无机材料学报 ›› 2019, Vol. 34 ›› Issue (1): 96-102.DOI: 10.15541/jim20180172
所属专题: MAX相和MXene材料; 光伏材料
熊浩1, 张渤昕1, 贾巍2, 张青红1, 谢华清3
收稿日期:2018-04-19
修回日期:2018-08-01
出版日期:2019-01-21
网络出版日期:2018-12-17
作者简介:熊浩(1990-),男,博士研究生. E-mail: xhqmlwhj@126.com
基金资助:XIONG Hao1, ZHANG Bo-Xin1, JIA Wei2, ZHANG Qing-Hong1, XIE Hua-Qing3
Received:2018-04-19
Revised:2018-08-01
Published:2019-01-21
Online:2018-12-17
About author:XIONG Hao. E-mail: xhqmlwhj@126.com
摘要:
作为一类新型薄膜太阳能电池, 近年来钙钛矿太阳电池的发展十分迅速, 其效率已接近商业化硅基太阳能电池, 但是钙钛矿薄膜在空气中稳定性较差, 严重限制了其进一步的商业化应用。本研究通过在钙钛矿薄膜中添加聚4-乙烯吡啶(PVP)来增强钙钛矿薄膜在空气中的稳定性。通过形貌、结构及性能测试, 发现相比于未添加PVP的钙钛矿薄膜, 添加PVP的钙钛矿薄膜形貌更均匀致密。添加0.4wt% PVP将钙钛矿太阳电池的光电效率从6.09%提升到13.07%, 而且, 存放在相对湿度超过50%的空气中, 其电池效率衰减为一半的时间由原来的3 d延长到3 w, 但是过多的PVP添加量会导致PbI2与CH3NH3I反应不完全。添加PVP工艺进一步优化后, 有望用于大面积、高稳定性的钙钛矿薄膜的制备。
中图分类号:
熊浩, 张渤昕, 贾巍, 张青红, 谢华清. 高分子PVP添加剂对钙钛矿太阳电池稳定性的提升[J]. 无机材料学报, 2019, 34(1): 96-102.
XIONG Hao, ZHANG Bo-Xin, JIA Wei, ZHANG Qing-Hong, XIE Hua-Qing. Polymer PVP Additive for Improving Stability of Perovskite Solar Cells[J]. Journal of Inorganic Materials, 2019, 34(1): 96-102.

图1 添加不同含量PVP的PbI2薄膜的SEM照片
Fig. 1 Surface SEM images of PbI2 films on the glass with or without polymer modification (a) Without PVP; (b) 0.2wt% PVP; (c) 0.4wt% PVP; (d) 0.6wt% PVP; (e) 0.8wt% PVP
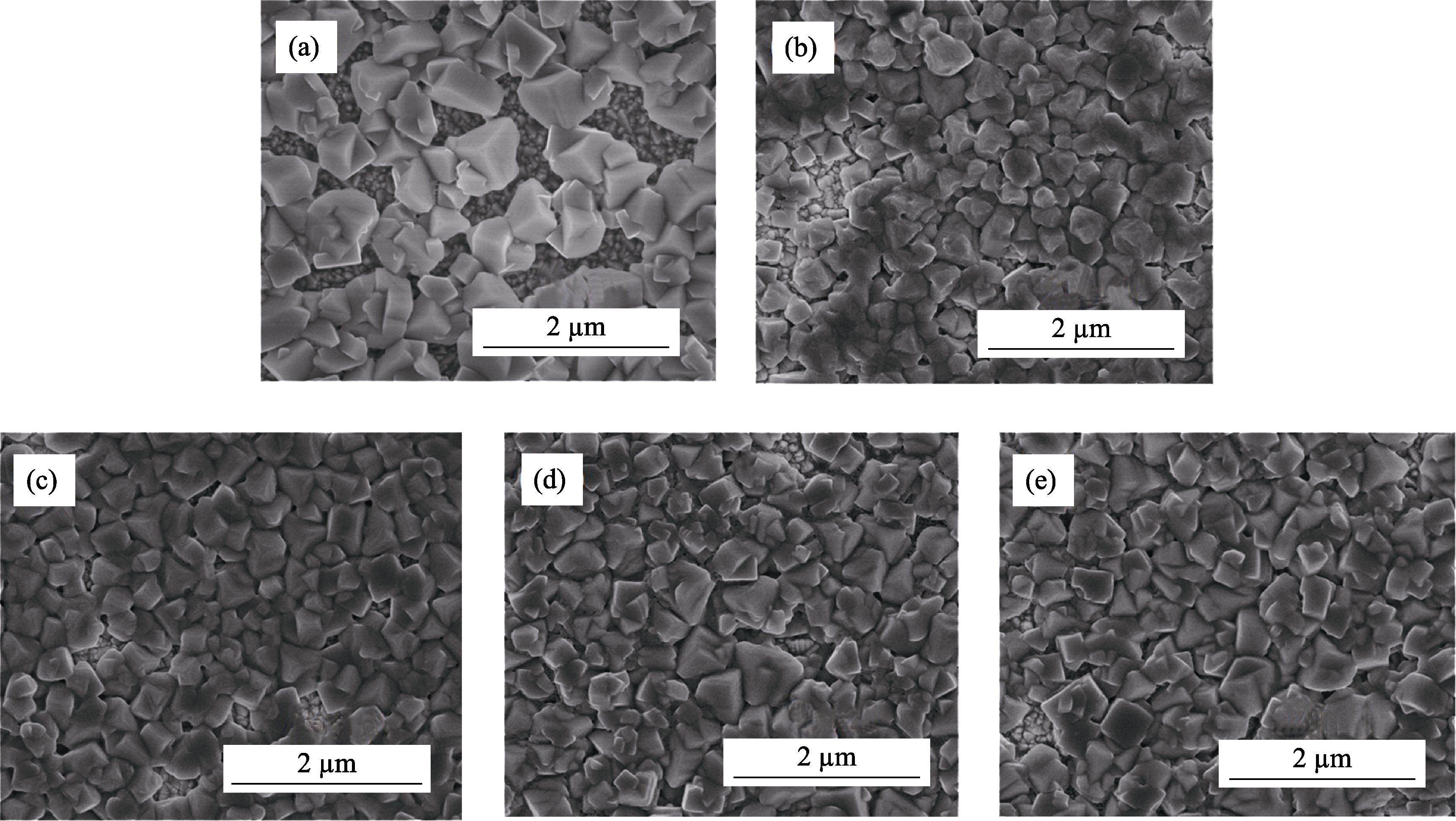
图2 添加不同含量PVP的钙钛矿薄膜的SEM照片
Fig. 2 Surface SEM images of perovskite films with various concentration of PVP (a) Without PVP; (b) 0.2wt% PVP; (c) 0.4wt% PVP; (d) 0.6wt% PVP; (e) 0.8wt% PVP
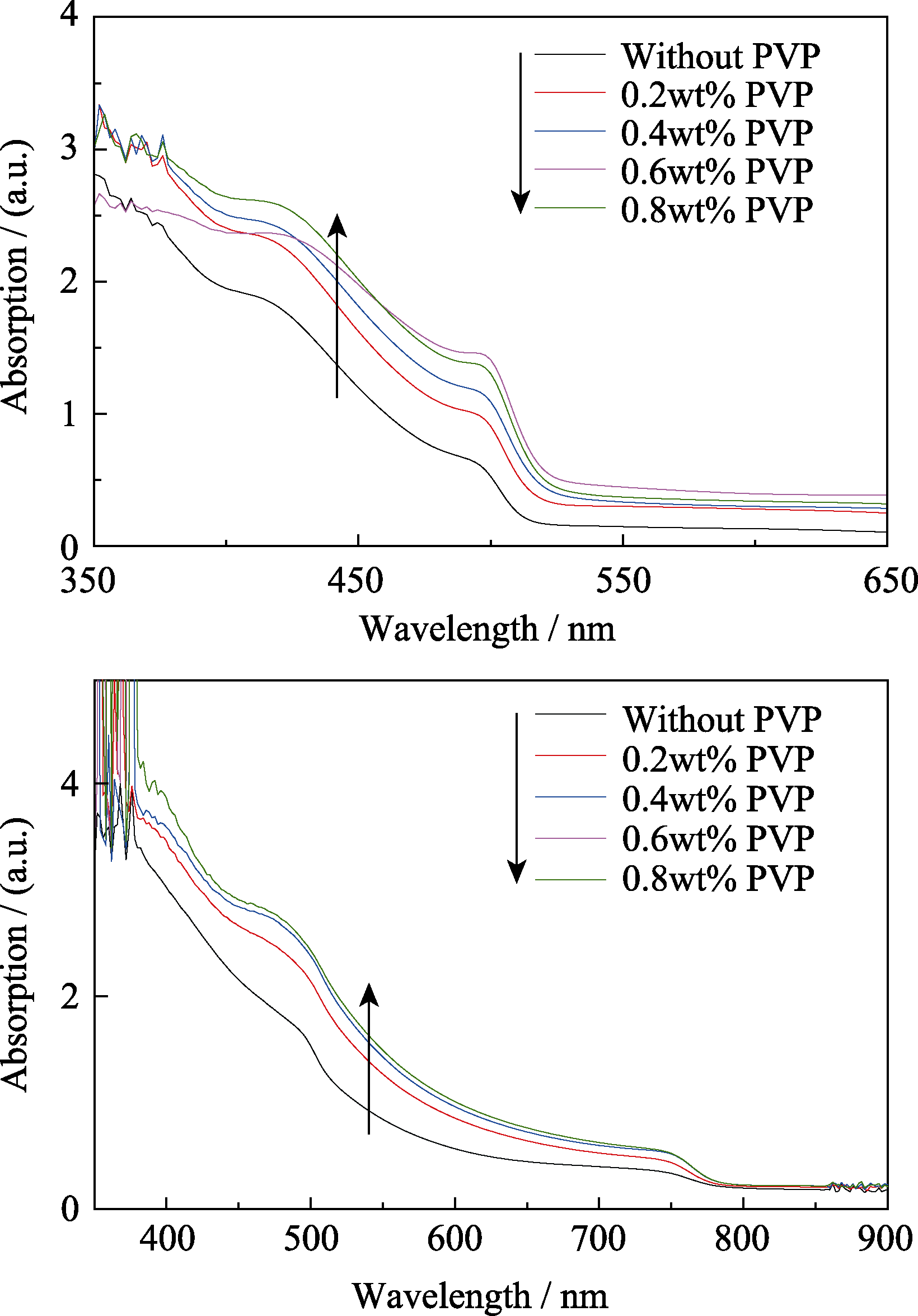
图3 添加不同含量PVP的薄膜紫外-可见吸收光谱图
Fig. 3 UV-visible absorption spectra of films in presence of varying concentration of PVP (a) PbI2 films; (b) CH3NH3PbI3 films

图5 添加不同含量PVP的新鲜钙钛矿薄膜的XRD图谱; 实物照片中PVP添加量从下到上依次为0, 0.2wt%, 0.4wt%, 0.6wt%, 0.8wt% (左边为碘化铅, 右边为钙钛矿)
