无机材料学报 ›› 2020, Vol. 35 ›› Issue (2): 145-157.DOI: 10.15541/jim20190108
所属专题: 2020年能源材料论文精选(二):超级电容器; 【虚拟专辑】超级电容器(2020~2021); 【虚拟专辑】柔性材料(2020~2021)
• 综述 • 下一篇
收稿日期:2019-03-12
修回日期:2019-05-17
出版日期:2020-02-20
网络出版日期:2019-06-17
作者简介:马丽娜(1988-), 女, 副教授. E-mail: malina@qdu.edu.cn
基金资助:
MA Li-Na1,SHI Chuan2,ZHAO Ning2,BI Zhi-Jie2,GUO Xiang-Xin2( ),HUANG Yu-Dong3
),HUANG Yu-Dong3
Received:2019-03-12
Revised:2019-05-17
Published:2020-02-20
Online:2019-06-17
Supported by:摘要:
细菌纤维素(Bacterial Cellulose, BC)是由微生物发酵获得的具有纳米尺寸的聚合物生物材料, 具有比表面积大、机械强度高、持水能力强、化学稳定性好及环境友好等特质, 可用于制备三维纳米碳材料的前驱体或支撑其他功能材料的柔性骨架。本文介绍了基于BC制备的各种碳纳米纤维(Carbon Nanofiber, CNF)及其复合材料, 包括掺杂CNF、CNF/金属氧化物、CNF/导电聚合物等材料。描述了这些材料在超级电容器中的应用, 关注BC用于可弯曲电极的设计和制备; 进一步阐述了当前BC应用于能源存储领域所面临的挑战和机遇, 并对其未来发展包括在高性能二次电池方面的应用等进行了展望。
中图分类号:
马丽娜,石川,赵宁,毕志杰,郭向欣,黄玉东. 细菌纤维素基纳米生物材料在储能领域的应用[J]. 无机材料学报, 2020, 35(2): 145-157.
MA Li-Na,SHI Chuan,ZHAO Ning,BI Zhi-Jie,GUO Xiang-Xin,HUANG Yu-Dong. Bacterial Cellulose Based Nano-biomaterials for Energy Storage Applications[J]. Journal of Inorganic Materials, 2020, 35(2): 145-157.
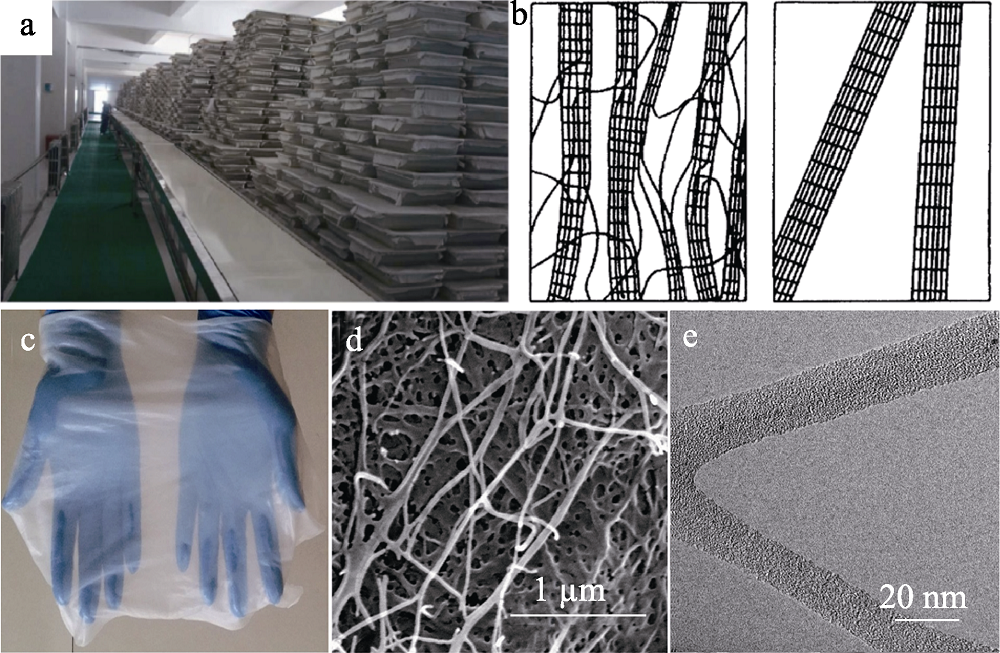
图1 (a) BC生产线照片[3]; (b)植物纤维素(左)和BC(右)模型的比较[5]; (c) BC膜的实物、(d) SEM及(e)TEM照片[4]
Fig. 1 (a) Photograph of the production line for BC[3]; (b) Schematic model of the plant cellulose fibrils (left) and the BC microfibers (right)[5]; (c) Photograph of BC slice; (d) SEM and (e)TEM images of BC[4]
| Material | Function of BC | Potential window/V | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density/ (Wh∙kg-1) | Highest power density/ (kW∙kg-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CO2 activated CNF | Active material | -0.2-0.2 (vs. Ag/AgCl) | 42 (1 mV∙s-1) (659 mF∙cm-2) | 70% (10 mV∙s-1) | — | — | — | [14] |
| CNF | Active material | -1-0 (vs. Ag/AgCl) | 108 (2 A∙g-1) | — | — | — | — | [15] |
| PCN/CNF | Active material | -1-0 (vs. Hg/HgO) | 261 (2 mV∙s-1) | 76.6 (500 mV∙s-1) | 97.6% (10000) | — | — | [18] |
| PCN/CNF// PCN/CNF | Active material | 0-1.8 | — | — | 94.8% (10000) | 20.4 | 17.8 | [18] |
| N,P-CNF// N,P-CNF | Active material | 0-1 | 204.9 (1 A∙g-1) | — | 100% (4000) | 7.76 | 26.1 | [2] |
| N-S-CNF-700 | Active material | 0-1 (vs. Ag/AgCl) | 171.2 (0.5 A∙g-1) | 105.2 (10 A∙g-1) | >90% (1000) | — | — | [20] |
| KOH activated N-CNF | Active material | 0.9-0.1 (vs. SHE) | 296 (2 mV∙s-1) | 75% (500 mV∙s-1) | 99% (10000) | — | — | [12] |
| N-CNF | Active material | -1-0 (vs. Ag/AgCl) | 120 (1 A∙g-1) | — | 98.2% (5000) | — | — | [13] |
| N,P-CNWs | Active material | -1-0 (vs. Hg/HgO) | 258 (1 A∙g-1) | 208 (10 A∙g-1) | 98% (30000) | — | — | [26] |
| N,P-CNWs// N,P-CNWs | Active material | 0-1 | 74 (0.5 A∙g-1) | — | 87% (6000) | 5.4 | 0.2 | [26] |
| CNF aerogels | Active material | -1-0 (vs. Ag/AgCl) | 194.7 (0.5 A∙g-1) | 108.7(10 A∙g-1) | 94% (5000) | — | — | [19] |
表1 BC基碳材料超级电容器电极的电化学性能
Table 1 BC-based carbon material electrodes for supercapacitor
| Material | Function of BC | Potential window/V | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density/ (Wh∙kg-1) | Highest power density/ (kW∙kg-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CO2 activated CNF | Active material | -0.2-0.2 (vs. Ag/AgCl) | 42 (1 mV∙s-1) (659 mF∙cm-2) | 70% (10 mV∙s-1) | — | — | — | [14] |
