无机材料学报 ›› 2018, Vol. 33 ›› Issue (8): 845-853.DOI: 10.15541/jim20170516
所属专题: 电催化研究
杨志宾1, 岳彤联1, 余向南1, 吴苗苗2
收稿日期:2017-10-28
修回日期:2017-12-14
出版日期:2018-08-28
网络出版日期:2018-07-17
基金资助:YANG Zhi-Bin1, YUE Tong-Lian1, YU Xiang-Nan1, WU Miao-Miao2
Received:2017-10-28
Revised:2017-12-14
Published:2018-08-28
Online:2018-07-17
摘要:
氧化铈的电子导电性较低、氧空位数量少, 难以单独用作为电催化剂。但是掺杂过渡金属或非金属元素可以提高氧化铈的CO催化能力, 同时在氧化物中掺杂钴可有效提高材料的电催化能力, 因此本工作开展了对钴掺杂的氧化铈电催化性能的研究。采用均相沉淀法制备了钴掺杂的氧化铈纳米粒子, 电化学测试发现当钴掺杂比例为20mol%时, 氧化铈纳米粒子对氧气还原反应(ORR)和氧气析出反应(OER)的综合催化能力最强。经过10 h的长时间催化作用, ORR、OER过程中的电流密度分别下降了20%、5%左右, 远优于贵金属和未掺杂氧化铈纳米粒子催化剂, 显示出良好的催化稳定性。拉曼光谱、阻抗图及XPS谱图等的测试分析表明钴掺杂后材料的电荷转移阻抗降低(电子导电性的提高)、氧活性物种和氧空位增加是氧化铈催化性能提高的主要原因。本工作通过钴掺杂大幅度提高了氧化铈的电催化性能, 同时为其它离子导体作为双功能电催化剂的使用提供了借鉴。
中图分类号:
杨志宾, 岳彤联, 余向南, 吴苗苗. 钴掺杂氧化铈纳米粒子电催化性能研究[J]. 无机材料学报, 2018, 33(8): 845-853.
YANG Zhi-Bin, YUE Tong-Lian, YU Xiang-Nan, WU Miao-Miao. Electrocatalytic Activity of Cobalt Doped Ceria Nanoparticles[J]. Journal of Inorganic Materials, 2018, 33(8): 845-853.
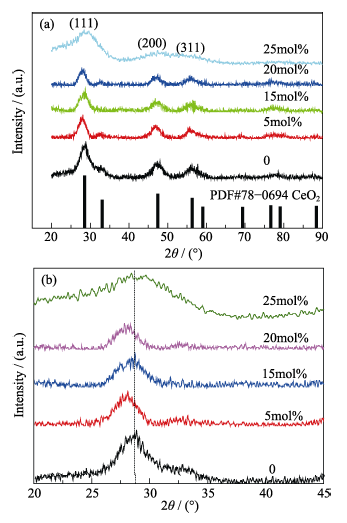
图1 不同钴掺杂比例的氧化铈纳米粒子的XRD图谱
Fig. 1 XRD patterns of ceria nanoparticles with different doping ratio of cobalt(a) Whole range patterns; (b) Magnified patterns of the highest peak (111)
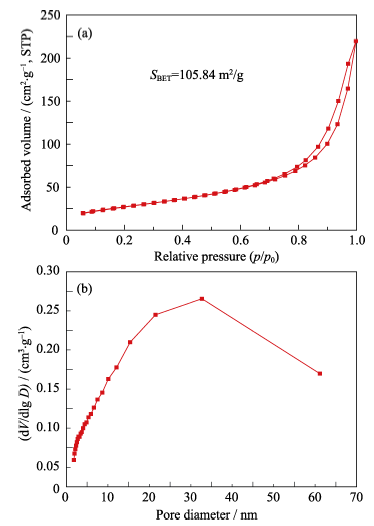
图2 (a)氧化铈纳米粒子的N2吸附-脱附曲线和(b)氧化铈纳米粒子的BJH孔径分布曲线
Fig. 2 (a) N2 adsorption-desorption isotherm of ceria nanoparticles and (b) BJH pore size distribution curve of ceria nanoparticles
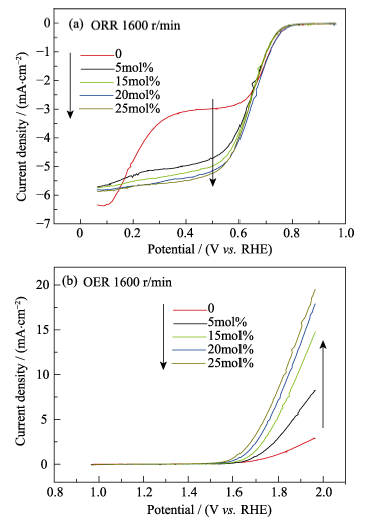
图3 不同掺杂比例的氧化铈纳米粒子在1600 r/min下的ORR (a)和OER (b)极化曲线
Fig. 3 Polarization curves at 1600 r/min of different of ceria nanoparticles with different doping ratio(a) (ORR) and (b) (OER)
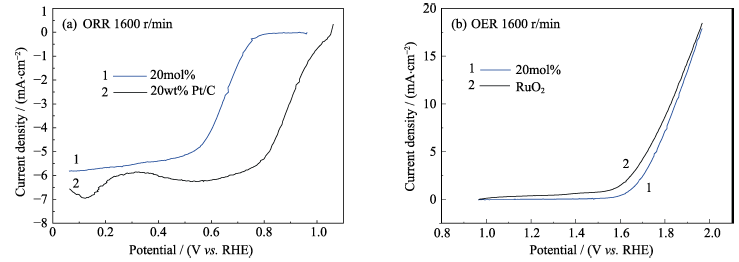
图4 (a)氧化铈纳米粒子和Pt/C在1600 r/min下的极化曲线(ORR); (b)氧化铈纳米粒子和RuO2在1600 r/min下的极化曲线(OER)
Fig. 4 Polarization curves at 1600 r/min of (a) ceria nanoparticles and Pt/C (ORR) and (b) ceria nanoparticles and RuO2 (OER)
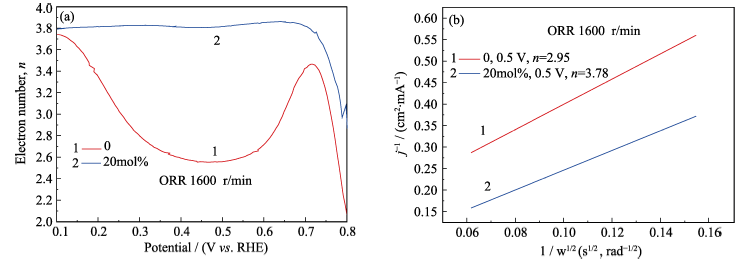
图5 (a) RRDE模式下测得相应掺杂比例的氧化铈纳米粒子转移电子数; (b) 0.5 V时相应掺杂比例的氧化铈纳米粒子的K-L曲线