Fig. 5 XRD patterns of fresh perovskite films doped with PVP of various quantities; The optical photos from bottom to top in the inserted are 0, 0.2wt%, 0.4wt%, 0.6wt%, 0.8wt% PVP (PbI2 on the left, perovskite on the right)

图6 在空气中放置3 w后添加不同含量PVP的钙钛矿薄膜的XRD图谱; 实物照片中PVP添加量从下到上依次为0, 0.2wt%, 0.4wt%, 0.6wt%, 0.8wt%(左边为碘化铅右边为钙钛矿)
Fig. 6 XRD patterns of CH3NH3PbI3 doped with PVP of various quantities after three weeks in the air; The optical photos from bottom to top in the inserted are 0, 0.2wt%, 0.4wt%, 0.6wt%, 0.8wt% PVP, respectively (PbI2 on the left, perovskite on the right)
| Sample | Voc/V | Jsc/(mA·cm-2) | FF/% | PCE/% |
|---|---|---|---|---|
| Without PVP | 0.95 | 16.17 | 39.40 | 6.09 |
| 0.2wt% PVP | 1.00 | 19.14 | 46.10 | 8.86 |
| 0.4wt% PVP | 1.04 | 19.60 | 64.00 | 13.07 |
| 0.6wt% PVP | 1.05 | 17.39 | 67.77 | 12.34 |
| 0.8wt% PVP | 1.04 | 14.83 | 67.38 | 10.42 |
表1 现场制备的添加不同含量PVP的钙钛矿太阳能电池性能参数
Table 1 The parameters of as-prepared perovskite solar cells doped with various concentration of PVP
| Sample | Voc/V | Jsc/(mA·cm-2) | FF/% | PCE/% |
|---|---|---|---|---|
| Without PVP | 0.95 | 16.17 | 39.40 | 6.09 |
| 0.2wt% PVP | 1.00 | 19.14 | 46.10 | 8.86 |
| 0.4wt% PVP | 1.04 | 19.60 | 64.00 | 13.07 |
| 0.6wt% PVP | 1.05 | 17.39 | 67.77 | 12.34 |
| 0.8wt% PVP | 1.04 | 14.83 | 67.38 | 10.42 |
| Sample | Voc/V | Jsc/(mA·cm-2) | FF/% | PCE/% |
|---|---|---|---|---|
| Without PVP | 1.09 | 12.05 | 31.64 | 4.17 |
| 0.2wt% PVP | 0.78 | 16.75 | 48.36 | 6.33 |
| 0.4wt% PVP | 1.04 | 21.05 | 53.05 | 11.60 |
| 0.6wt% PVP | 0.99 | 21.44 | 47.12 | 10.02 |
| 0.8wt% PVP | 0.84 | 17.20 | 66.10 | 9.52 |
表2 在空气中放置3 d的添加不同含量PVP的钙钛矿太阳能电池性能参数
Table 2 The parameters of perovskite solar cells doped with various concentration of PVP after three days in the air
| Sample | Voc/V | Jsc/(mA·cm-2) | FF/% | PCE/% |
|---|---|---|---|---|
| Without PVP | 1.09 | 12.05 | 31.64 | 4.17 |
| 0.2wt% PVP | 0.78 | 16.75 | 48.36 | 6.33 |
