无机材料学报 ›› 2019, Vol. 34 ›› Issue (2): 145-151.DOI: 10.15541/jim20180203
胡茜1,刘洪波1,2,夏笑虹1,2,谷智强1
收稿日期:2018-05-02
修回日期:2018-05-30
出版日期:2019-02-20
网络出版日期:2019-01-24
作者简介:胡茜(1993-),女,硕士研究生.E-mail: xihuzwc@163.com
基金资助:HU Xi1, LIU Hong-Bo1, 2, XIA Xiao-Hong1, 2, GU Zhi-Qiang1
Received:2018-05-02
Revised:2018-05-30
Published:2019-02-20
Online:2019-01-24
About author:HU Xi. E-mail: xihuzwc@163.com
Supported by:摘要:
通过真空抽滤诱导自组装及热解还原处理, 制备出具有柱撑结构的聚苯胺炭/石墨烯复合材料(PGR)。采用X射线衍射(XRD)、透射电子显微镜(TEM)、X射线电子能谱(XPS)和电化学测试等表征技术考察了聚苯胺单体(AN)与氧化石墨烯(GO)质量比对PGR结构和电化学性能的影响。结果表明, 聚苯胺炭均匀分布在石墨烯(GR)片层间形成三维导电网络, 有效地增大了GR的层间距, 且实现了氮掺杂, 显著提高了GR的结构稳定性和电化学性能; AN与GO质量比为1 : 1时制备的样品PGR1在100 mA/g电流密度下的首次脱锂比容量为653 mAh/g, 当电流密度增大至1 A/g时, 仍具有高达343 mAh/g的脱锂比容量, 远高于GR的脱锂比容量(101 mAh/g), 表现出优异的倍率性能。
中图分类号:
胡茜,刘洪波,夏笑虹,谷智强. 聚苯胺炭柱撑石墨烯复合材料的制备及其电化学性能的研究[J]. 无机材料学报, 2019, 34(2): 145-151.
HU Xi, LIU Hong-Bo, XIA Xiao-Hong, GU Zhi-Qiang. Polyaniline-carbon Pillared Graphene Composite: Preparation and Electrochemical Performance[J]. Journal of Inorganic Materials, 2019, 34(2): 145-151.
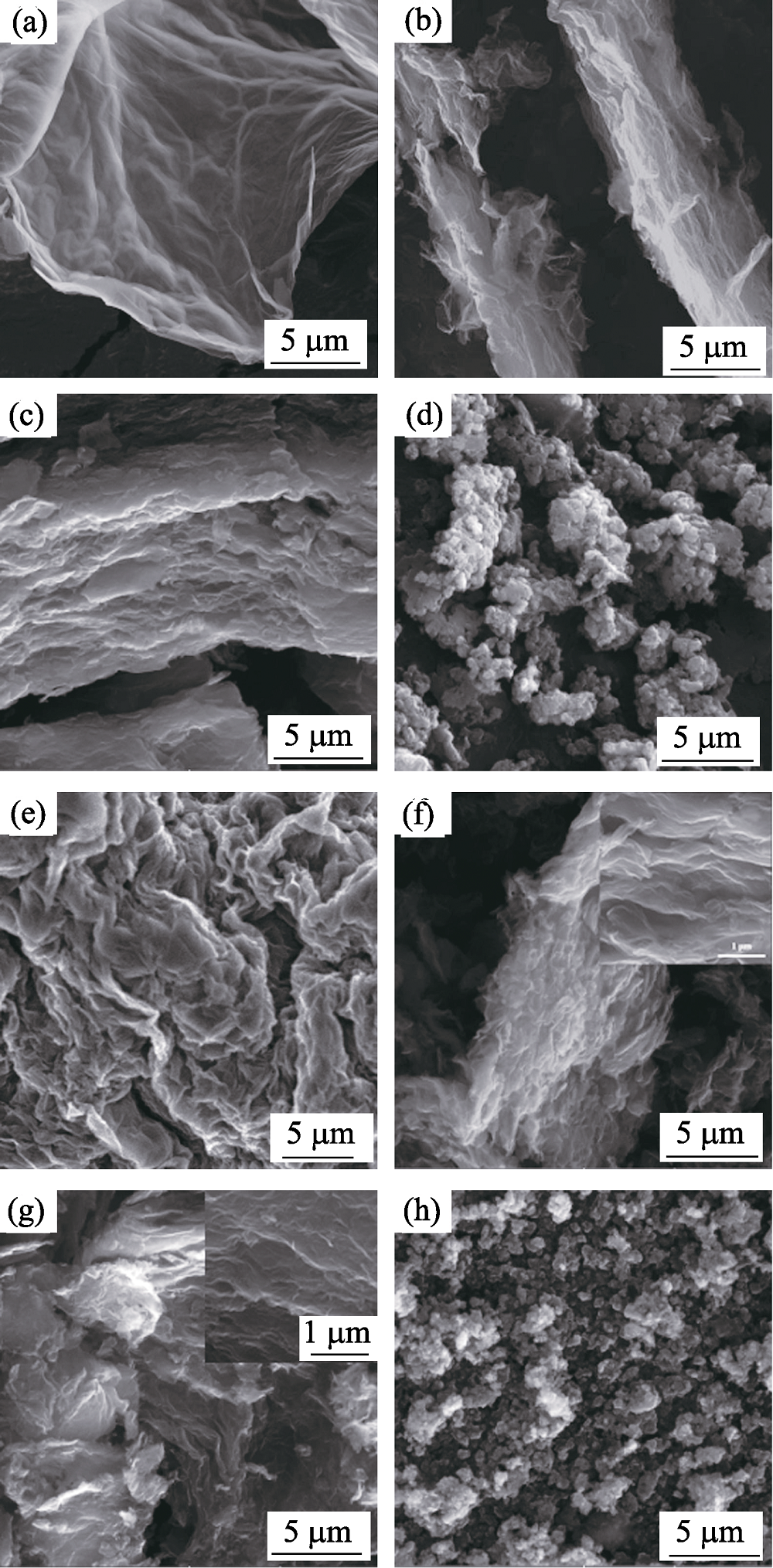
图3 (a) GO, (b) PGO0.5, (c) PGO1, (d) PANI, (e) GR, (f) PGR0.5, (g) PGR1和(h) CP的SEM照片
Fig. 3 SEM images of (a) GO, (b) PGO0.5, (c) PGO1, (d) PANI, (e) GR, (f) PGR0.5, (g) PGR1 and (h) CP with insets showing magnified photos
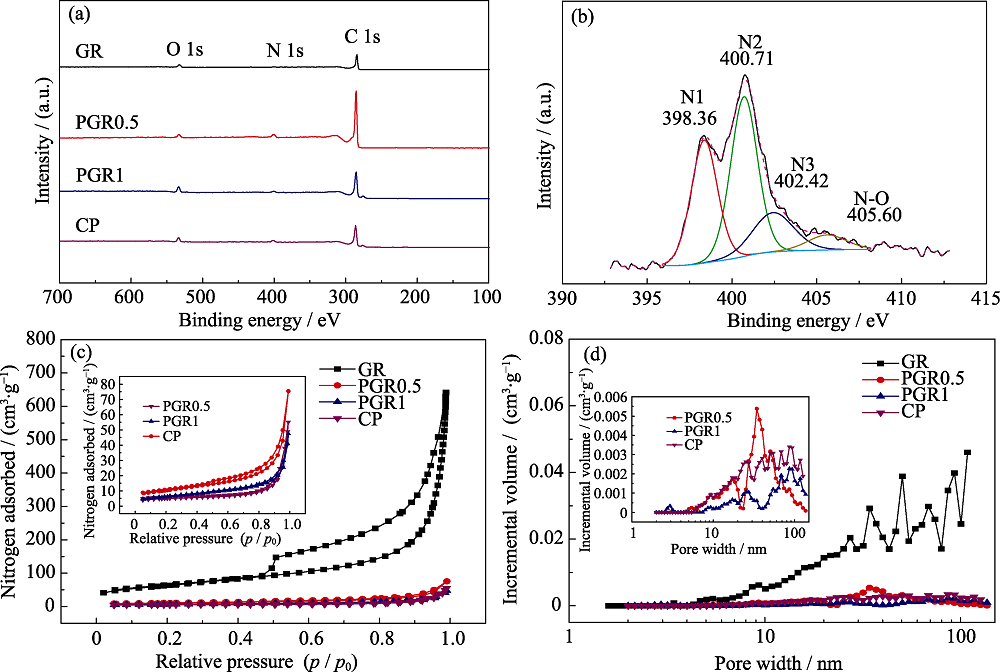
图6 (a) GR、PGR0.5、PGR1和CP的XPS图谱; (b) PGR1的N1s峰的分峰曲线; (c) GR、PGR0.5、PGR1和CP的氮气吸脱附等温线和(d)孔径分布图
Fig. 6 (a) XPS general spectra of GR, PGRs and CP, (b) curve fitting of N1s spectrum of the sample PGR1, (c) nitrogen sorption isotherms, and (d) DFT pore size distributions of GR, PGRs and CP
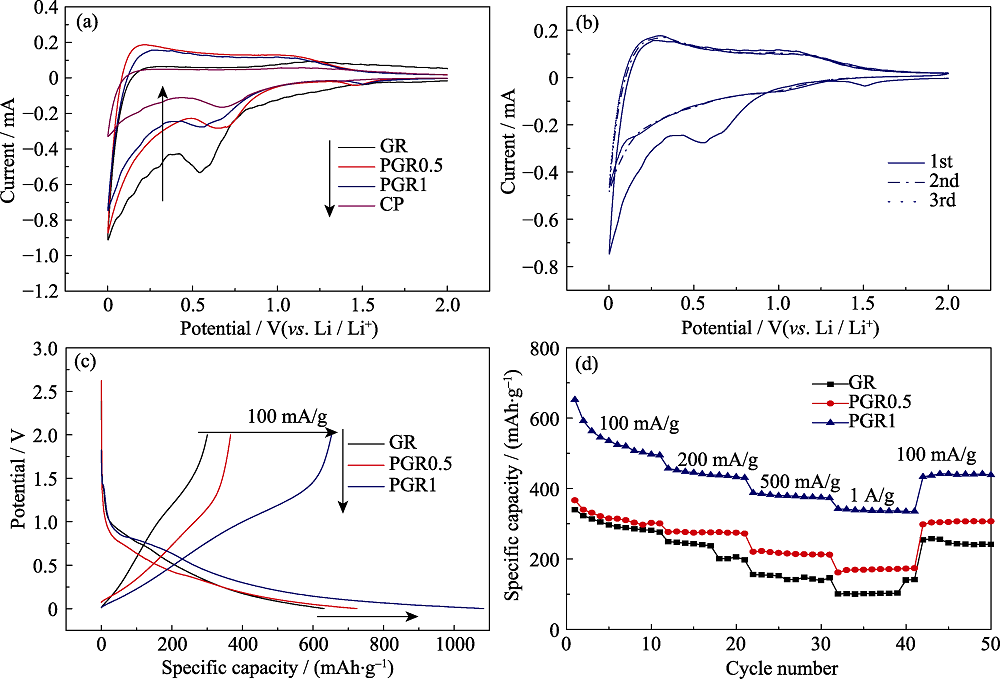
图7 GR、PGR0.5和PGR1的电化学性能
Fig. 7 Electrochemical performance of GR, PGR0.5, and PGR1(a) The first CV curve of the samples; (b) The first to the third CV curves of PGR1; (c) The first charge/discharge profile cycle and (d) rate capability