Fig. 5 (a) Corresponding transfer electron number of ceria nanoparticles with different doping amount in RRDE model, and (b) corresponding K-L plots of ceria nanoparticles with different doping ratio at 0.5 V
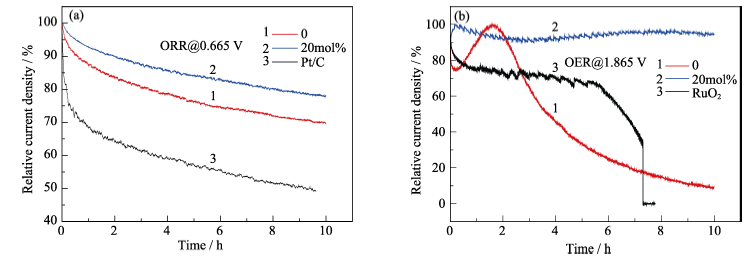
图6 (a)二种不同掺杂比例的氧化铈纳米粒子和Pt/C对应的计时电流曲线; (b)二种不同掺杂比例氧化铈纳米粒子和RuO2对应的计时电流曲线
Fig. 6 Current-time(I-t) chronoamperometric responses for ceria nanoparticles (a) at two different doping ratio and Pt/C, and (b) at two different doping ratio and RuO2
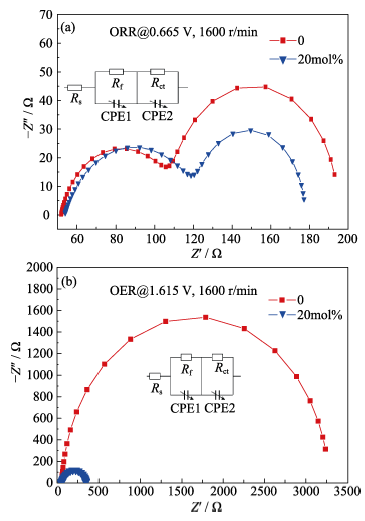
图7 (a)二种不同掺杂比例下对应氧化铈的ORR (a)和OER (b)的极化阻抗图谱
Fig. 7 (a) Corresponding ORR(a) and OER (b) electrochemical impedance spectra of Ceria at two different doping ratio
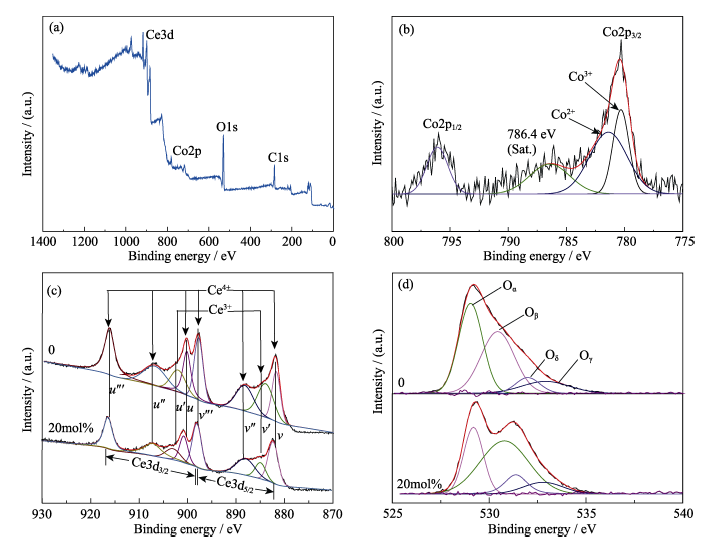
图10 Co掺杂比例为20mol%时CeO2的(a) XPS全谱和(b) Co2p的高分辨XPS图谱; 不同掺杂比例下CeO2中Ce3d (c)和O1s(d)的高分辨XPS图谱
Fig. 10 (a) XPS survey spectra and (b) high-resolution XPS spectra of Co2p of CeO2 doping with 20mol% Co; High-resolution XPS spectra of Ce3d (c) and O1s (d) of CeO2 with different doping ratio
| Sample | Content | |||||
|---|---|---|---|---|---|---|
| Oα/at% | Oβ/at% | Oδ/at% | Oγ/at% | Oβ/Oα | [Ce3+/(Ce4++Ce3+)]/% | |
| 0 | 48.5 | 29.2 | 10.4 | 11.8 | 0.60 | 20.1 |
| 20mol% | 27.2 | 53.1 | 10.5 | 9.2 | 1.95 | 24.1 |
表1 不同掺杂比例下对应的XPS结果
Table 1 Corresponding XPS results of different doping ratio
| Sample | Content | |||||
|---|---|---|---|---|---|---|
| Oα/at% | Oβ/at% | Oδ/at% | Oγ/at% | Oβ/Oα | [Ce3+/(Ce4++Ce3+)]/% | |
| 0 | 48.5 | 29.2 | 10.4 | 11.8 | 0.60 | 20.1 |
| 20mol% | 27.2 | 53.1 | 10.5 | 9.2 | 1.95 | 24.1 |
| [1] | JIE S, KEVIN J M, HUBERT A G,et al. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles.Science, 2011, 334(6061): 1383-1385. |
| [2] | JIN S, HUBERT A G, YABUUCHI N,et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries.Nature Chemistry, 2011, 3(8): 647. |
| [3] | WU G, KARREN L M, JOHNSTON C M,et al. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt.Science, 2011, 332(6028): 443-447. |