| 0.4wt% PVP | 1.04 | 21.05 | 53.05 | 11.60 |
| 0.6wt% PVP | 0.99 | 21.44 | 47.12 | 10.02 |
| 0.8wt% PVP | 0.84 | 17.20 | 66.10 | 9.52 |
| Sample | Voc/V | Jsc/(mA·cm-2) | FF/% | PCE/% |
|---|---|---|---|---|
| Without PVP | 0.95 | 6.18 | 26.35 | 1.55 |
| 0.2wt% PVP | 1.03 | 15.53 | 29.75 | 4.75 |
| 0.4wt% PVP | 0.80 | 15.12 | 54.84 | 6.63 |
| 0.6wt% PVP | 0.82 | 10.88 | 68.36 | 6.11 |
| 0.8wt% PVP | 0.97 | 21.13 | 34.29 | 7.04 |
表3 在空气中放置3 w的添加不同含量PVP的钙钛矿太阳能电池性能参数
Table 3 The parameters of perovskite solar cells doped with various concentration of PVP after three weeks in the air
| Sample | Voc/V | Jsc/(mA·cm-2) | FF/% | PCE/% |
|---|---|---|---|---|
| Without PVP | 0.95 | 6.18 | 26.35 | 1.55 |
| 0.2wt% PVP | 1.03 | 15.53 | 29.75 | 4.75 |
| 0.4wt% PVP | 0.80 | 15.12 | 54.84 | 6.63 |
| 0.6wt% PVP | 0.82 | 10.88 | 68.36 | 6.11 |
| 0.8wt% PVP | 0.97 | 21.13 | 34.29 | 7.04 |
| [1] | HADADIAN M, CORREA-BAENA J P, GOHARSHADI E K, et al. Enhancing efficiency of perovskite solar cells via N-doped graphene: crystal modification and surface passivation. Adv. Mater., 2016, 28(39): 8681-8686. |
| [2] | KIM H S, LEE C R, IM J H, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep., 2012, 2: 591-1-7. |
| [3] | MENARD E, MEITL M A, SUN Y, et al.Micro and nanopatterning techniques for organic electronic and optoelectronic systems. Chem. Rev., 2007, 107(4): 1117-1160. |
| [4] | JEON N J, NOH J H, KIM Y C, et al.Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nature Mater., 2014, 13(9): 897-903. |
| [5] | GUO XIU-BIN, YU WEI, LI JING, et al.Improving microstructure and photoelectric performance of the perovskite material via mixed solvents. [J]. Inorg. Mater., 2017, 32(8): 870-876. |
| [6] | CHANG C Y, CHU C Y, HUANG Y C, et al.Tuning perovskite morphology by polymer additive for high efficiency solar cell. ACS Appl. Mater. Interfaces, 2015, 7(8): 4955-4961. |
