无机材料学报 ›› 2019, Vol. 34 ›› Issue (1): 65-71.DOI: 10.15541/jim20180255
所属专题: MAX相和MXene材料
刘小元1,2, 刘宝丹1, 姜亚南1, 王柯1,2, 周洋1,2, 杨兵1, 张兴来1, 姜辛1
收稿日期:2018-06-06
出版日期:2019-01-21
网络出版日期:2018-12-17
作者简介:刘小元. E-mail: xyliu13s@imr.ac.cn
LIU Xiao-Yuan1,2, LIU Bao-Dan1, JIANG Ya-Nan1, WANG Ke1,2, ZHOU Yang1,2, YANG Bing1, ZHANG Xing-Lai1, JIANG Xin1
Received:2018-06-06
Published:2019-01-21
Online:2018-12-17
About author:LIU Xiao-Yuan (1990-), male, candidate of PhD. E-mail: xyliu13s@imr.ac.cn
Supported by:摘要:
钙钛矿相SrTiO3在太阳能电池、光催化、燃料电池, 超导等领域均有广泛应用, 这些应用均与其晶体质量、形貌、暴露晶面和光学吸收等特性息息相关。本文通过微弧氧化-水热两步法原位制备了两种典型形貌的SrTiO3纳米晶。结果表明, 随着微弧氧化电解液锶源浓度的降低, SrTiO3形貌从立方块状转变为超薄片状。进一步分析表明, 所得的SrTiO3立方块和Sr1-δTiO3纳米片均为结晶质量良好的单晶体, 通过分析两种形貌样品的紫外-可见漫反射光谱, 发现Sr1-δTiO3纳米片相对于SrTiO3立方块, 具有明显的尺寸效应诱导的光学吸收蓝移特性。最后, 本研究提出了SrTiO3的原位生长及形貌演变机制。
中图分类号:
刘小元, 刘宝丹, 姜亚南, 王柯, 周洋, 杨兵, 张兴来, 姜辛. 形貌可控及光学吸收性能可调的钙钛矿型SrTiO3纳米结构的原位生长[J]. 无机材料学报, 2019, 34(1): 65-71.
LIU Xiao-Yuan, LIU Bao-Dan, JIANG Ya-Nan, WANG Ke, ZHOU Yang, YANG Bing, ZHANG Xing-Lai, JIANG Xin. In-situ Synthesis of Perovskite SrTiO3 Nanostructures with Modified Morphology and Tunable Optical Absorption Property[J]. Journal of Inorganic Materials, 2019, 34(1): 65-71.
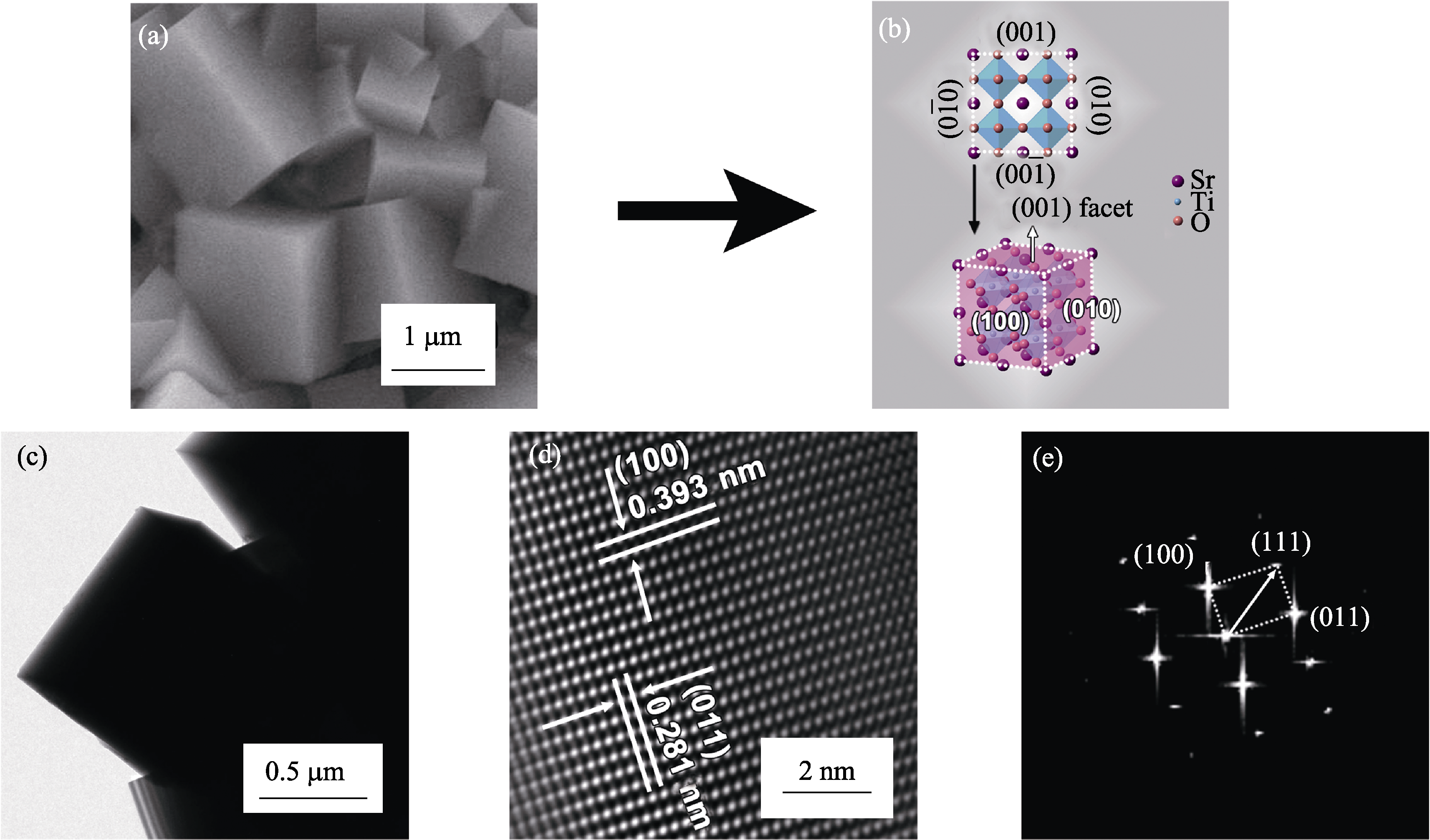
Fig. 2 (a,c) SEM image and TEM bright field image of SrTiO3 microcubes; (b) Crystallographic model of cube-like SrTiO3 nanostructure; (d,e) HRTEM image and FFT pattern of SrTiO3 microcubes
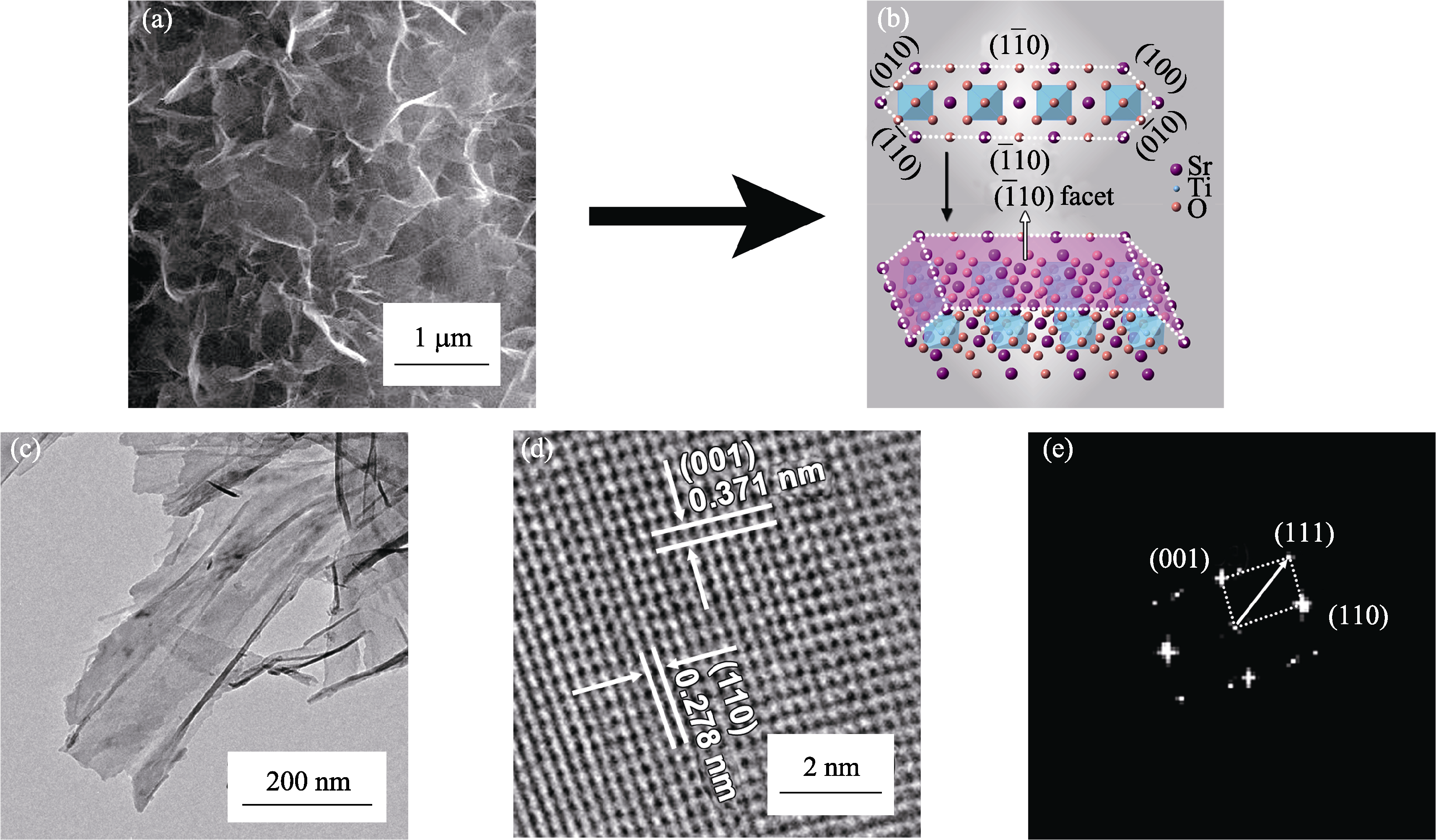
Fig. 3 (a,c) SEM image and TEM bright field image of Sr1-δTiO3 nanosheets; (b) Crystallographic model of sheet-like Sr1-δTiO3; (d,e) HRTEM image and FFT pattern of Sr1-δTiO3 nanosheets