| [4] | LEFÈVRE M, PROIETTI E, JAOUEN F,et al. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells.Science, 2009, 324(5923): 71-74. |
| [5] | CAO Z X, DING Y M, WANG Z C,et al. Porous calcium manganese oxide: preparation and electrocatalytic activity of oxygen reduction reaction.Journal of Inorganic Materials, 2017, 32(5): 535-542. |
| [6] | HOU F, LI H, YANG Y,et al. Preparation and catalytic oxidation of CO with specific morphology and porous nano CeO2.Chemical Industry and Engineering Progress, 2017, 36(7): 2481-2487. |
| [7] | KE J, XIAO J W, ZHU W,et al. Dopant-induced modification of active site structure and surface bonding mode for high-performance nanocatalysts: CO oxidation on capping-free(110)-oriented CeO2: Ln (Ln=La-Lu) nanowires.Journal of the American Chemical Society, 2013, 135(40): 15191-15200. |
| [8] | XU B, ZHANG Q, YUAN S,et al. Synthesis and photocatalytic performance of yttrium-doped CeO2 with a porous broom-like hierarchical structure.Applied Catalysis B: Environmental, 2016, 183: 361-370. |
| [9] | HAO L, YU J, XU X,et al. Nitrogen-doped MoS2/carbon as highly oxygen-permeable and stable catalysts for oxygen reduction reaction in microbial fuel cells.Journal of Power Sources, 2017, 339: 68-79. |
| [10] | CHEN P, ZHOU T, XING L,et al. Atomically dispersed iron-nitrogen species as electrocatalysts for bifunctional oxygen evolution and reduction reactions.Angewandte Chemie International Edition, 2017, 56(2): 610-614. |
| [11] | ZHU Q L, XIA W, AKITA T,et al. Metal-organic framework-derived honeycomb-like open porous nanostructures as precious-metal-free catalysts for highly efficient oxygen electroreduction.Advanced Materials, 2016, 28(30): 6391-6398. |
| [12] | FENG J, LIANG Y G, WANG H L,et al. Engineering manganese oxide/nanocarbon hybrid materials for oxygen reduction electrocatalysis.Nano Research, 2012, 5(10): 718-725. |