| Sample | Rs/? | Rsei/? | Rct/? |
|---|---|---|---|
| GR | 7.23 | 141.3 | 90.67 |
| PGR0.5 | 6.03 | 47.13 | 82.87 |
| PGR1 | 2.02 | 54.50 | 0.10 |
表1 GR、PGR0.5和PGR1负极材料的交流阻抗拟合数据
Table 1 EIS fitting results of GR, PGR0.5, and PGR1 electrodes
| Sample | Rs/? | Rsei/? | Rct/? |
|---|---|---|---|
| GR | 7.23 | 141.3 | 90.67 |
| PGR0.5 | 6.03 | 47.13 | 82.87 |
| PGR1 | 2.02 | 54.50 | 0.10 |
| [1] | NOVOSELOV K S, GEIM A K, MOROZOV S V,et al. Two-dimensional gas of massless Dirac fermions in graphene. Nature, 2005, 438(7065): 197-200. |
| [2] | HAN P X, YUE Y H, ZHANG L X,et al. Nitrogen-doping of chemically reduced mesocarbon microbead oxide for the improved performance of lithium ion batteries. Carbon, 2012, 50(3): 1355-1362. |
| [3] | LI D, MULLER M B, GILJE S,et al. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol., 2008, 3(2): 101-105. |
| [4] | WANG G X, SHEN X P, YAO J,et al. Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon, 2009, 47(8): 2049-2053. |
| [5] | BONACCORSO F, SUN Z, HASAN T,et al. Graphene photonics and optoelectronics. Nat. Photonics, 2010, 4(9): 611-622. |
| [6] | WAN L J, REN Z Y, WANG H,et al. Graphene nanosheets based on controlled exfoliation process for enhanced lithium storage in lithium-ion battery. Diam. Relat. Mater., 2011, 20(5/6): 756-761. |
| [7] | LEE S H, SEO S D, PARK K S,et al. Synthesis of graphene nanosheets by the electrolytic exfoliation of graphite and their direct assembly for lithium ion battery anodes. Mater. Chem. Phys., 2012, 135(2/3): 309-316. |
| [8] | SANG Y, ZHOU Y, XIE H,et al. Assembling and nanocutting graphene/CNT sponge for improved lithium-ion batteries. Ionics, 2017, 23(5): 1329-1336. |
| [9] | KIM C, KIM J W, KIM H,et al. Graphene oxide assisted synthesis of self-assembled zinc oxide for lithium-ion battery anode. Chemistry of Materials, 2016, 28(23): 8498-8503. |