| CNF | Active material | -1-0 (vs. Ag/AgCl) | 108 (2 A∙g-1) | — | — | — | — | [15] |
| PCN/CNF | Active material | -1-0 (vs. Hg/HgO) | 261 (2 mV∙s-1) | 76.6 (500 mV∙s-1) | 97.6% (10000) | — | — | [18] |
| PCN/CNF// PCN/CNF | Active material | 0-1.8 | — | — | 94.8% (10000) | 20.4 | 17.8 | [18] |
| N,P-CNF// N,P-CNF | Active material | 0-1 | 204.9 (1 A∙g-1) | — | 100% (4000) | 7.76 | 26.1 | [2] |
| N-S-CNF-700 | Active material | 0-1 (vs. Ag/AgCl) | 171.2 (0.5 A∙g-1) | 105.2 (10 A∙g-1) | >90% (1000) | — | — | [20] |
| KOH activated N-CNF | Active material | 0.9-0.1 (vs. SHE) | 296 (2 mV∙s-1) | 75% (500 mV∙s-1) | 99% (10000) | — | — | [12] |
| N-CNF | Active material | -1-0 (vs. Ag/AgCl) | 120 (1 A∙g-1) | — | 98.2% (5000) | — | — | [13] |
| N,P-CNWs | Active material | -1-0 (vs. Hg/HgO) | 258 (1 A∙g-1) | 208 (10 A∙g-1) | 98% (30000) | — | — | [26] |
| N,P-CNWs// N,P-CNWs | Active material | 0-1 | 74 (0.5 A∙g-1) | — | 87% (6000) | 5.4 | 0.2 | [26] |
| CNF aerogels | Active material | -1-0 (vs. Ag/AgCl) | 194.7 (0.5 A∙g-1) | 108.7(10 A∙g-1) | 94% (5000) | — | — | [19] |
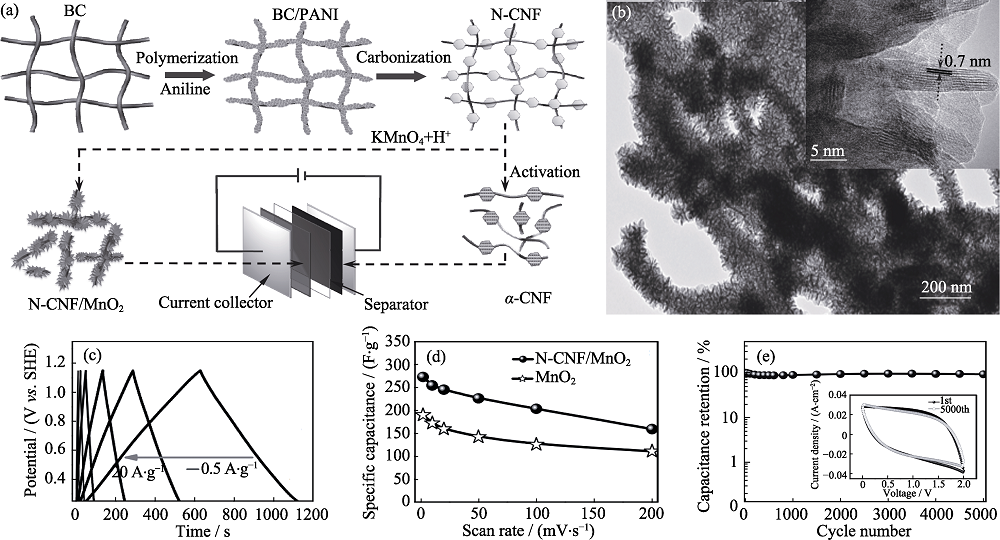
图2 (a)非对称型超级电容器的原理图, (b)N-CNF/MnO2的TEM照片, (c)N-CNF/MnO2电极的GCD曲线, (d)质量比电容和(e)循环稳定性测试[12]
Fig. 2 (a) Schematic diagram for asymmetric supercapacitor device; (b) TEM images, (c) GCD curves, (d) specific capacitance and (e) cycle performance of N-CNF/MnO2[12]
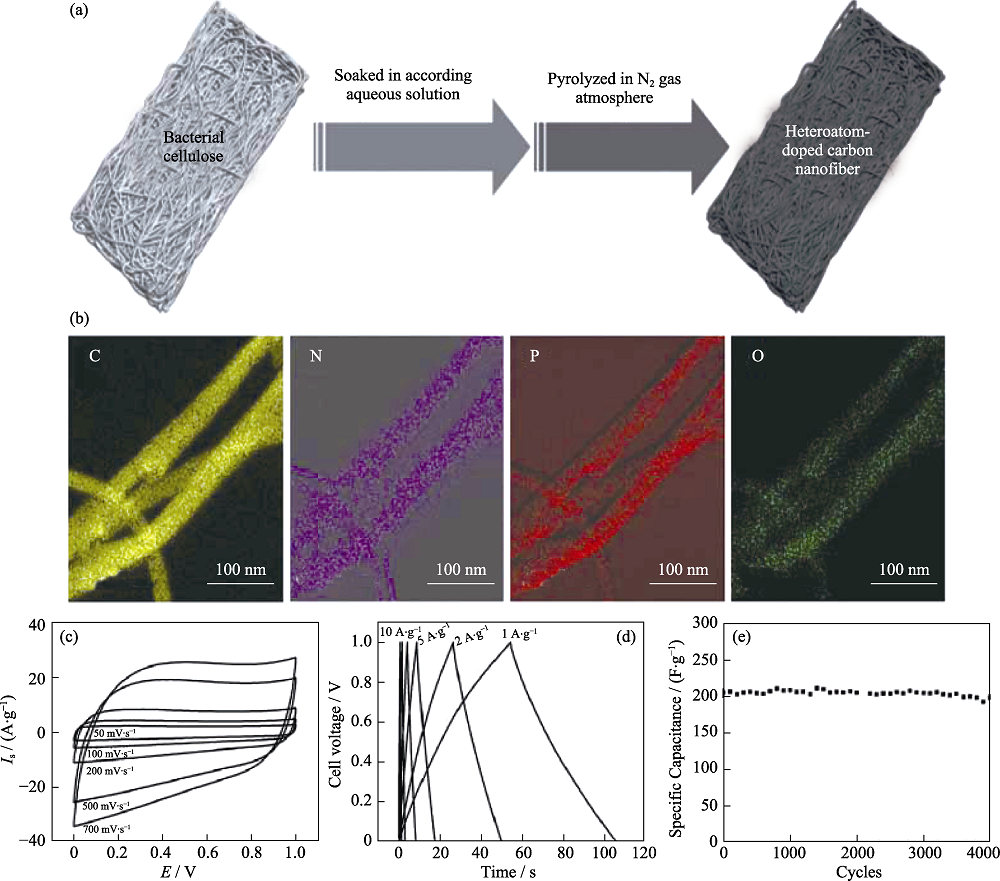
图3 (a)杂原子掺杂CNF的制备流程图, (b)映射图像, (c) CV曲线, (d) GCD曲线和(e)循环稳定性测试[2]
Fig. 3 (a) Fabrication process of heteroatom-doped CNF; (b) Elemental mapping images of C, N, P, and O for N, P-CNF; (c) CV curves, (d) GCD curves and (e) cycling stability test of N,P-CNF supercapacitor[2]
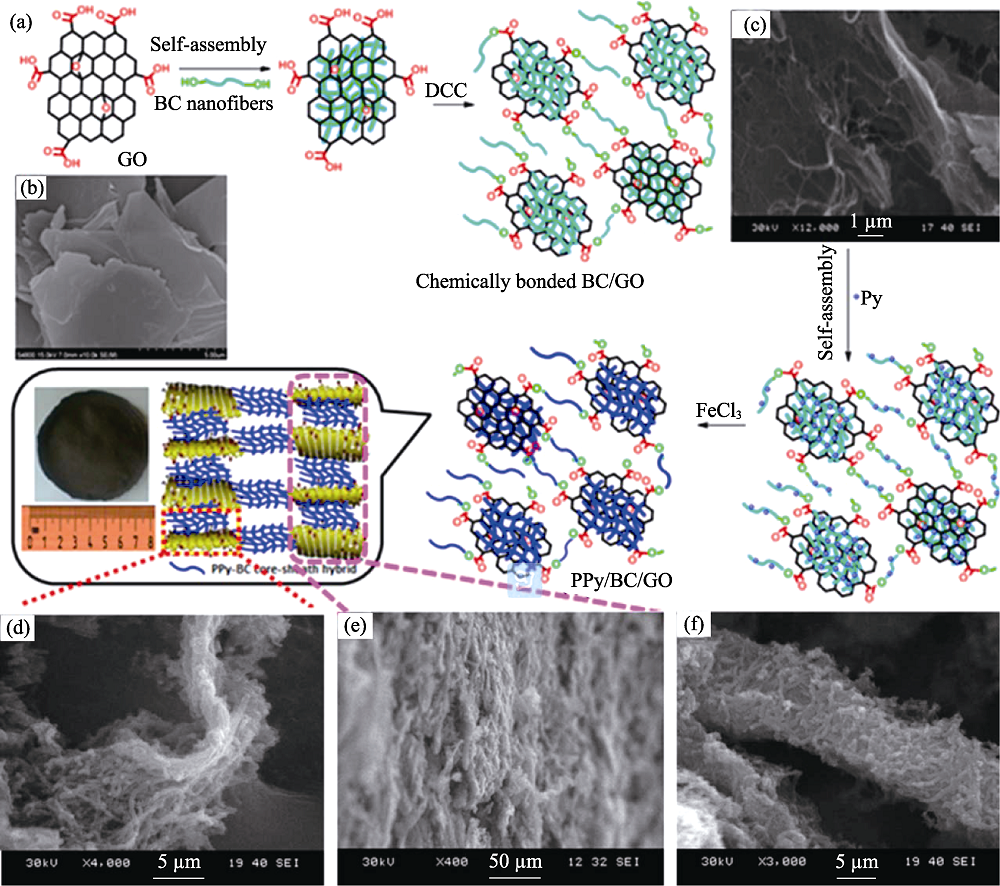
图4 (a)PPY/BC/GO复合物的制备流程图, (b~f)不同物质的SEM照片[38]
Fig. 4 (a) Synthesis scheme of PPY/BC/GO composites; SEM images of (b) pristine GO, (c) cross-linked BC/GO, (d) a single layer and (e) multilayers of PPY/BC/GO hybrid, and (f) PPY/BC core-sheath hybrid[38]
| Material | Function of BC | Potential window/V | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density/ (Wh∙kg-1) | Highest power density/ (kW∙kg-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CNF@MnO2 | Active material | 0-1 (vs. Ag/AgCl) | 254.64 (1 A∙g-1) | 77.53% (10 A∙g-1) | — | — | — | [24] |
| CNF@MnO2// N-CNF | Active material | 0-2 | — | — | 95.4% (2000) | 32.91 | 284.63 | [24] |
| Ni3S2/CNF | Active material | 0-0.6 (vs. Ag/AgCl) | 957 (1 A∙g-1) | 703 (8 A∙g-1) | 16.5% (1000) | — | — | [15] |
| Ni3S2/CNF//CNF | Active material | 0-1.7 | 56.6 (1 A∙g-1) | 35.4 (10 A∙g-1) | 97% (2500) | 25.8 | 0.425 | [15] |
| CNF/MnO2 | Active material | 0.15-1.15 (vs. SCE) | 273 (2 mV∙s-1) | 75% (100 mV∙s-1) | — | — | — | [12] |
| CNF//CNF/MnO2 | Active material | 0-2 | 113 (20 mV∙s-1) | 53% (10~200 mV∙s-1) | 92% (5000) | 63 | 8 | [12] |
| N-CNF@LDH | Active material | 0-0.5 (Ag/AgCl) | 1949.5 (1 A∙g-1) | 54.7 (10 A∙g-1) | 74.4% (5000) | — | — | [23] |