| [13] | TAN Y M, XU C F, CHEN G X,et al. Facile synthesis of manganese-oxide-containing mesoporous nitrogen-doped carbon for efficient oxygen reduction.Advanced Functional Materials, 2012, 22(21): 4584-4591. |
| [14] | DONG C, LIU Z W, LIU Y J, ,et al.Modest oxygen-defective amorphous manganese-based nanoparticle mullite with superior overall electrocatalytic performance for oxygen reduction reaction. Small, 2017,13(16): 1603903-1-9. |
| [15] | LIANG Y Y, WANG H L, ZHOU J G,et al. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts.Journal of the American Chemical Society, 2012, 134(7): 3517-3523. |
| [16] | PENDASHTEH A, PALAMA J, ANDERSON M,et al. NiCoMnO4 nanoparticles on N-doped graphene: highly efficient bifunctional electrocatalyst for oxygen reduction/evolution reactions.Applied Catalysis B: Environmental, 2017, 201: 241-252. |
| [17] | HARDIN W G, MEFFORD J T, SLANAC D A,et al. Tuning the electrocatalytic activity of perovskites through active site variation and support interactions.Chemistry of Materials, 2014, 26(11): 3368-3376. |
| [18] | HARDIN W G, SLANAC D A, WANG X,et al. Highly active, nonprecious metal perovskite electrocatalysts for bifunctional metal-air battery electrodes.The Journal of Physical Chemistry Letters, 2013, 4(8): 1254-1259. |
| [19] | HARTMANN P, BREZESINSKI T, SANN J,et al. Defect chemistry of oxide nanomaterials with high surface area: ordered mesoporous thin films of the oxygen storage catalyst CeO2-ZrO2.ACS Nano. 2013, 7(4): 2999-3013. |
| [20] | XU X M, SU C, ZHOU W, ,et al.3 Co-doping strategy for developing perovskite oxides as highly efficient electrocatalysts for oxygen evolution reaction.Advanced Science., 2016, 3(2): 1500187-1-6. |
| [21] | ZHU Y L, ZHOU W, SUNARSO J,et al. Phosphorus-doped perovskite oxide as highly efficient water oxidation electrocatalyst in alkaline solution.Advanced Functional Materials, 2016, 26(32): 5862-5872. |
| [22] | HE Y, ZHANG J F, HE G W,et al. Ultrathin Co3O4 nanofilm as an efficient bifunctional catalyst for oxygen evolution and reduction reaction in rechargeable zinc-air batteries.Nanoscale, 2017, 9(25): 8623-8630. |