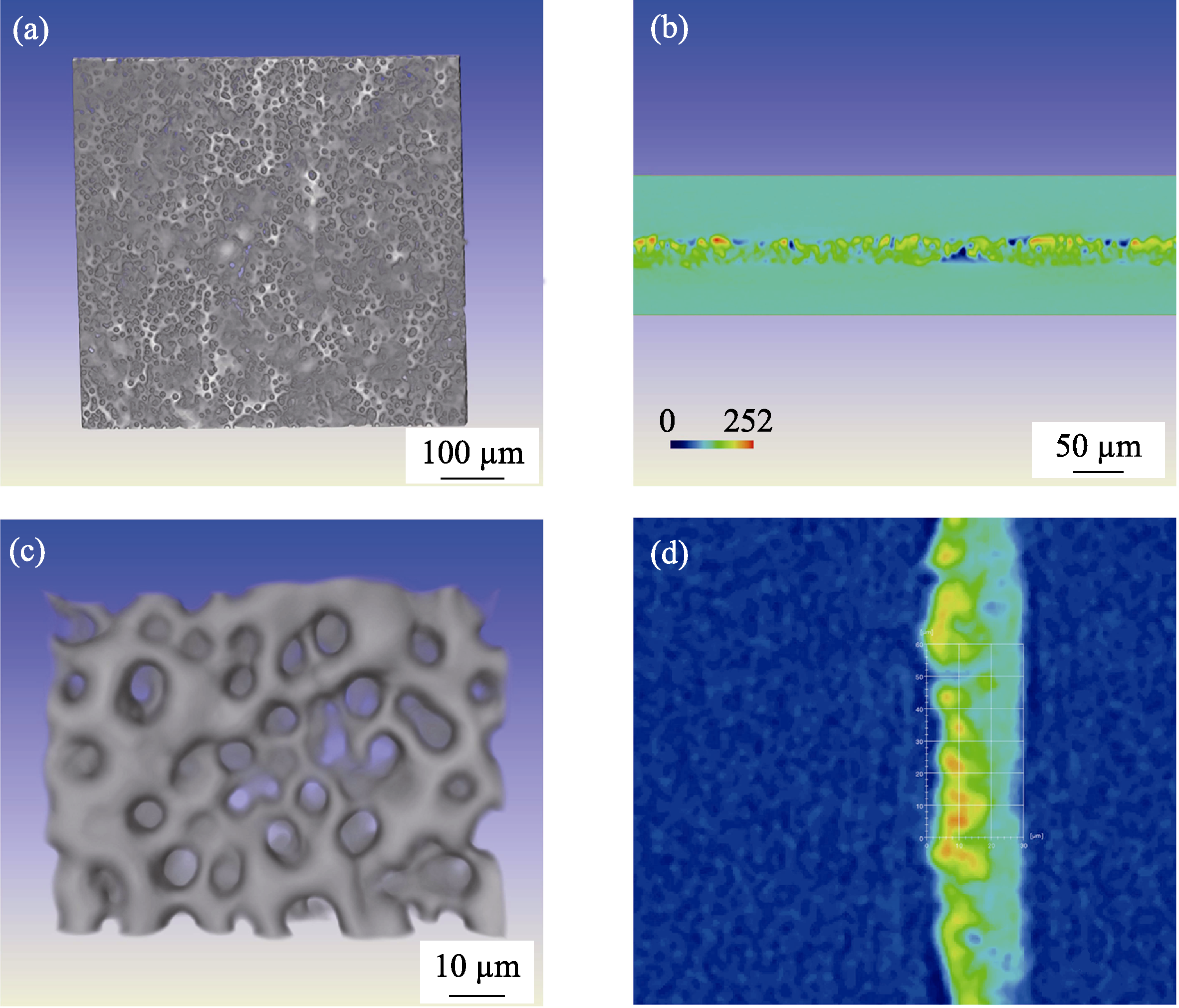
Fig. s2 (a, c) X-ray diffraction topography (XRT) images of PEO film surface morphology and (b, d) cross section images Table S1 EDS results of PEO film prepared in high Sr concentration electrolyte (HSCE) and low Sr concentration electrolyte (LSCE)
| Sample | O/at% | Ti/at% | Sr/at% | n(Sr) : n(Ti) |
|---|---|---|---|---|
| HSCE | 79.35 | 11.51 | 9.14 | 0.79 |
| LSCE | 53.93 | 37.32 | 8.75 | 0.23 |
Table s1 EDS results of PEO film prepared in high Sr concentration electrolyte (HSCE) and low Sr concentration electrolyte (LSCE)
| Sample | O/at% | Ti/at% | Sr/at% | n(Sr) : n(Ti) |
|---|---|---|---|---|
| HSCE | 79.35 | 11.51 | 9.14 | 0.79 |
| LSCE | 53.93 | 37.32 | 8.75 | 0.23 |
| Sample | O/at% | Ti/at% | Sr/at% | n(Sr) : n(Ti) |
|---|---|---|---|---|
| Microcube | 55.06 | 24.16 | 20.77 | 0.86 |
| Nanosheet | 61.65 | 31.65 | 6.7 | 0.21 |
Table s2 EDS results of SrTiO3 microcubes and Sr1-δTiO3 nanosheets
| Sample | O/at% | Ti/at% | Sr/at% | n(Sr) : n(Ti) |
|---|---|---|---|---|
| Microcube | 55.06 | 24.16 | 20.77 | 0.86 |
| Nanosheet | 61.65 | 31.65 | 6.7 | 0.21 |
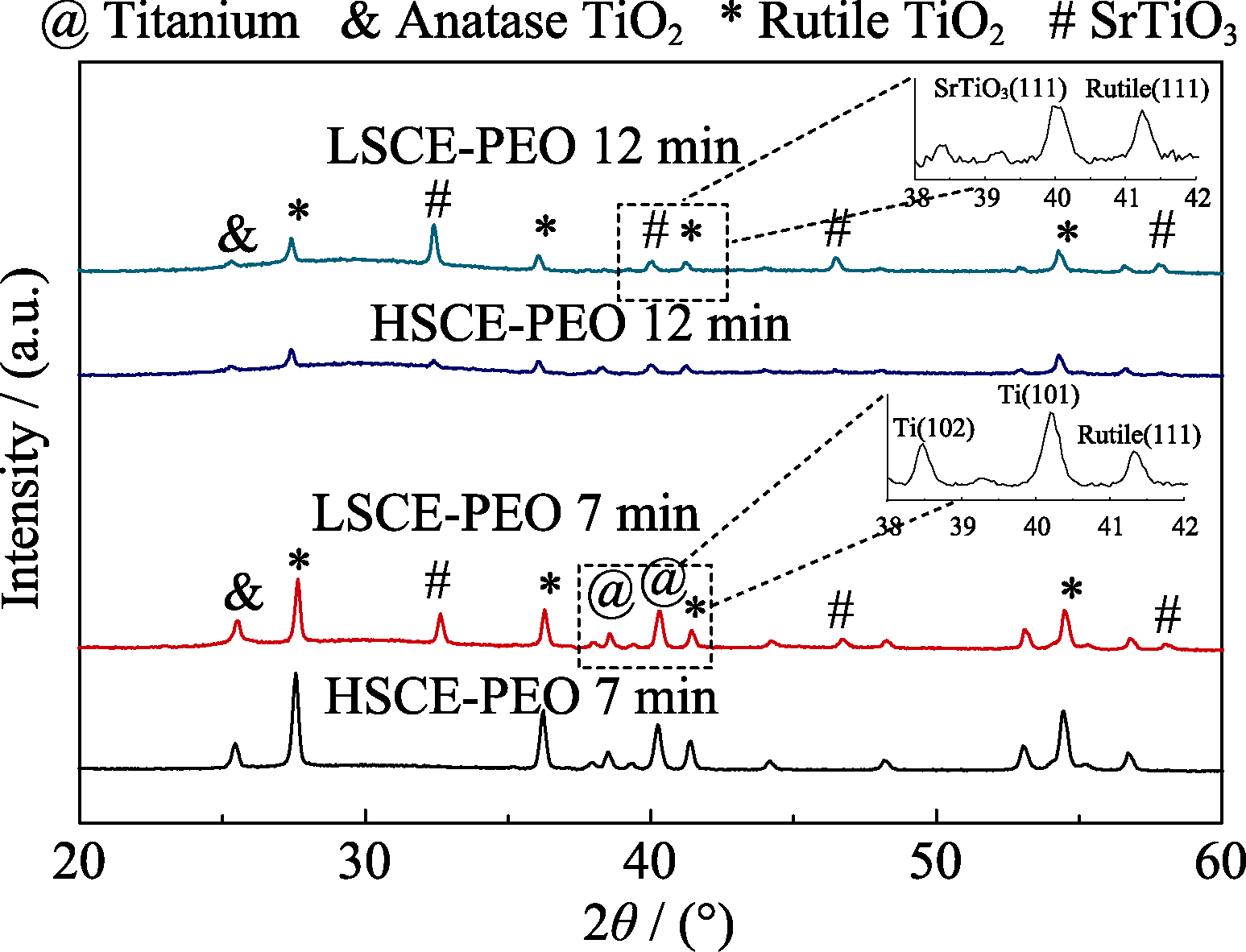
Fig. s3 XRD patterns of PEO films prepared under different conditions LSCE, 7 min PEO treating time; LSCE, 12 min PEO treating time, HSCE, 7 min PEO treating time and HSCE, 12 min PEO treating time
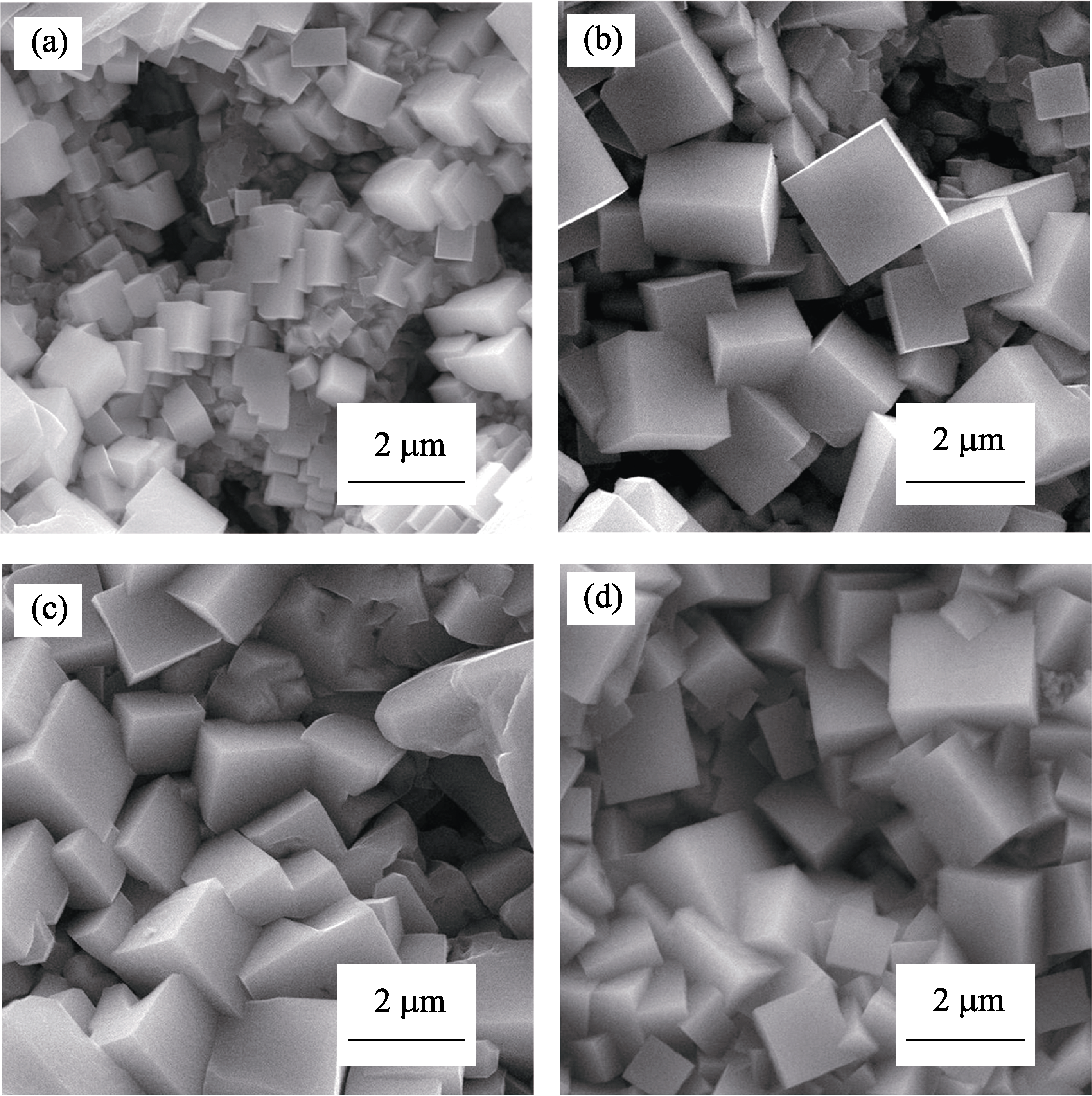
Fig. s4 SEM images of SrTiO3 microcubes obtained on PEO film under HSCE and in 0.5 mol/L NaOH with different durations (a) 180℃, 1 h; (b) 180℃, 4 h; (c) 180℃, 6 h; (d) 180℃, 8 h
| Sample | O/at% | B/at% | Ti/at% | Sr/at% | n(Sr) : n(Ti) |