| N-CNF@LDH// N-CNF | Active material | 0-1.6 | 101.9 (1 A∙g-1) | 63.8 (10 A∙g-1) | 89.3% (2500) | 36.3 | 8 | [23] |
表2 BC基复合材料超级电容器电极的电化学性能
Table 2 BC-based composites electrodes for supercapacitor
| Material | Function of BC | Potential window/V | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density/ (Wh∙kg-1) | Highest power density/ (kW∙kg-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CNF@MnO2 | Active material | 0-1 (vs. Ag/AgCl) | 254.64 (1 A∙g-1) | 77.53% (10 A∙g-1) | — | — | — | [24] |
| CNF@MnO2// N-CNF | Active material | 0-2 | — | — | 95.4% (2000) | 32.91 | 284.63 | [24] |
| Ni3S2/CNF | Active material | 0-0.6 (vs. Ag/AgCl) | 957 (1 A∙g-1) | 703 (8 A∙g-1) | 16.5% (1000) | — | — | [15] |
| Ni3S2/CNF//CNF | Active material | 0-1.7 | 56.6 (1 A∙g-1) | 35.4 (10 A∙g-1) | 97% (2500) | 25.8 | 0.425 | [15] |
| CNF/MnO2 | Active material | 0.15-1.15 (vs. SCE) | 273 (2 mV∙s-1) | 75% (100 mV∙s-1) | — | — | — | [12] |
| CNF//CNF/MnO2 | Active material | 0-2 | 113 (20 mV∙s-1) | 53% (10~200 mV∙s-1) | 92% (5000) | 63 | 8 | [12] |
| N-CNF@LDH | Active material | 0-0.5 (Ag/AgCl) | 1949.5 (1 A∙g-1) | 54.7 (10 A∙g-1) | 74.4% (5000) | — | — | [23] |
| N-CNF@LDH// N-CNF | Active material | 0-1.6 | 101.9 (1 A∙g-1) | 63.8 (10 A∙g-1) | 89.3% (2500) | 36.3 | 8 | [23] |
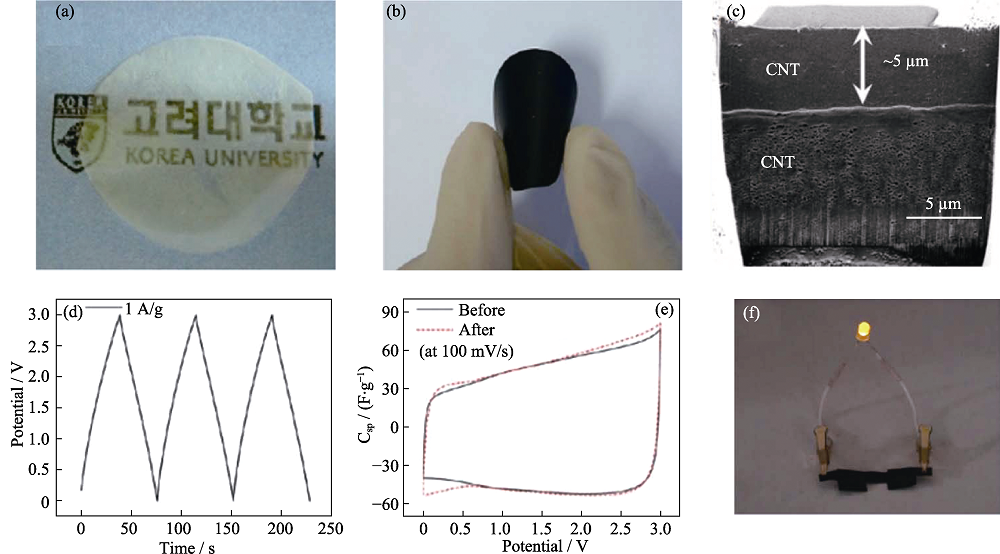
图5 (a)BC纸和(b)CNT/BC纸的照片; (c)CNT/BC纸的SEM截面照片; 柔性超级电容器的 (d)GCD曲线和(e)CV曲线; (f)柔性超级电容器照片[41]
Fig. 5 Photographs of (a) BC paper and (b) flexible CNT/BC paper; (c) Cross-sectional image of CNT/BC paper; (d) GCD curve and (e) CV curves for CNT/BC/ion gel flexible supercapacitors; (f) Photograph of a LED turned on by the flexible supercapacitors[41]
| Material | Function of BC | Potential window/V | Capacitance/ (mF∙cm-2) | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density | Highest power density | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| N-CNF/RGO/BC | Active material & substrate | -0.8-0.2 (vs. Hg/HgO) | 2106 (1 mV∙s-1) | 263 | 76% (50 mV∙s-1) | 100% (2×104) | — | — | [45] |
| N-CNF/RGO/BC//N-CNF/RGO/BC | Active material & substrate | 0-1 | 810 (2 mV∙s-1) | — | 755 (50 mV∙s-1) | 99.6% (104) | 0.11 mWh∙cm-2 | 27 mW∙cm-2 | [46] |
| N,P-CNF/RGO/BC | Active material & substrate | -0.8-0.2 (vs. Hg/HgO) | 1900 (2 mV∙s-1) | 244.8 | 1554 (50 mV∙s-1) | 100% (2×104) | — | — | [16] |
| N,P-CNF/RGO/ BC//N,P-CNF/ RGO/BC | Active material & substrate | 0-1 | 690 (2 mV∙s-1) | — | 620 (40 mV∙s-1) | 99.6% (1×104) | 0.096 mWh∙cm-2 | 19.98 mW∙cm-2 | [16] |
| BC/GO electrode | Scaffold | -0.2-0.8 (vs. SCE) | — | 160 (0.4 A∙g-1) | 68 (2 A∙g-1) | 90.3% (2×103) | — | — | [44] |
| BC/CNT/ion gel supercapacitors | Substrate | 0-3 | 18.8 (100 mV∙s-1) | 46.9 | 42.0 (500 mV∙s-1) | 99.5% (5×104) | 15.5 Wh∙kg-1 | 1.5 kW∙kg-1 | [41] |
| a-CNF//BC gel// a-CNF supercapacitors | Active material & electrolyte & separator | 0-1 | 289 (0.1 mA∙cm-2) | — | 70% (10 mA∙cm-2) | 66.7% (100) | — | — | [60] |
表3 BC基柔性超级电容器电极的电化学性能
Table 3 BC-based electrodes for flexible EDLCs