| [10] | ZHANG Q Q, LI R, ZHANG M M,et al. TiO2 nanocrystals/ graphene hybrids with enhanced Li-ion storage performance. J. Energy Chem., 2014, 23(3): 403-410. |
| [11] | LI L, RAJI A R, TOUR J M.Graphene-wrapped MnO2-graphene nanoribbons as anode materials for high-performance lithium ion batteries.Advanced Materials, 2013, 25(43): 6298-6302. |
| [12] | PING, G Z, ZHANG J Y, CHENG J,et al. Graphene nanosheets prepared by low-temperature exfoliation and reduction technique toward fabrication of high-performance poly(1-butene)/graphene films. Iran. Polym. J., 2017, 26(1): 55-69. |
| [13] | SHEN B, LU D D, ZHAI W T,et al. Synthesis of graphene by low-temperature exfoliation and reduction of graphite oxide under ambient atmosphere. J. Mater. Chem. C, 2013, 1(1): 50-53. |
| [14] | MENG L Y, PARK S J. Synthesis of graphene nanosheets via thermal exfoliation of pretreated graphite at low temperature. Adv. Mater. Res-Switz, 2010, 123-125: 787-790. |
| [15] | NIU S Z, LV W, ZHANG C,et al. One-pot self-assembly of graphene/ carbon nanotube/sulfur hybrid with three dimensionally interconnected structure for lithium-sulfur batteries. Journal of Power Sources, 2015, 295: 182-189. |
| [16] | WU H W, HUANG Y, ZONG M,et al.Electrostatic self-assembly of graphene oxide wrapped sulfur particles for lithium-sulfur batteries. Mater. Res. Bull., 2015, 64: 12-16. |
| [17] | TANG J J, YANG J, ZHOU L M,et al. Layer-by-layer self- assembly of a sandwich-like graphene wrapped SnOx@graphene composite as an anode material for lithium ion batteries. J. Mater. Chem. A, 2014, 2(18): 6292-6295. |
| [18] | BAI X J, LIU C, HOU M,et al. Silicon/CNTs/graphene free-standing anode material for lithium-ion battery. Journal of Inorganic Materials, 2017, 32(7): 705-712. |
| [19] | HU A P, CHEN X H, TANG Y H,et al. Self-assembly of Fe3O4 nanorods on graphene for lithium ion batteries with high rate capacity and cycle stability. Electrochemistry Communications, 2013, 28: 139-142. |