| [7] | JEON N J, NOH J H, YANG W S, et al.Compositional engineering of perovskite materials for high-performance solar cells. Nature, 2015, 517(7535): 476-480. |
| [8] | ZHOU Z, PANG S, LIU Z, et al.Interface engineering for high-performance perovskite hybrid solar cells. J. Mater. Chem. A, 2015, 3(38): 19205-19217. |
| [9] | ZHANG MIN, WANG ZENG-HUA, ZHENG XIAO-JIA, et al.Structural effect of TiO2 on the performance of MAPbBr3 solar cells. [J]. Inorg. Mater., 2018, 33(2): 245-250. |
| [10] | YANG G, TAO H, QIN P, et al.Recent progress in electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A, 2016, 4(11): 3970-3990. |
| [11] | MALI S S, HONG C K.Pin/nip type planar hybrid structure of highly efficient perovskite solar cells towards improved air stability: synthetic strategies and the role of p-type hole transport layer (HTL) and n-type electron transport layer (ETL) metal oxides. Nanoscale, 2016, 8(20): 10528-10540. |
| [12] | JIANG WEN-LONG, ZHOU WEI, YING JI-FEI, et al.Thermal stable perovskite solar cells improved by ZnO/graphene oxide as electron transfer layers. [J]. Inorg. Mater., 2017, 32(1): 96-100. |
| [13] | LIU CHANG, YUAN SHUAI, ZHANG HAI-LIANG, et al.p-type CuI films grown by iodination of copper and their application as hole transporting layers for inverted perovskite solar cells. [J]. Inorg. Mater., 2016, 31(4): 358-364. |
| [14] | HUANG X, ZHU C, ZHANG S, et al.Porphyrin-dithienothiophene π-conjugated copolymers: synthesis and their applications in field- effect transistors and solar cells. Macromolecules, 2008, 41(19): 6895-6902. |
| [15] | XIONG H, RUI Y, LI Y, et al.Hydrophobic coating over a CH3NH3PbI3 absorbing layer towards air stable perovskite solar cells. J. Mater. Chem. C, 2016, 4(28): 6848-6854. |