| Material | Function of BC | Potential window/V | Capacitance/ (mF∙cm-2) | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density | Highest power density | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| N-CNF/RGO/BC | Active material & substrate | -0.8-0.2 (vs. Hg/HgO) | 2106 (1 mV∙s-1) | 263 | 76% (50 mV∙s-1) | 100% (2×104) | — | — | [45] |
| N-CNF/RGO/BC//N-CNF/RGO/BC | Active material & substrate | 0-1 | 810 (2 mV∙s-1) | — | 755 (50 mV∙s-1) | 99.6% (104) | 0.11 mWh∙cm-2 | 27 mW∙cm-2 | [46] |
| N,P-CNF/RGO/BC | Active material & substrate | -0.8-0.2 (vs. Hg/HgO) | 1900 (2 mV∙s-1) | 244.8 | 1554 (50 mV∙s-1) | 100% (2×104) | — | — | [16] |
| N,P-CNF/RGO/ BC//N,P-CNF/ RGO/BC | Active material & substrate | 0-1 | 690 (2 mV∙s-1) | — | 620 (40 mV∙s-1) | 99.6% (1×104) | 0.096 mWh∙cm-2 | 19.98 mW∙cm-2 | [16] |
| BC/GO electrode | Scaffold | -0.2-0.8 (vs. SCE) | — | 160 (0.4 A∙g-1) | 68 (2 A∙g-1) | 90.3% (2×103) | — | — | [44] |
| BC/CNT/ion gel supercapacitors | Substrate | 0-3 | 18.8 (100 mV∙s-1) | 46.9 | 42.0 (500 mV∙s-1) | 99.5% (5×104) | 15.5 Wh∙kg-1 | 1.5 kW∙kg-1 | [41] |
| a-CNF//BC gel// a-CNF supercapacitors | Active material & electrolyte & separator | 0-1 | 289 (0.1 mA∙cm-2) | — | 70% (10 mA∙cm-2) | 66.7% (100) | — | — | [60] |
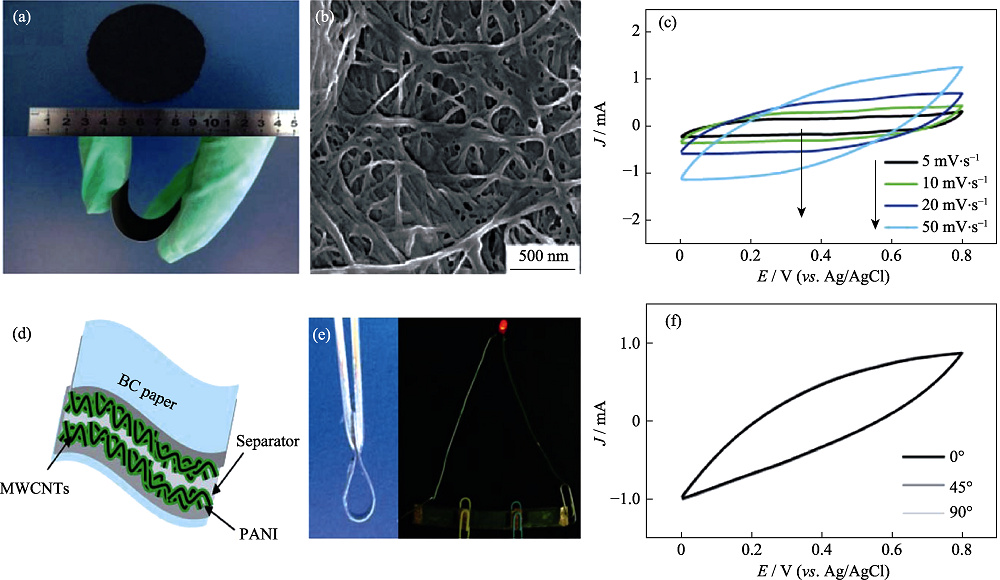
图6 (a) CNT/BC和PANI/CNT/BC的照片; (b) PANI/CNT/BC的SEM照片和(c) CV曲线; (d) PANI/CNT/BC柔性超级电容器的结构示意图, (e)电子照片和(f)不同弯折角度的CV曲线[1]
Fig. 6 (a) Photographs of CNT/BC paper and PANI/CNT/BC paper; (b) SEM image and (c) CV curves of PANI/CNT/BC electrode; (d) Schematic structure, (e) digital images and (f) CV curves for flexible supercapacitor[1]
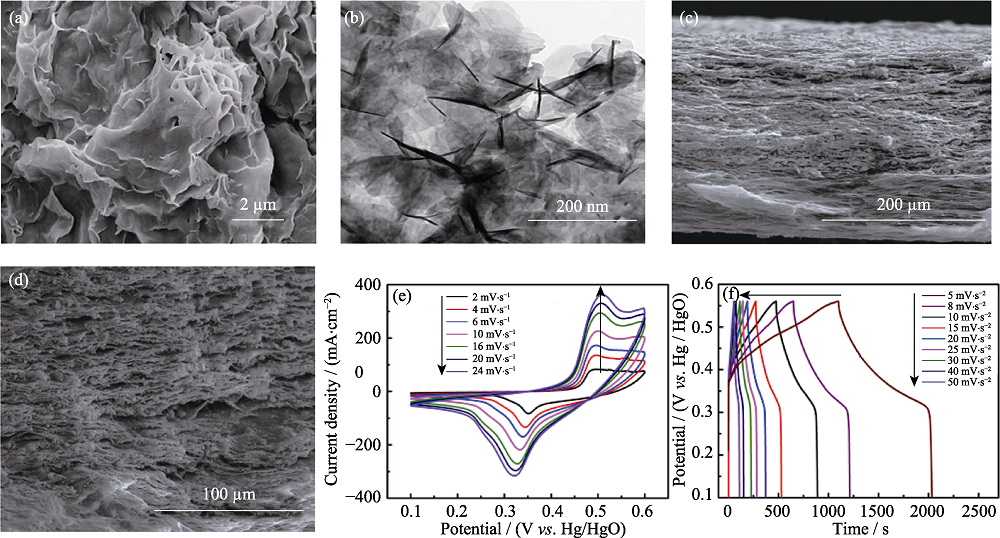
图7 Ni(OH)2/RGO/BC的(a)SEM和(b) TEM照片; (c,d)Ni(OH)2/RGO/BC的SEM截面照片; Ni(OH)2/RGO/BC电极的(e)CV和(f)GCD曲线[58]
Fig. 7 (a) SEM and (b) TEM images of Ni(OH)2/RGO/BC; (c, d) Cross-sectional SEM micrographs of Ni(OH)2/RGO/BC; (e) CV and (f) GCD curves of Ni(OH)2/RGO/BC electrode[58]
| [1] | LI S H, HUANG D K, ZHANG B Y , et al. Flexible supercapacitors based on bacterial cellulose paper electrodes. Adv. Energy Mater., 2014,4(10):1301655. |
| [2] | CHEN L F, HUANG Z H, LIANG H W , et al. Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors. Adv. Funct. Mater., 2014,24(32):5104-5111. |
| [3] | WU Z Y, LIANG H W, CHEN L F , et al. Bacterial cellulose: a robust platform for design of three dimensional carbon-based functional nanomaterials. Acc. Chem. Res., 2016,49(1):96-105. |
| [4] | MA L N, LIU R, NIU H J , et al. Flexible and freestanding electrode based on polypyrrole/graphene/bacterial cellulose paper for supercapacitor. Compos. Sci. Technol., 2016,137(12):87-93. |
| [5] | IGUCHI M, YAMANAKA S, BUDHIOKO A . Bacterial cellulose— a masterpiece of nature’s arts. J. Mater. Sci., 2000,35(2):261-270. |
| [6] | TIAN X D, LI X, YANG T , et al. Recent advances on synthesis and supercapacitor application of binary metal oxide. J. Inorg. Mater., 2017,32(5):459-468. |
| [7] | ZENG Y F, XIN G X, BU L C K , et al. One-step preparation and electrochemical performance of 3D reduced graphene oxide/NiO as supercapacitor electrodes materials. J. Inorg. Mater., 2018,33(10):1070-1076. |
