2iv—Pd3—Se2i | 87.388 (17) |
| Se2ii—Nb1—Pd3vi | 50.210 (11) | Se2x—Pd3—Se2i | 180.0 |
| Se2iii—Nb1—Pd3vi | 50.210 (11) | Se2—Pd3—Se2xi | 180.0 |
| Se2iv—Nb1—Pd3vi | 129.790 (11) | Se2ix—Pd3—Se2xi | 87.388 (17) |
| Se2—Nb1—Pd3vi | 129.790 (11) | Se2iv—Pd3—Se2xi | 92.612 (17) |
| Se2v—Nb1—Pd3vi | 50.210 (11) | Se2x—Pd3—Se2xi | 87.388 (17) |
| Se2i—Nb1—Pd3 | 50.210 (11) | Se2i—Pd3—Se2xi | 92.612 (17) |
| Se2ii—Nb1—Pd3 | 129.790 (11) | Se2—Pd3—Nb1 | 52.909 (12) |
| Se2iii—Nb1—Pd3 | 129.790 (11) | Se2ix—Pd3—Nb1 | 127.092 (12) |
| Se2iv—Nb1—Pd3 | 50.210 (11) | Se2iv—Pd3—Nb1 | 52.908 (12) |
| Se2—Nb1—Pd3 | 50.210 (11) | Se2x—Pd3—Nb1 | 127.092 (12) |
| Se2v—Nb1—Pd3 | 129.790 (11) | Se2i—Pd3—Nb1 | 52.908 (12) |
| Pd3vi—Nb1—Pd3 | 180.0 | Se2xi—Pd3—Nb1 | 127.091 (12) |
| Pd3vii—Se2—Pd3 | 87.388 (17) | Se2—Pd3—Nb1xi | 127.091 (12) |
| Pd3vii—Se2—Pd3viii | 87.388 (17) | Se2ix—Pd3—Nb1xi | 52.908 (12) |
| Pd3—Se2—Pd3viii | 87.388 (17) | Se2iv—Pd3—Nb1xi | 127.092 (12) |
| Pd3vii—Se2—Nb1viii | 133.822 (2) | Se2x—Pd3—Nb1xi | 52.908 (12) |
| Pd3—Se2—Nb1viii | 133.822 (3) | Se2i—Pd3—Nb1xi | 127.092 (12) |
| Pd3viii—Se2—Nb1viii | 76.881 (6) | Se2xi—Pd3—Nb1xi | 52.909 (12) |
| Pd3vii—Se2—Nb1 | 133.822 (2) | Nb1—Pd3—Nb1xi | 180.0 |
| Pd3—Se2—Nb1 | 76.881 (6) |
| [1] | TAN CHAO-LIANG, CAO XIE-HONG, WU XUE-JUN , et al. Recent advances in ultrathin two-dimensional nanomaterials. Chemical Reviews, 2017,117(9):6225-6331. |
| [2] | GOPALAKRISHNAN D, LEE A, THANGAVEL N K , et al. Facile synthesis of electrocatalytically active NbS2 nanoflakes for an enhanced hydrogen evolution reaction. Sustainable Energy & Fuels, 2018,2(1):96-102. |
| [3] | JIN HUAN-YU, GUO CHUN-XIAN, LIU XIN , et al. Emerging two-dimensional nanomaterials for electrocatalysis. Chemical Reviews, 2018,118(13):6337-6408. |
| [4] | WANG REN-YAN, GAN LIN, ZHAI TIAN-YOU . ReX2 (X=S, Se): a new opportunity for development of two-dimensional anisotropic materials. Journal of Inorganic Materials, 2019,34(1):1-16. |
| [5] | ZHAO DE-RUI, ZHAI YING-JIAO, LI JIN-HUA , et al. Preparation and properties of glucose biosensor based on flower-like MoS2 micrometer material. Journal of Inorganic Materials, 2016,31(2):153-158. |
| [6] | CHIA X, AMBROSI A, LAZAR P , et al. Electrocatalysis of layered group 5 metallic transition metal dichalcogenides (MX2, M=V, Nb, and Ta; X = S, Se, and Te). Journal of Materials Chemistry A, 2016,4(37):14241-14253. |