| [20] | ZHAO M Q, LIU X F, ZHANG Q,et al. Graphene/single-walled carbon nanotube hybrids: one-step catalytic growth and applications for high-rate Li-S batteries. ACS Nano, 2012, 6(12): 10759-10769. |
| [21] | KUMAR N A, CHOI H J, SHIN Y R.Polyaniline-grafted reduced graphene oxide for efficient electrochemical supercapacitors.ACS Nano, 2012, 6(2): 1715-1723. |
| [22] | ZHANG K, ZHANG L L, ZHAO X S,et al. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chemistry of Materials, 2010, 22(4): 1392-1401. |
| [23] | ZHU B Y, DENG Z, YANG W L,et al. Pyrolyzed polyaniline and graphene nano sheet composite with improved rate and cycle performance for lithium storage. Carbon, 2015, 92: 354-361. |
| [24] | HUMMERS W S, OFFEMAN R E.Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80: 1339-1339. |
| [25] | YANG F, XU M, BAO S J,et al. Self-assembled hierarchical graphene/ polyaniline hybrid aerogels for electrochemical capacitive energy storage. Electrochimica Acta, 2014, 137: 381-387. |
| [26] | SUN Y B, SHAO D, CHEN C,et al. Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environmental Science & Technology, 2013, 47(17): 9904-9910. |
| [27] | FAN W, XIA Y Y, TJIU W W,et al. Nitrogen-doped graphene hollow nanospheres as novel electrode materials for supercapacitor applications. Journal of Power Sources, 2013, 243: 973-981. |
| [28] | WANG C, WANG Y L, ZHAN L,et al. Synthesis of nitrogen doped graphene through microwave irradiation. Journal of Inorganic Materials, 2012, 27(2): 146-150. |
| [29] | LI X, GENG D, ZHANG Y,et al. Superior cycle stability of nitrogen- doped graphene nanosheets as anodes for lithium ion batteries. Electrochemistry Communications, 2011, 13(8): 822-825. |
| [30] | WANG D W, LI F, LIU M,et al. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed., 2008, 47(2): 373-376. |