|---|---|---|---|---|---|
| Nanosheet | 69.95 | 0.70 | 22.39 | 6.96 | 0.31 |
Table s3 XPS element analysis of Sr1-δTiO3 nanosheets
| Sample | O/at% | B/at% | Ti/at% | Sr/at% | n(Sr) : n(Ti) |
|---|---|---|---|---|---|
| Nanosheet | 69.95 | 0.70 | 22.39 | 6.96 | 0.31 |

Fig. S6 SEM images of SrTiO3 microcubes obtained on PEO film under HSCE and in 1.0 mol/L NaOH with different durations (a) 180℃, 0.5 h; (b) 180℃, 1 h; (c) 180℃, 2 h; (d) 180℃, 8 h
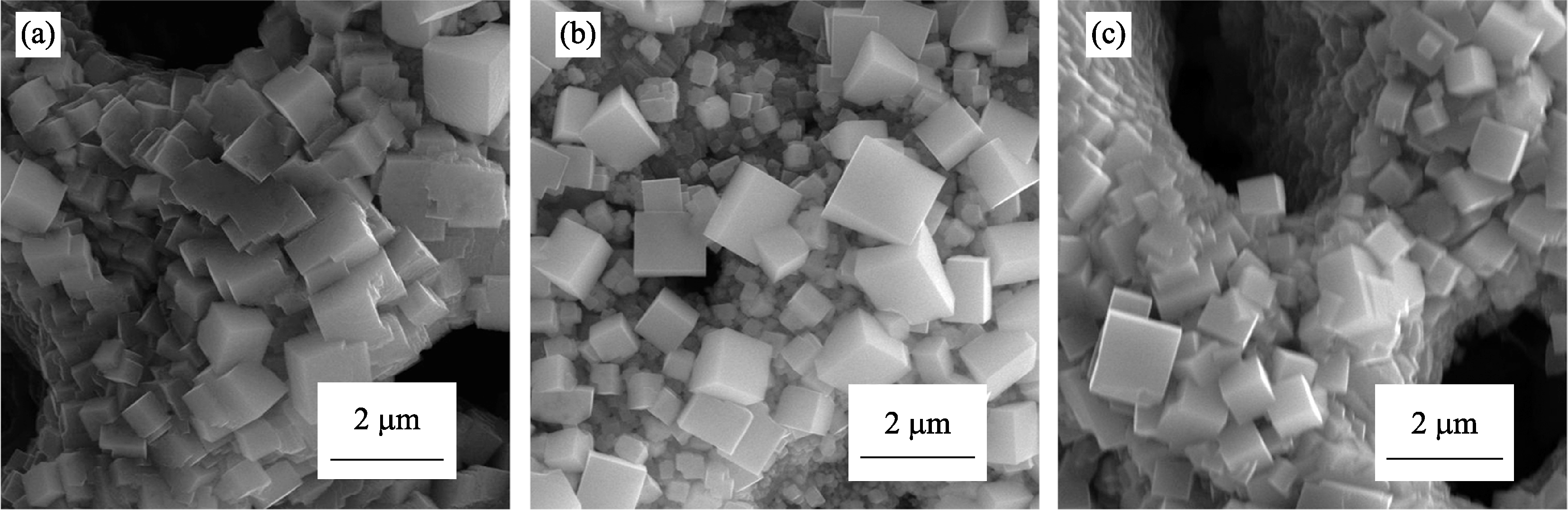
Fig. S7 SEM images of SrTiO3 microcubes obtained on PEO film under HSCE and in 1.5 mol/L NaOH with different durations (a) 180℃, 4 h; (b) 180℃, 6 h; (c) 180℃, 8 h
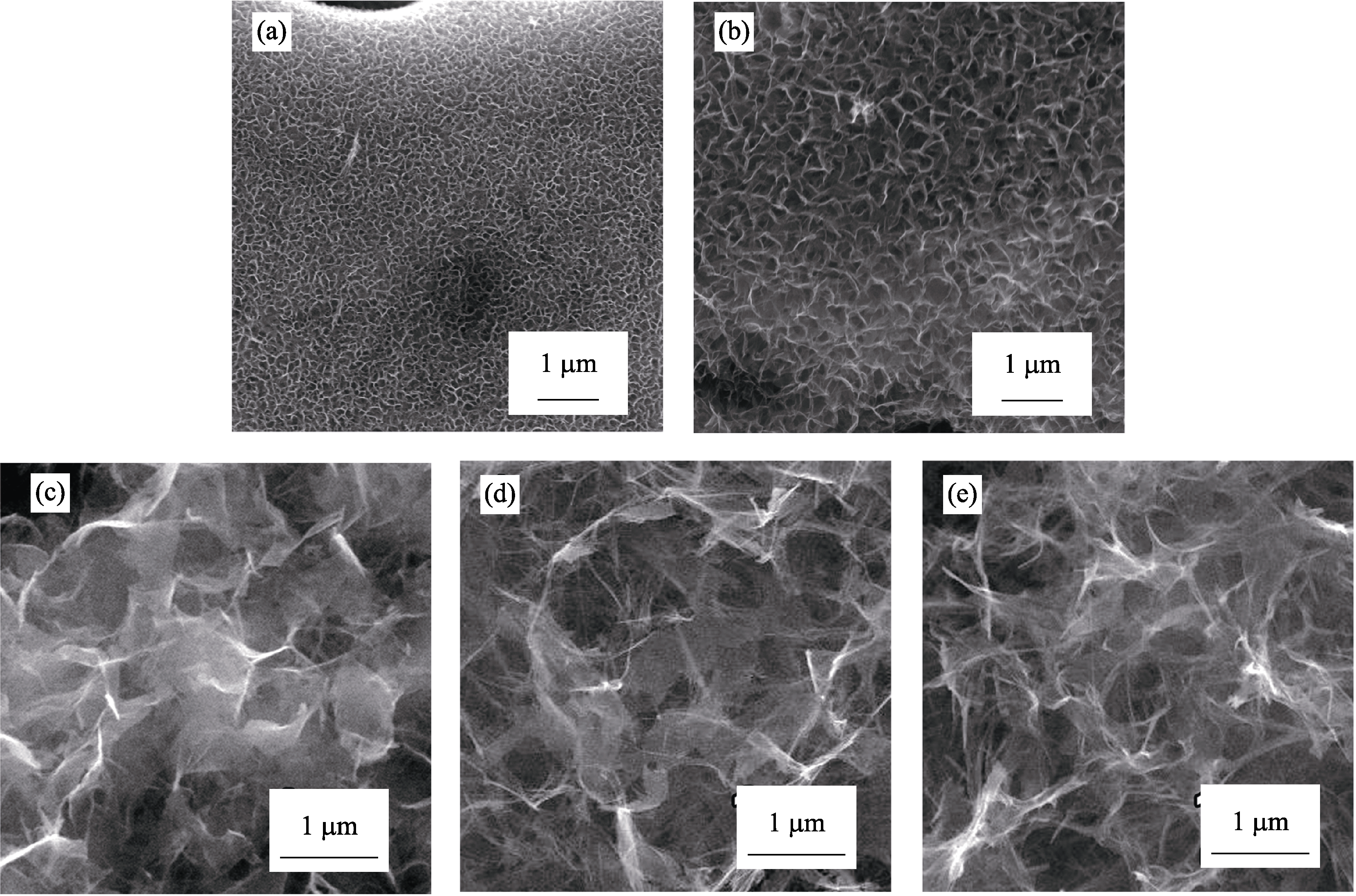
Fig. S8 SEM images of Sr1-δTiO3 nanosheets obtained on PEO film under LSCE and in 1.0 mol/L NaOH with different durations (a) 180℃, 0.5 h; (b) 180℃, 1 h; (c) 180℃, 2 h; (d) 180℃, 4 h; (e) 180℃, 8 h
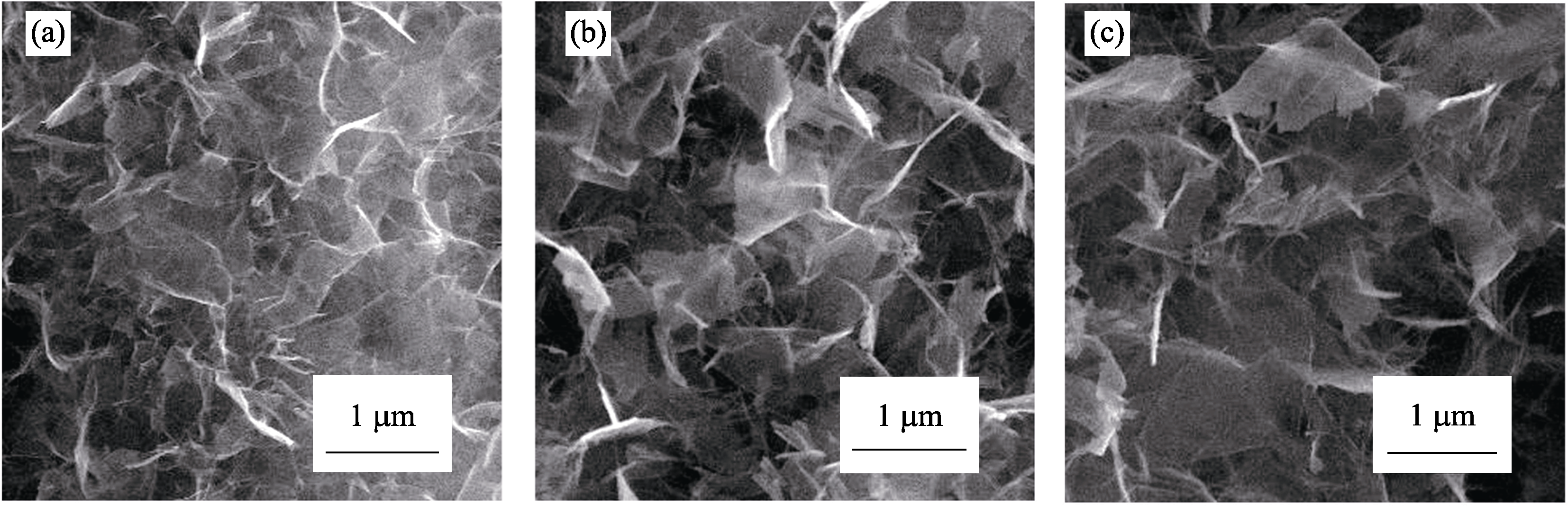
Fig. S9 SEM images of Sr1-δTiO3 nanosheets obtained on PEO film under LSCE and in 0.5 mol/L NaOH with different durations (a) 180℃, 2 h; (b) 180℃, 4 h; (c) 180℃, 8 h
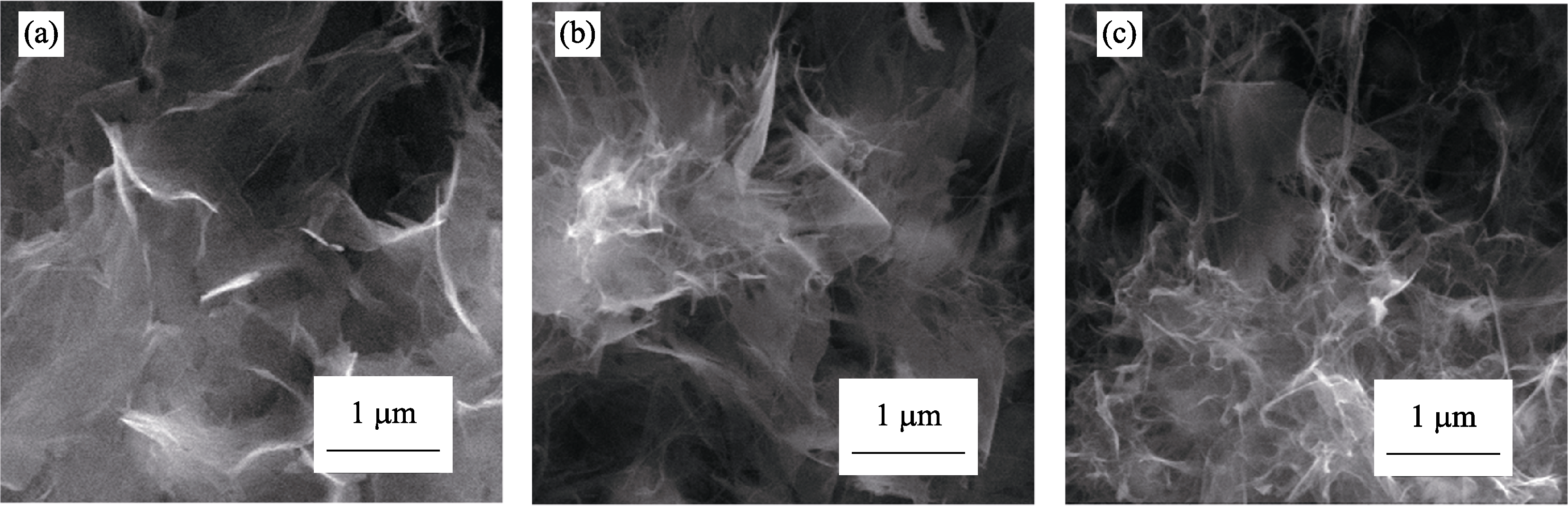
Fig. S10 SEM images of Sr1-δTiO3 nanosheets obtained on PEO film under LSCE and in 1.5 mol/L NaOH with different durations (a) 180℃, 2 h; (b) 180℃, 4 h; (c) 180℃, 8 h
| [1] | KAZIM S, NAZEERUDDIN M K, GRATZEL M, et al.Perovskite as light harvester: a game changer in photovoltaics. Angew Chem. Int. Edit., 2014, 53(11): 2812-2824. |
| [2] | SULAEMAN U, YIN S, SATO T.Solvothermal synthesis and photocatalytic properties of chromium-doped SrTiO3 nanoparticles. Appl Catal B-Environ., 2011, 105(1/2): 206-210. |