| [7] | SIPOS B, KUSMARTSEVA A F, AKRAP A , et al. From Mott state to superconductivity in 1T-TaS2. Nature Materials, 2008,7(12):960-965. |
| [8] | LUO HUI-XIA, STRYCHALSKA-NOWAK J, LI JUN , et al. S-shaped suppression of the superconducting transition temperature in Cu-intercalated NbSe2. Chemistry of Materials, 2017,29(8):3704-3712. |
| [9] | WANG MENG-JING, WILLIAMS D, LAHTI G , et al. Chemical intercalation of heavy metal, semimetal, and semiconductor atoms into 2D layered chalcogenides. 2D Materials, 2018,5(4):045005. |
| [10] | HAO QIAO-YAN, WANG DA-KE, ZHU BAI-CHUAN ,et al. Facile synthesis, structure and physical properties of 3R-AxNbS2(A=Li, Na). Journal of Alloys and Compounds, 2016,663:225-229. |
| [11] | PRODAN A, MARINKOVIC V, ROJSEK M , et al. The surface superstructures in niobium disulfide and diselenide intercalated by Cu, Co and Fe. Surface Science, 2001,476(1):71-77. |
| [12] | HUGHES T A, KEVAN S D, COX D E , et al. Synthesis of superlattices of intercalated transition metal dichalcogenides. Journal of the American Chemical Society, 2000,122(37):8910-8915. |
| [13] | LIAN CHAO-SHENG, SI CHEN, WU JIAN , et al. First-principles study of Na-intercalated bilayer NbSe2: suppressed charge-density wave and strain-enhanced superconductivity. Physical Review B, 2017,96(23):235426. |
| [14] | WANG DONG, WANG XIN, LU YUE , et al. Atom-scale dispersed palladium in a conductive Pd0.1TaS2 lattice with a unique electronic structure for efficient hydrogen evolution. Journal of Materials Chemistry A, 2017,5(43):22618-22624. |
| [15] | BHOI D, KHIM S, NAM W , et al. Interplay of charge density wave and multiband superconductivity in 2H-PdxTaSe2. Scientific Reports, 2016,6:24068. |
| [16] | MOMMA K, IZUMI F . VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. Journal of Applied Crystallography, 2011,44(6):1272-1276. |
| [17] | MAREZIO M, DERNIER P, MENTH A , et al. The crystal structure of NbSe2 at 15 K. Journal of Solid State Chemistry, 1972,4(3):425-429. |
| [18] | HAMIDANI A, BENNECER B, ZANAT K . Structural and electronic properties of the pseudo-binary compounds PdX2 (X=P, S and Se). Journal of Physics and Chemistry of Solids, 2010,71(1):42-46. |
| [19] | ZHAO BEN-LIANG, HUANG JIAN, FU QI , et al. MoS2/NbSe2 hybrid nanobelts for enhanced hydrogen evolution. Journal of The Electrochemical Society, 2016,163(6):H384-H387. |