| [8] | QU L, PEI H M, KONG R M , et al. Novel turn-on fluorescent detection of alkaline phosphatase based on green synthesized carbon dots and MnO2 nanosheets. Talanta, 2017,165:136-142. |
| [9] | ZHAO J, GONG X B, ZHANG R M , et al. Enhanced biosensing platform constructed using urchin-like ZnO-Au@CdS microspheres based on the combination of photoelectrochemical and bioetching strategies. Sens. Actuators B: Chem., 2018,255:1753-1761. |
| [10] | YAO J J, JI P, NAN SHENG N , et al. Hierarchical core-sheath polypyrrole@carbon nanotube/bacterial cellulose macrofibers with high electrochemical performance for allsolid-state supercapacitors. Electrochim. Acta, 2018,283:1578-1588. |
| [11] | LI J, ZHANG G F, CHEN N , et al. Built structure of ordered vertically aligned codoped carbon nanowire arrays for supercapacitors. ACS Appl. Mater. Interfaces, 2017,9:24840-24845. |
| [12] | LONG C L, QI D P, WEI T , et al. Nitrogen-doped carbon networks for high energy density supercapacitors derived from polyaniline coated bacterial cellulose. Adv. Funct. Mater., 2014,24(25):3953-3961. |
| [13] | LEI W, HAN L L, XUAN C J , et al. Nitrogen-doped carbon nanofibers derived from polypyrrole coated bacterial cellulose as high-performance electrode materials for supercapacitors and Li-ion batteries. Electrochim. Acta, 2016,210:130-137. |
| [14] | LEE K Y, QIAN H, TAY F H , et al. Bacterial cellulose as source for activated nanosized carbon for electric double layer capacitors. J. Mater. Sci., 2013,48(1):367-376. |
| [15] | YU W D, LIN W R, SHAO X F , et al. High performance supercapacitor based on Ni3S2/carbon nanofibers and carbon nanofibers electrodes derived from bacterial cellulose. J. Power Sour., 2014,272:137-143. |
| [16] | LIU R, MA L N, MEI J , et al. Large areal mass, mechanically tough and freestanding electrode based on heteroatom-doped carbon nanofibers for flexible supercapacitors. Chem-Eur. J., 2017,23(11):2610-2618. |
| [17] | YUAN D, HUANG X, YAN J , et al. Porous carbon nanofibers derived from bacterial cellulose for sustainable energy storage. Science of Advanced Materials, 2013,5(11):1694-1700. |
| [18] | JIANG Y T, YAN J, WU X L , et al. Facile synthesis of carbon nanofibers-bridged porous carbon nanosheets for high-performance supercapacitors. J. Power Sour., 2016,307:190-198. |
| [19] | LAI F L, MIAO Y E, ZUO L Z , et al. Carbon aerogels derived from bacterial cellulose/polyimide composites as versatile adsorbents and supercapacitor electrodes. ChemNanoMat, 2016,2(3):212-219. |
| [20] | WU Z Y, LIANG H W, LI C , et al. Dyeing bacterial cellulose pellicles for energetic heteroatom doped carbon nanofiber aerogel. Nano Res., 2014,7(12):1861-1872. |
| [21] | LUO H L, DONG J J, ZHANG Y , et al. Constructing 3D bacterial cellulose/graphene/polyaniline nanocomposites by novel layer-by- layer in situ culture toward mechanically robust and highly flexible freestanding electrodes for supercapacitors. Chem. Eng. J., 2018,334:1148-1158. |