| [3] | IWASHINA K, KUDO A.Rh-doped SrTiO3 photocatalyst electrode showing cathodic photocurrent for water splitting under visible- light irradiation. [J]. Am. Chem. Soc., 2011, 133(34): 13272-13275. |
| [4] | COMES R B, SMOLIN S Y, KASPAR T C, et al. Visible light carrier generation in co-doped epitaxial titanate films. Appl. Phys. Lett., 2015, 106(9): 092901-1-5. |
| [5] | PARK K I, XU S, LIU Y, et al.Piezoelectric BaTiO3 thin film nanogenerator on plastic substrates. Nano Letters, 2010, 10(12): 4939-4943. |
| [6] | GRABOWSKA E.Selected perovskite oxides: characterization, preparation and photocatalytic properties—a review. Applied Catalysis B: Environmental, 2016, 186: 97-126. |
| [7] | MADHAVAN B, ASHOK A.Review on nanoperovskites: materials, synthesis, and applications for proton and oxide ion conductivity. Ionics, 2014, 21(3): 1-10. |
| [8] | KUDO A, MISEKI Y.Heterogeneous photocatalyst materials for water splitting. Chemical Society Reviews, 2009, 38(1): 253-278. |
| [9] | DIAMANT Y, CHEN S G, MELAMED O, et al.Core-shell nanoporous electrode for dye sensitized solar cells: the effect of the SrTiO3 shell on the electronic properties of the TiO2 core. J. Phys. Chem. B, 2003, 107(9): 1977-1981. |
| [10] | LENZMANN F, KRUEGER J, BURNSIDE S, et al.Surface photovoltage spectroscopy of dye-sensitized solar cells with TiO2, Nb2O5, and SrTiO3 nanocrystalline photoanodes: indication for electron injection from higher excited dye states. J. Phys. Chem. B, 2001, 105(27): 6347-6352. |
| [11] | KANG Q, WANG T, LI P, et al.Photocatalytic reduction of carbon dioxide by hydrous hydrazine over Au-Cu alloy nanoparticles supported on SrTiO/TiO coaxial nanotube arrays. Angewandte Chemie International Edition, 2014, 54(3): 841-845. |