| [16] | MÜLLER C, GLASER T, PLOGMYER M, et al. Water infiltration in methylammonium leadiodide perovskite: fast and inconspicuous. Chem. Mater., 2015, 27(22): 7835-7841. |
| [17] | AMEEN S, RUB M A, KOSA S A, et al.Perovskite solar cells: influence of hole transporting materials on power conversion efficiency. ChemSusChem, 2016, 9(1): 10-27. |
| [18] | LI B, LI Y, ZHENG C, et al.Advancements in the stability of perovskite solar cells: degradation mechanisms and improvement approaches. RSC Adv., 2016, 6(44): 38079-38091. |
| [19] | ZHANG M, LYU M, YU H, et al.Stable and low-cost mesoscopic CH3NH3PbI2Br perovskite solar cells by using a thin poly (3-hexylthiophene) layer as a hole transporter. Chem-Eur J., 2015, 21(1): 434-439. |
| [20] | CHAUDHARY B, KULKARNI A, JENA A K, et al.Poly (4-vinylpyridine)-based interfacial passivation to enhance voltage and moisture stability of lead halide perovskite solar cells. ChemSusChem, 2017, 10(11): 2473-2479. |
| [21] | PALOMARES E, CLIFFORD J N, HAQUE S A, et al.Control of charge recombination dynamics in dye sensitized solar cells by the use of conformally deposited metal oxide blocking layers. [J]. Am. Chem. Soc., 2003, 125(2): 475-482. |
| [22] | CORBETT J D, VON WINBUSH S, ALBERS F C.The solubility of the post-transition metals in their molten halides. [J]. Am. Chem. Soc., 1957, 79(12): 3020-3024. |
| [23] | MABROUK S, DUBEY A, ZHANG W, et al.Increased efficiency for perovskite photovoltaics via doping the PbI2 layer. J. Phys. Chem. C, 2016, 120(43): 24577-24582. |
| [24] | OKU T. Crystal Structures of CH3NH3PbI3 and Related Perovskite Compounds Used for Solar Cells, Solar Cells-New Approaches and Reviews, ed. L. A. Kosyachenko, InTech, ISBN: 78-953-51-2184-8, DOI: 10.5772/59284. 2015. |
| [25] | LIU T, HU Q, WU J, et al. Mesoporous PbI2 scaffold for high-performance planar heterojunction perovskite solar cells. Adv. Energy Mater., 2016, 6(3): 1501890-1-7. |