| [23] | MENG Y T, SONG W Q, HUANG H,et al. Structure-property relationship of bifunctional MnO2 nanostructures: highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media.Journal of the American Chemical Society, 2014, 136(32): 11452-11464. |
| [24] | ZHAO X, FU Y, WANG J,et al. Ni-doped CoFe2O4 hollow nanospheres as efficient bi-functional catalysts.Electrochimica Acta, 2016, 201: 172-178. |
| [25] | XU B, ZHANG Q T, YUAN S S,et al. Synthesis and photocatalytic performance of yttrium-doped CeO2 with a hollow sphere structure.Catalysis Today, 2017, 281: 135-143. |
| [26] | LI H, MENG F, GONG J,et al. Structural, morphological and optical properties of shuttle-like CeO2 synthesized by a facile hydrothermal method.Journal of Alloys and Compounds, 2017, 722: 489-498. |
| [27] | DENG W, DAI Q G, LAO Y J,et al. Low temperature catalytic combustion of 1, 2-dichlorobenzene over CeO2-TiO2 mixed oxide catalysts.Applied Catalysis B: Environmental, 2016, 181: 848-861. |
| [28] | ZANG C J, ZHANG X S, HU S Y,et al. The role of exposed facets in the Fenton-like reactivity of CeO2 nanocrystal to the Orange II.Applied Catalysis B: Environmental, 2017, 216: 106-113. |
| [29] | FENG J, YE S H, XU H,et al. Design and synthesis of FeOOH/ CeO2 heterolayered nanotube electrocatalysts for the oxygen evolution reaction.Advanced Materials, 2016, 28(23): 4698-4703. |
| [30] | BECHE E, CHARVIN P, PERARNAU D,et al. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (C.xTiyOz). Surface and Interface Analysis, 2008, 40(3/4): 264-267. |
| [31] | LI L L, ZHANG L, MA K L,et al. Ultra-low loading of copper modified TiO2/CeO2 catalysts for low-temperature selective catalytic reduction of NO by NH3.Applied Catalysis B: Environmental, 2017, 207: 366-375. |
| [32] | LIANG F L, YU Y, ZHOU W,et al. Highly defective CeO2 as a promoter for efficient and stable water oxidation.Journal of Material Chemistry, 2015, 3(2): 634-640. |