| [12] | CAO T, LI Y, WANG C, et al.A facile in situ hydrothermal method to SrTiO3/TiO2 nanofiber heterostructures with high photocatalytic activity. Langmuir, 2011, 27(6): 2946-2952. |
| [13] | LONG Z, WEI X H, QIU X Q.Preparation of SrTiO3 cubes by molten salt method and its surface ions modification with Cu(II) clusters. J. Inorg. Mater., 2013, 28(10): 1103-1107. |
| [14] | ZHU Y R, TANG Y G, YAN J H, et al.Preparation and photocatalytic hydrogen generation activity of nitrogen doped SrTiO3 under visible light irradiation. [J]. Inorg. Mater., 2008, 23(3): 443-448. |
| [15] | JI L, MCDANIEL M D, WANG S, et al.A silicon-based photocathode for water reduction with an epitaxial SrTiO3 protection layer and a nanostructured catalyst. Nat. Nano, 2014, 10(1): 84-90. |
| [16] | YAN J H, ZHANG L, ZHU Y R, et al.Preparation and photocatalytic hydrogen production of NiO(CoO)/N-SrTiO3 heterojunction complex catalyst under simulated sunlight irradiation. J. Inorg. Mater., 2009, 24(4): 666-670. |
| [17] | KOVALEVSKY A V, POPULOH S, PATRÍCIO S G, et al. Design of SrTiO3-based thermoelectrics by tungsten substitution. The Journal of Physical Chemistry C, 2015, 119(9): 4466-4478. |
| [18] | OHTOMO A, HWANG H Y.A high-mobility electron gas at the LaAlO3/SrTiO3 heterointerface. Nature, 2004, 427(6973): 423-426. |
| [19] | KUANG Q, YANG S.Template synthesis of single-crystal-like porous SrTiO3 nanocube assemblies and their enhanced photocatalytic hydrogen evolution. ACS Applied Materials & Interfaces, 2013, 5(9): 3683-3690. |