| [26] | KIM Y C, JEON N J, NOH J H, et al. Beneficial effects of PbI2 incorporated in organo-lead halide perovskite solar cells. Adv. Energy Mater., 2016, 6(4): 1502104-1-8. |
| [27] | ZUO L, GUO H, JARIWALA S, et al. Polymer-modified halide perovskite films for efficient and stable planar heterojunction solar cells. Sci. Adv., 2017, 3(8): e1700106-1-12. |
| [1] | 汪波, 余健, 李存成, 聂晓蕾, 朱婉婷, 魏平, 赵文俞, 张清杰. Gd/Bi0.5Sb1.5Te3热电磁梯度复合材料的服役稳定性[J]. 无机材料学报, 2023, 38(6): 663-670. |
| [2] | 王世怡, 冯爱虎, 李晓燕, 于云. Fe3O4负载Ti3C2Tx对Pb(II)的吸附性能研究[J]. 无机材料学报, 2023, 38(5): 521-528. |
| [3] | 李悦, 张旭良, 景芳丽, 胡章贵, 吴以成. 铈掺杂硼酸钙镧晶体的生长与性能研究[J]. 无机材料学报, 2023, 38(5): 583-588. |
| [4] | 张万文, 罗建强, 刘淑娟, 马建国, 张小平, 杨松旺. 氧化锆间隔层的低温喷涂制备及其三层结构钙钛矿太阳能电池应用性能[J]. 无机材料学报, 2023, 38(2): 213-218. |
| [5] | 王红宁, 黄丽, 清江, 马腾洲, 黄维秋, 陈若愚. 有机-无机氧化硅空心球的合成及VOCs吸附应用[J]. 无机材料学报, 2022, 37(9): 991-1000. |
| [6] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [7] | 罗世淋, 张胜泰, 许保亮, 王凌坤, 段思逸菡, 丁艺, 赵倩, 段涛. YIG陶瓷对三价锕系模拟核素的固化行为研究[J]. 无机材料学报, 2022, 37(7): 757-763. |
| [8] | 刘鼎伟, 曾江涛, 郑嘹赢, 满振勇, 阮学政, 时雪, 李国荣. BiAlO3掺杂PZT陶瓷的高压电性能和低电场应变滞后[J]. 无机材料学报, 2022, 37(12): 1365-1370. |
| [9] | 王烈林, 谢华, 谢宇骐, 胡平涛, 尹雯, 任馨玥, 丁芸. Nd2Zr2O7烧绿石A、B位晶格固化钍的结构演化及化学稳定性研究[J]. 无机材料学报, 2022, 37(10): 1073-1078. |
| [10] | 杨新月, 董庆顺, 赵伟冬, 史彦涛. 基于对氯苄胺的2D/3D钙钛矿太阳能电池[J]. 无机材料学报, 2022, 37(1): 72-78. |
| [11] | 范君, 江雪, 焦毅, 陈宇圣, 王健礼, 陈耀强. 不同碱辅助的沉积沉淀法对三效催化剂稳定性的影响[J]. 无机材料学报, 2021, 36(6): 659-664. |
| [12] | 王艳香, 高培养, 范学运, 李家科, 郭平春, 黄丽群, 孙健. SnO2退火温度对钙钛矿太阳能电池性能的影响[J]. 无机材料学报, 2021, 36(2): 168-174. |
| [13] | 曾凡鑫, 刘创, 曹余良. 去合金化制备具有高循环稳定性的纳米多孔Sb/MCNT储钠负极材料[J]. 无机材料学报, 2021, 36(11): 1137-1144. |
| [14] | 段涛, 丁艺, 罗世淋, 张胜泰, 刘建. 回归自然: 人造岩石固化核素的思考与进展[J]. 无机材料学报, 2021, 36(1): 25-35. |
| [15] | 张志刚,卢晓通,刘金莉. NiFe2O4陶瓷U型套管的注浆成型和无压烧结[J]. 无机材料学报, 2020, 35(6): 661-668. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||