| [31] | LI X F, GENG D S, ZHANG Y,et al. Superior cycle stability of nitrogen-doped graphene nanosheets as anodes for lithium ion batteries. Electrochemistry Communications, 2011, 13: 822-825. |
| [32] | WANG Z, CHEN T, CHEN W X,et al. CTAB-assisted synthesis of single-layer MoS2-graphene composites as anode materials of Li-ion batteries. J. Mater. Chem. A, 2013, 1(6): 2202-2210. |
| [1] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [2] | 陈赛赛, 庞雅莉, 王娇娜, 龚䶮, 王锐, 栾筱婉, 李昕. 绿-黄可逆电热致变色织物的制备及其性能[J]. 无机材料学报, 2022, 37(9): 954-960. |
| [3] | 宿拿拿, 韩静茹, 郭印毫, 王晨宇, 石文华, 吴亮, 胡执一, 刘婧, 李昱, 苏宝连. 基于ZIF-8的三维网络硅碳复合材料锂离子电池性能研究[J]. 无机材料学报, 2022, 37(9): 1016-1022. |
| [4] | 王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029. |
| [5] | 朱河圳, 王选朋, 韩康, 杨晨, 万睿哲, 吴黎明, 麦立强. 超高镍LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2正极材料的储锂稳定性的提升机制[J]. 无机材料学报, 2022, 37(9): 1030-1036. |
| [6] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [7] | 陈莹, 栾伟玲, 陈浩峰, 朱轩辰. 基于应力场的锂离子电池正极多尺度失效研究[J]. 无机材料学报, 2022, 37(8): 918-924. |
| [8] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [9] | 苏东良, 崔锦, 翟朋博, 郭向欣. 石榴石型Li6.4La3Zr1.4Ta0.6O12对Si/C负极表面固体电解质中间相的调控机制研究[J]. 无机材料学报, 2022, 37(7): 802-808. |
| [10] | 孙铭, 邵溥真, 孙凯, 黄建华, 张强, 修子扬, 肖海英, 武高辉. RGO/Al复合材料界面性质第一性原理研究[J]. 无机材料学报, 2022, 37(6): 651-659. |
| [11] | 肖美霞, 李苗苗, 宋二红, 宋海洋, 李钊, 毕佳颖. 表面端基卤化Ti3C2 MXene应用于锂离子电池高容量电极材料的研究[J]. 无机材料学报, 2022, 37(6): 660-668. |
| [12] | 王禹桐, 张非凡, 许乃才, 王春霞, 崔立山, 黄国勇. 水系锂离子电池负极材料LiTi2(PO4)3的研究进展[J]. 无机材料学报, 2022, 37(5): 481-492. |
| [13] | 安琳, 吴淏, 韩鑫, 李耀刚, 王宏志, 张青红. 非贵金属Co5.47N/N-rGO助催化剂增强TiO2光催化制氢性能[J]. 无机材料学报, 2022, 37(5): 534-540. |
| [14] | 王虹力, 王男, 王丽莹, 宋二红, 赵占奎. 功能化石墨烯担载型AuPd纳米催化剂增强甲酸制氢反应[J]. 无机材料学报, 2022, 37(5): 547-553. |
| [15] | 董淑蕊, 赵笛, 赵静, 金万勤. 离子化氨基酸对氧化石墨烯膜渗透汽化过程中水选择性渗透的影响[J]. 无机材料学报, 2022, 37(4): 387-394. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||