| [20] | ZHAN H, CHEN Z G, ZHUANG J, et al.Correlation between multiple growth stages and photocatalysis of SrTiO3 nanocrystals. The Journal of Physical Chemistry C, 2015, 119(7): 3530-3537. |
| [21] | SREEDHAR G, SIVANANTHAM A, BASKARAN T, et al.A role of lithiated sarcosine TFSI on the formation of single crystalline SrTiO3 nanocubes via hydrothermal method. Materials Letters, 2014, 133: 127-131. |
| [22] | DONG L, LUO Q, CHENG K, et al. Facet-specific assembly of proteins on SrTiO3 polyhedral nanocrystals. Sci. Rep., 2014, 4: 5084-1-5. |
| [23] | JIANG Y, LIU B, ZHAI Z, et al.A general strategy toward the rational synthesis of metal tungstate nanostructures using plasma electrolytic oxidation method. Appl. Surf. Sci., 2015, 356: 273-281. |
| [24] | JIANG Y N, LIU B D, YANG W J, et al.New strategy for the in situ synthesis of single-crystalline MnWO4/TiO2 photocatalysts for efficient and cyclic photodegradation of organic pollutants. CrystEngComm., 2016, 18(10): 1832-1841. |
| [25] | JIANG Y, LIU B, YANG L, et al. Size-controllable Ni5TiO7 nanowires as promising catalysts for CO oxidation. Sci. Rep., 2015, 5: 14330-1-10. |
| [26] | JIANG Y, LIU B, YANG W, et al.Crystalline (Ni1-xCox)5TiO7 nanostructures grown in situ on a flexible metal substrate used towards efficient CO oxidation. Nanoscale, 2017, 9(32): 11713-11719. |
| [27] | JIANG X, ZHANG L, WYBORNOV S, et al.Highly efficient nanoarchitectured Ni5TiO7 catalyst for biomass gasification. ACS Applied Materials & Interfaces, 2012, 4(8): 4062-4066. |
| [28] | BARATI N, YEROKHIN A, GOLESTANIFARD F, et al.Alumina- zirconia coatings produced by plasma electrolytic oxidation on Al alloy for corrosion resistance improvement. [J]. Alloy Compd., 2017, 724: 435-442. |