| [33] | XU L, JIANG Q Q, XIAO Z H,et al. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction.Angewandte Chemie International Edition, 2016, 55(17): 5277-5281. |
| [34] | ZHUANG L Z, GE L, YANG Y S, ,et al..Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Advanced Materials, 2017, 29(17): 1606793- 1-7. |
| [35] | WANG F, ZHANG L, XU L,et al. Low temperature CO oxidation and CH4 combustion over Co3O4 nanosheets.Fuel, 2017, 203: 419-429. |
| [1] | 孔国强, 冷明哲, 周战荣, 夏池, 沈晓芳. Sb掺杂O3型Na0.9Ni0.5Mn0.3Ti0.2O2钠离子电池正极材料[J]. 无机材料学报, 2023, 38(6): 656-662. |
| [2] | 杨颖康, 邵怡晴, 李柏良, 吕志伟, 王路路, 王亮君, 曹逊, 吴宇宁, 黄荣, 杨长. Cl掺杂对CuI薄膜发光性能增强研究[J]. 无机材料学报, 2023, 38(6): 687-692. |
| [3] | 李悦, 张旭良, 景芳丽, 胡章贵, 吴以成. 铈掺杂硼酸钙镧晶体的生长与性能研究[J]. 无机材料学报, 2023, 38(5): 583-588. |
| [4] | 齐占国, 刘磊, 王守志, 王国栋, 俞娇仙, 王忠新, 段秀兰, 徐现刚, 张雷. GaN单晶的HVPE生长与掺杂进展[J]. 无机材料学报, 2023, 38(3): 243-255. |
| [5] | 王志强, 吴济安, 陈昆峰, 薛冬峰. 大尺寸Er,Yb:YAG单晶的生长及其性能[J]. 无机材料学报, 2023, 38(3): 329-334. |
| [6] | 陆晨辉, 葛万银, 宋盼盼, 张盼锋, 徐美美, 张伟. 用于白光LED稀土Eu掺杂SiAlON基荧光粉的发光性能[J]. 无机材料学报, 2023, 38(1): 97-104. |
| [7] | 王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029. |
| [8] | 柳琪, 朱璨, 谢贵震, 王俊, 张东明, 邵刚勤. Ce掺杂SrMgF4超结构多晶体的吸收/光致发光光谱[J]. 无机材料学报, 2022, 37(8): 897-902. |
| [9] | 邹顺, 何夕云, 曾霞, 仇萍荪, 凌亮, 孙大志. 掺铋钇铁石榴石磁光陶瓷的热压烧结及其性能研究[J]. 无机材料学报, 2022, 37(7): 773-779. |
| [10] | 焦博新, 刘兴翀, 全子威, 彭永姗, 周若男, 李海敏. L-精氨酸掺杂钙钛矿太阳电池性能研究[J]. 无机材料学报, 2022, 37(6): 669-675. |
| [11] | 王新健, 朱逸璇, 张鹏, 杨文龙, 王挺, 郇宇. (Ba0.85Ca0.15)(Ti0.9Zr0.1-xSnx)O3无铅压电陶瓷的相结构与压电性能[J]. 无机材料学报, 2022, 37(5): 513-519. |
| [12] | 安琳, 吴淏, 韩鑫, 李耀刚, 王宏志, 张青红. 非贵金属Co5.47N/N-rGO助催化剂增强TiO2光催化制氢性能[J]. 无机材料学报, 2022, 37(5): 534-540. |
| [13] | 李高然, 李红阳, 曾海波. 硼基材料在锂硫电池中的研究进展[J]. 无机材料学报, 2022, 37(2): 152-162. |
| [14] | 娄许诺, 邓后权, 李爽, 张青堂, 熊文杰, 唐国栋. Ge掺杂MnTe材料的热电输运性能[J]. 无机材料学报, 2022, 37(2): 209-214. |
| [15] | 刘鼎伟, 曾江涛, 郑嘹赢, 满振勇, 阮学政, 时雪, 李国荣. BiAlO3掺杂PZT陶瓷的高压电性能和低电场应变滞后[J]. 无机材料学报, 2022, 37(12): 1365-1370. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||