| [22] | WU H, ZHANG Y Z, YUAN W Y , et al. Highly flexible, foldable and stretchable Ni-Co layered double hydroxide/polyaniline/bacterial cellulose electrodes for high performance all-solid-state supercapacitors. J. Mater. Chem. A, 2018,6(34):16617-16626. |
| [23] | LAI F L, MIAO Y, ZUO L Z , et al. Biomass-derived nitrogen- doped carbon nanofiber network: a facile template for decoration of ultrathin nickel-cobalt layered double hydroxide nanosheets as high-performance asymmetric supercapacitor electrode. Small, 2016,12(24):3235-3244. |
| [24] | CHEN L F, HUANG Z H, LIANG H W , et al. Bacterial-cellulose- derived carbon nanofiber@MnO2 and nitrogen-doped carbon nanofiber electrode materials: an asymmetric supercapacitor with high energy and power density. Adv. Mater., 2013,25(34):4746-4752. |
| [25] | CHEN L F, HUANG Z H, LIANG H W , et al. Flexible all-solid-state high-power supercapacitor fabricated with nitrogen- doped carbon nanofiber electrode material derived from bacterial cellulose. Energy Environ. Sci., 2013,6(11):3331-3338. |
| [26] | HU Z X, LI S S, CHENG P P , et al. N,P-co-doped carbon nanowires prepared from bacterial cellulose for supercapacitor. J. Mater. Sci., 2016,51(5):2627-2633. |
| [27] | LI S M, YANG S Y, WANG Y S , et al. N-doped structures and surface functional groups of reduced graphene oxide and their effect on the electrochemical performance of supercapacitor with organic electrolyte. J. Power Sources, 2015,278:218-229. |
| [28] | ZHAO L, HU Y S, LI H , et al. Porous Li4Ti5O12 coated with N-doped carbon from ionic liquids for Li-Ion batteries. Advanced Materials, 2011,23(11):1385-1388. |
| [29] | HAO L, LUO B, LI X , et al. Terephthalonitrile-derived nitrogen- rich networks for high performance supercapacitors. Energy & Environmental Science, 2012,5(12):9747-9751. |
| [30] | LIU Y Q, YAN Y, LI K , et al. A high-areal-capacity lithium-sulfur cathode achieved by a boron-doped carbon-sulfur aerogel with consecutive core-shell structures. Chem. Commun., 2019,55(8):1084-1087. |
| [31] | DING L G, YAO B J, LI F , et al. Ionic liquid-decorated COF and its covalent composite aerogel for selective CO2 adsorption and catalytic conversion. J. Mater. Chem. A, 2019, DOI: 10.1039/C8TA12046C. |
| [32] | LIN C, HUANG Q, ZHANG D , et al. Temperature programmed surface reaction test of Co-Ni bimetallic aerogel catalysts for methane reforming. React. Kinet. Mech. Cat., 2019, DOI: 10.1007/s11144-018-01531-3. |
| [33] | GIGOT A, FONTANA M, PIRRI C F , et al. Graphene/ruthenium active species aerogel as electrode for supercapacitor applications. Materials, 2018,11(1):57. |
| [34] | XU X Z, ZHOU J, NAGARAJU D H , et al. Flexible, highly graphitized carbon aerogels based on bacterial cellulose/lignin: catalyst-free synthesis and its application in energy storage devices. Adv. Funct. Mater., 2015,25(21):3193-3202. |
| [35] | MA L N, LIU R, NIU H J , et al. Freestanding conductive film based on polypyrrole/bacterial cellulose/graphene paper for flexible supercapacitor: large areal mass exhibits excellent areal capacitance. Electrochim. Acta, 2016,222(20):429-437. |