| [29] | HAASCH R T, BRECKENFELD E, MARTIN L W.Single crystal perovskites analyzed using X-ray photoelectron spectroscopy: 1. SrTiO3(001). Surface Science Spectra, 2014, 21(1): 87-94. |
| [30] | MOCKEL H, GIERSIG M, WILLIG F.Formation of uniform size anatase nanocrystals from bis(ammoniumlactato)titanium dihydroxide by thermohydrolysis. Journal of Materials Chemistry, 1999, 9(12): 3051-3056. |
| [31] | KATO K, DANG F, MIMURA K I, et al.Nano-sized cube-shaped single crystalline oxides and their potentials, composition, assembly and functions. Advanced Powder Technology, 2014, 25(5): 1401-1414. |
| [32] | FUJINAMI K, KATAGIRI K, KAMIYA J, et al.Sub-10 nm strontium titanate nanocubes highly dispersed in non-polar organic solvents. Nanoscale, 2010, 2(10): 2080-2083. |
| [33] | GUO Y, LIU G, REN Z, et al.Single crystalline brookite titanium dioxide nanorod arrays rooted on ceramic monoliths: a hybrid nanocatalyst support with ultra-high surface area and thermal stability. CrystEngComm., 2013, 15(41): 8345-8352. |
| [34] | AKKERMAN Q A, MOTTI S G, KANDADA A R S, et al. Solution synthesis approach to colloidal cesium lead halide perovskite nanoplatelets with monolayer-level thickness control. [J]. Am. Chem. Soc., 2016, 138(3): 1010-1016. |
| [35] | XU Z T, MITZI D B, DIMITRAKOPOULOS C D, et al.Semiconducting perovskites (2-XC6H4C2H4NH3)2SnI4 (X = F, Cl, Br): steric interaction between the organic and inorganic layers. Inorganic Chemistry, 2003, 42(6): 2031-2039. |
| [1] | 李悦, 张旭良, 景芳丽, 胡章贵, 吴以成. 铈掺杂硼酸钙镧晶体的生长与性能研究[J]. 无机材料学报, 2023, 38(5): 583-588. |