| [20] | HALASYAMANI P S, O'HARE D . Synthesis and characterization of Se4Nb2O13: a new ternary Se4+-Nb5+-oxide with monoselenite and diselenite groups . Chemistry of Materials, 1998,10(2):646-649. |
| [21] | BU KE-JUN, HUANG JIAN, LUO MENG-JIA , et al. Observation of high Seebeck coefficient and low thermal conductivity in [SrO]- intercalated CuSbSe2 compound. Chemistry of Materials, 2018,30(16):5539-5543. |
| [22] | WANG QIN-CHAO, MENG JING-KE, YUE XIN-YANG , et al. Tuning P2-structured cathode material by Na-site Mg substitution for Na-Ion batteries. Journal of the American Chemical Society, 2019,141(2):840-848. |
| [23] | NAIK I, RASTOGI A K . Transport properties of 2H-NbSe2: effect of Ga-intercalation. Physica B: Condensed Matter, 2010,405(3):955-957. |
| [24] | HAUDER J J, ROBBINS M, DISALVO F J . Effect of 3d impurities on the superconducting transition temperature of the layered compound NbSe2. Physical Review B, 1973,8(3):1038-1042. |
| [1] | 赵伟, 徐阳, 万颖杰, 蔡天逊, 穆金潇, 黄富强. 金属氰胺化合物的结构、合成及电化学储能应用[J]. 无机材料学报, 2022, 37(2): 140-151. |
| [2] | 彭帆, 曾毅. 利用菊池衍射花样鉴定晶体结构的方法研究[J]. 无机材料学报, 2021, 36(11): 1193-1198. |
| [3] | 李淑芳,赵爽,周潇,李满荣. Zn3-xMnxTeO6的晶体结构与吸收光谱和磁性研究[J]. 无机材料学报, 2020, 35(8): 895-901. |
| [4] | 李淑芳, 赵爽, 李满荣. 助熔剂法合成钨氧氯化合物Li23CuW10O40Cl5[J]. 无机材料学报, 2020, 35(7): 834-838. |
| [5] | 曾祥雄, 杨进超, 左联, 杨奔奔, 秦峻, 彭志航. Li/Ce/La共掺杂对CaBi2Nb2O9陶瓷晶体结构及电学性能的影响[J]. 无机材料学报, 2019, 34(4): 379-386. |
| [6] | 罗志永, 廖楚剑, 蔡传兵, 刘志勇, 李敏娟, 鲁玉明. 氩离子刻蚀对TFA-MOD法YBa2Cu3O7-x薄膜性能的影响研究[J]. 无机材料学报, 2019, 34(12): 1279-1284. |
| [7] | 黄龙, 丁士华, 张晓云, 严欣堪, 李超, 朱惠. Li2O-B2O3-SiO2玻璃相对BaAl2Si2O8结构及微波介电性能的影响[J]. 无机材料学报, 2019, 34(10): 1091-1096. |
| [8] | 周鑫, 马垒, 刘涛, 郭永斌, 王岛, 董培林. Si3N4/FePd/Si3N4薄膜的晶体结构和磁性能研究[J]. 无机材料学报, 2018, 33(8): 909-913. |
| [9] | 左珺凉, 赵跃, 吴蔚, 储静远, 张智巍, 洪智勇, 金之俭. 无氟MOD法制备YBCO超导薄膜工艺中物相的演变过程[J]. 无机材料学报, 2018, 33(7): 773-778. |
| [10] | 孟凡斌, 马晓帆, 张 炜, 吴光恒, 张玉洁. Mn和Fe掺杂对尖晶石氧化物Co2MnO4结构和磁性的影响[J]. 无机材料学报, 2017, 32(6): 609-614. |
| [11] | 王晴晴, 史 坚, 李焕英, 陈晓峰, 潘尚可, 卞建江, 任国浩. Cs2LiYCl6:Ce闪烁晶体的光学及闪烁性能[J]. 无机材料学报, 2017, 32(2): 175-179. |
| [12] | 淡猛, 张骞, 钟云倩, 周莹. 不同晶相MnS制备及光解H2S制氢性能研究[J]. 无机材料学报, 2017, 32(12): 1308-1314. |
| [13] | 杨志胜, 柯蔚芳, 王艳香, 黄丽群, 郭平春, 朱 华. 杂化钙钛矿(HOC2H4NH3)2CuCl4的制备与表征[J]. 无机材料学报, 2017, 32(10): 1063-1067. |
| [14] | 张 瑶, 丁士华, 刘杨琼, 段绍英, 肖 鹏, 韩林材. Ba1-xMgxAl2Si2O8晶体结构与微波介电性能的研究[J]. 无机材料学报, 2017, 32(1): 91-95. |
| [15] | 刘 鑫, 熊 杰, 张 飞, 赵晓辉, 赵睿鹏, 陶伯万. Ba源配比对MOCVD法制备YBCO薄膜性能的影响研究[J]. 无机材料学报, 2014, 29(3): 275-278. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||