| [36] | LIU R, MA L N, HUANG S , et al. Large areal mass, flexible and freestanding polyaniline/bacterial cellulose/graphene film for high- performance supercapacitors. RSC Adv., 2016,6(109):107426-107432. |
| [37] | MULLER D, RECOUVREUX D O S, PORTO L M, , et al. Chemical in situ polymerization of polypyrrole on bacterial cellulose nanofibers. Synthetic Met., 2011,161(1/2):106-111. |
| [38] | LIU Y, ZHOU J, TANG J , et al. Three-dimensional, chemically bonded polypyrrole/bacterial cellulose/graphene composites for high- performance supercapacitors. Chem. Mater., 2015,27(20):7034-7041. |
| [39] | WU H, HUANG Y A, XU F , et al. Energy harvesters for wearable and stretchable electronics: from flexibility to stretchability. Adv. Mater., 2016,28(45):9881-9919. |
| [40] | ZHENG Y, YANG Y B, CHEN S S , et al. Smart, stretchable and wearable supercapacitors: prospects and challenges. CrystEngComm, 2016,18(23):4218-4235. |
| [41] | KANG Y J, CHUN S J, LEE S S , et al. All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers, carbon nanotubes, and triblock-copolymer ion gels. ACS Nano, 2012,6(7):6400-6406. |
| [42] | SUMBOJA A, FOO C Y, WANG X , et al. Large areal mass, flexible and free-standing reduced graphene oxide/manganese dioxide paper for asymmetric supercapacitor device. Adv. Mater., 2013,25(20):2809-2815. |
| [43] | XIONG Z Y, LIAO C L, HAN W H , et al. Mechanically tough large-area hierarchical porous graphene films for high-performance flexible supercapacitor applications. Adv. Mater., 2015,27(30):4469-4475. |
| [44] | LIU Y, ZHOU J, ZHU E W , et al. Facile synthesis of bacterial cellulose fibres covalently intercalated with graphene oxide by one- step cross-linking for robust supercapacitors. J. Mater. Chem. C, 2015,3(5):1011-1017. |
| [45] | MA L N, LIU R, NIU, H J , et al. Flexible and freestanding supercapacitor electrodes based on nitrogen-doped carbon networks/ graphene/bacterial cellulose with ultrahigh areal capacitance. ACS Appl. Mater. Interfaces, 2016,8(49):33608-33618. |
| [46] | XIA C, CHEN W, WANG X B , et al. Highly stable supercapacitors with conducting polymer core-shell electrodes for energy storage applications. Adv. Energy Mater., 2015,5(8):1401805. |
| [47] | YANG C Y, SHEN J L, WANG C Y , et al. All-solid-state asymmetric supercapacitor based on reduced graphene oxide/carbon nanotube and carbon fiber paper/polypyrrole electrodes. J. Mater. Chem. A, 2014,2(5):1458-1464. |
| [48] | ZHAO Y, LIU J, HU Y , et al. Highly compression-tolerant supercapacitor based on polypyrrole-mediated graphene foam electrodes. Adv. Mater., 2013,25(4):591-595. |
| [49] | SONG Y, XU J L, LIU X X . Electrochemical anchoring of dual doping polypyrrole on graphene sheets partially exfoliated from graphite foil for high-performance supercapacitor electrode. J. Power Sour., 2014,249:48-58. |
| [50] | LEE H J, CHUANG T J, KWON H J , et al. Fabrication and evaluation of bacterial cellulose-polyaniline composites by interfacial polymerization. Cellulose, 2012,19:1251-1258. |