| [2] | 姚仪帅, 郭瑞华, 安胜利, 张捷宇, 周国治, 张国芳, 黄雅荣, 潘高飞. 原位负载Pt-Co高指数晶面催化剂的制备及其电催化性能[J]. 无机材料学报, 2023, 38(1): 71-78. |
| [3] | 李文俊, 王皓, 涂兵田, 谌强国, 郑凯平, 王为民, 傅正义. 宽光谱透过Mg0.9Al2.08O3.97N0.03透明陶瓷的制备与性能研究[J]. 无机材料学报, 2022, 37(9): 969-975. |
| [4] | 刘强, 王倩, 陈鹏辉, 李晓英, 章立轩, 谢腾飞, 李江. 两步烧结法制备红色Ce:8YSZ透明陶瓷及其性能研究[J]. 无机材料学报, 2022, 37(8): 911-917. |
| [5] | 刘子玉, TOCI Guido, PIRRI Angela, PATRIZI Barbara, 冯亚刚, 陈肖朴, 胡殿君, 田丰, 吴乐翔, VANNINIMatteo, 李江. 固体激光用Nd:Lu2O3透明陶瓷的制备和光学性能研究[J]. 无机材料学报, 2021, 36(2): 210-216. |
| [6] | 黄新友, 刘玉敏, 刘洋, 李晓英, 冯亚刚, 陈肖朴, 陈鹏辉, 刘欣, 谢腾飞, 李江. 醇水共沉淀法制备Yb:YAG透明陶瓷及其性能研究[J]. 无机材料学报, 2021, 36(2): 217-224. |
| [7] | 韦家蓓, TOCIGuido, PIRRIAngela, PATRIZIBarbara, 冯亚刚, VANNINIMatteo, 李江. 共沉淀纳米粉体制备Yb:CaF2激光陶瓷及其性能研究[J]. 无机材料学报, 2019, 34(12): 1341-1348. |
| [8] | 闫明洋, 杨敏, 李红, 任慕苏, 俞鸣明, 孙晋良. 原位生长的气相生长碳纤维增强C/C复合材料的制备及其弯曲性能[J]. 无机材料学报, 2018, 33(11): 1161-1166. |
| [9] | 吕玉珍, 孙 倩,李 超, 单秉亮, 齐 波, 李成榕. 油酸修饰TiO2纳米棒的溶剂热合成及形貌调控研究[J]. 无机材料学报, 2017, 32(7): 719-724. |
| [10] | 杨锁龙, 王晓方, 蒋春丽, 赵雅文, 曾荣光, 王怀胜, 赖新春. InP量子点的掺杂及其光学性能[J]. 无机材料学报, 2016, 31(10): 1051-1057. |
| [11] | 周 鼎, 施 鹰, 范灵聪, 林德宝, 孙泽清, 徐家跃. Ce, Pr离子双掺LuAG透明陶瓷制备及光学性能[J]. 无机材料学报, 2016, 31(10): 1099-1102. |
| [12] | 杨雨佳, 王 晶, 何慧芬. 铽离子掺杂锆酸钡粉体制备及其光学性能研究[J]. 无机材料学报, 2016, 31(1): 27-33. |
| [13] | 刘 婧, 刘 军, 李 江, 林 丽, 潘裕柏, 程晓农, 郭景坤. 球磨转速对Nd:YAG透明陶瓷的显微结构及光学性能的影响[J]. 无机材料学报, 2015, 30(6): 581-587. |
| [14] | 杨 睿, 介万奇, 孙晓燕, 杨 敏, 呼 唤, 蔺 云. 温度梯度溶液法生长的Cr掺杂的ZnTe晶体的表征[J]. 无机材料学报, 2015, 30(4): 401-407. |
| [15] | 向卫东, 赵斌宇, 梁晓娟, 陈兆平, 谢翠萍, 骆 乐, 张志敏, 张景峰, 钟家松. 白光LED用Ce:YAG单晶光学性能及封装工艺的研究[J]. 无机材料学报, 2014, 29(6): 614-620. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||