| [51] | XU J, ZHU L G, BAI Z K , et al. Conductive polypyrrole-bacterial cellulose nanocomposite membranes as flexible supercapacitor electrode. Org. Electron., 2013,14(12):3331-3338. |
| [52] | PENG S, FAN L L, WEI C Z , et al. Flexible polypyrrole/copper sulfide/bacterial cellulose nanofibrous composite membranes as supercapacitor electrodes. Carbohyd. Polym., 2017,157:344-352. |
| [53] | PENG S, XU Q, FAN L L , et al. Flexible polypyrrole/cobalt sulfide/ bacterial cellulose composite membranes for supercapacitor application. Synthetic Met., 2016,222:285-292. |
| [54] | PENG S, FAN L L, WEI C Z , et al. Polypyrrole/nickel sulfide/ bacterial cellulose nanofibrous composite membranes for flexible supercapacitor electrodes. Cellulose, 2016,23(4):2639-2651. |
| [55] | WANG F, KIM H J, PARK S , et al. Bendable and flexible supercapacitor based on polypyrrole-coated bacterial cellulose core-shell composite network. Compos. Sci. Technol., 2016,128(18):33-40. |
| [56] | YUAN L, YAO B, HU B , et al. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci., 2013,6(2):470-476. |
| [57] | LIU R, MA L N, HUANG S , et al. A flexible polyaniline/ graphene/bacterial cellulose supercapacitor electrode. New J. Chem., 2017,41(2):857-864. |
| [58] | MA L N, LIU R, WANG F , et al. Facile synthesis of Ni(OH)2/graphene/bacterial cellulose paper for large areal mass, mechanically tough and flexible supercapacitor electrodes. J. Power Sour., 2016,335(15):76-83. |
| [59] | LIU R, MA L N, HUANG S , et al. Large areal mass and high scalable and flexible cobalt oxide/graphene/bacterial cellulose electrode for supercapacitors. J. Phys. Chem. C, 2016,120(50):28480-28488. |
| [60] | WANG X, KONG D, ZHANG Y , et al. All-biomaterial supercapacitor derived from bacterial cellulose. Nanoscale, 2016,8(17):9146-9150. |
| [1] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| [2] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [3] | 陈强, 白书欣, 叶益聪. 热管理用高导热碳化硅陶瓷基复合材料研究进展[J]. 无机材料学报, 2023, 38(6): 634-646. |
| [4] | 林俊良, 王占杰. 铁电超晶格的研究进展[J]. 无机材料学报, 2023, 38(6): 606-618. |
| [5] | 牛嘉雪, 孙思, 柳鹏飞, 张晓东, 穆晓宇. 铜基纳米酶的特性及其生物医学应用[J]. 无机材料学报, 2023, 38(5): 489-502. |
| [6] | 苑景坤, 熊书锋, 陈张伟. 聚合物前驱体转化陶瓷增材制造技术研究趋势与挑战[J]. 无机材料学报, 2023, 38(5): 477-488. |
| [7] | 游钧淇, 李策, 杨栋梁, 孙林锋. 氧化物双介质层忆阻器的设计及应用[J]. 无机材料学报, 2023, 38(4): 387-398. |
| [8] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [9] | 杨洋, 崔航源, 祝影, 万昌锦, 万青. 柔性神经形态晶体管研究进展[J]. 无机材料学报, 2023, 38(4): 367-377. |
| [10] | 齐占国, 刘磊, 王守志, 王国栋, 俞娇仙, 王忠新, 段秀兰, 徐现刚, 张雷. GaN单晶的HVPE生长与掺杂进展[J]. 无机材料学报, 2023, 38(3): 243-255. |
| [11] | 林思琪, 李艾燃, 付晨光, 李荣斌, 金敏. Zintl相Mg3X2(X=Sb, Bi)基晶体生长及热电性能研究进展[J]. 无机材料学报, 2023, 38(3): 270-279. |
| [12] | 张超逸, 唐慧丽, 李宪珂, 王庆国, 罗平, 吴锋, 张晨波, 薛艳艳, 徐军, 韩建峰, 逯占文. 新型GaN与ZnO衬底ScAlMgO4晶体的研究进展[J]. 无机材料学报, 2023, 38(3): 228-242. |
| [13] | 陈昆峰, 胡乾宇, 刘锋, 薛冬峰. 多尺度晶体材料的原位表征技术与计算模拟研究进展[J]. 无机材料学报, 2023, 38(3): 256-269. |
| [14] | 刘岩, 张珂颖, 李天宇, 周菠, 刘学建, 黄政仁. 陶瓷材料电场辅助连接技术研究现状及发展趋势[J]. 无机材料学报, 2023, 38(2): 113-124. |
| [15] | 谢兵, 蔡金峡, 王铜铜, 刘智勇, 姜胜林, 张海波. 高储能密度聚合物基多层复合电介质的研究进展[J]. 无机材料学报, 2023, 38(2): 137-147. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||