无机材料学报 ›› 2018, Vol. 33 ›› Issue (8): 811-824.DOI: 10.15541/jim20170529
• • 下一篇
何前军, 陈丹阳, 范明俭
收稿日期:2017-11-09
修回日期:2017-12-27
出版日期:2018-08-28
网络出版日期:2018-07-17
基金资助:HE Qian-Jun, CHEN Dan-Yang, FAN Ming-Jian
Received:2017-11-09
Revised:2017-12-27
Published:2018-08-28
Online:2018-07-17
Supported by:摘要:
精准纳米气体治疗具有低毒高效等特性, 作为一种新兴的疾病治疗手段受到越来越多的关注。研究表明, 纳米气体治疗不仅能在特定疾病部位选择性杀死癌细胞, 还能保护正常细胞。本文总结了国际最新研究成果, 对精准纳米气体治疗的最新研究进展进行了总结归纳和展望。首先, 阐述了纳米气体治疗的治疗作用和特点; 然后, 总结了实现精准纳米气体治疗的主要途径, 包括靶向气体传输、可控气体释放、医学成像引导和监控气体治疗、基于治疗性气体的多模式联合治疗等; 最后, 对纳米气体治疗存在的问题和发展前景做出了总结和展望。
中图分类号:
何前军, 陈丹阳, 范明俭. 精准纳米气体治疗研究进展[J]. 无机材料学报, 2018, 33(8): 811-824.
HE Qian-Jun, CHEN Dan-Yang, FAN Ming-Jian. Progress of Precision Nanomedicine-mediated Gas Therapy[J]. Journal of Inorganic Materials, 2018, 33(8): 811-824.
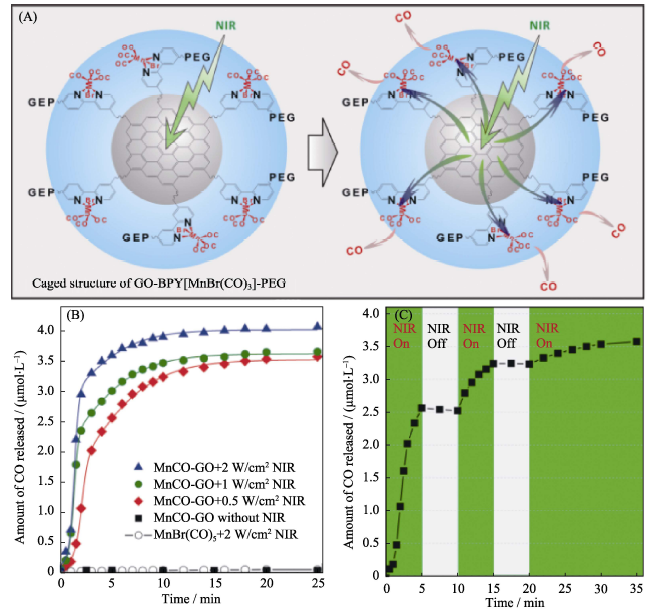
图1 (A)具有“笼状”结构MnCO-GON纳米药物的NIR光响应性释放CO气体的原理示意图, (B)纳米药物可控释放CO的NIR光响应性和(C)纳米药物可控释放CO的NIR光可控性[27]
Fig. 1 (A) NIR-responsive CO release mechanism of the MnCO-GON nanomedicine with a caged structure, (B) NIR responsive for CO release profiles of MnCO-GON, and (C) NIR-controllability of MnCO-GON for CO release[27] GON: Graphene Oxide Nanosheet
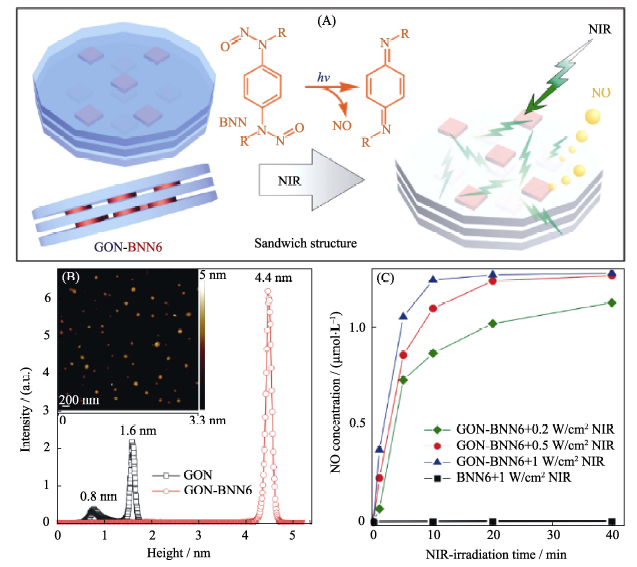
图2 (A)具有“三明治”结构的GON-BNN6纳米药物的π?π共轭自组装, 及其NIR光响应性释放NO气体的原理示意图, (B)纳米药物的AFM形貌表征和(C)纳米药物的NIR可控释放NO行为[28]
Fig. 2 (A) The sandwich structure of GO-BNN6 self-assembled by GO nanosheets and BNN6 molecules through the π-π stacking, and the mechanism of NIR-responsive NO release; (B) AFM data of GO-BNN6 and (C) NIR-controlled NO release profiles of the GO-BNN6 nanomedicine[28] BNN: bis-N-nitroso
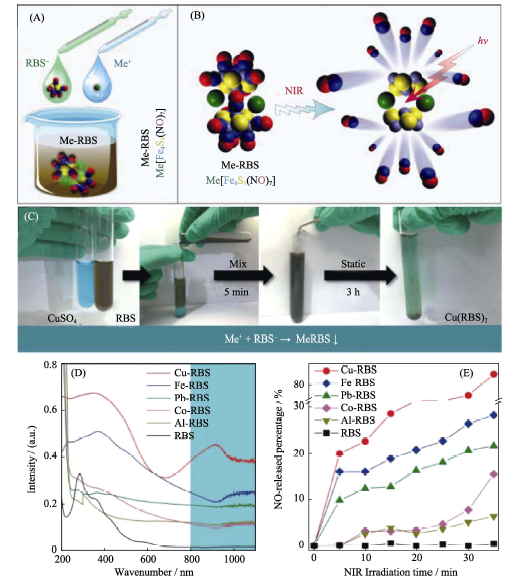
图3 (A)不溶性金属配位型罗森黑盐Me-RBS的配位络合-沉淀法示意图; (B)Me-RBS的NIR光响应性释放NO气体的原理示意图; (C)Me-RBS的紫外吸收行为比较和(D)NIR光控释放NO行为比较[38]
Fig. 3 (A) Schematic illustration of the coordination-precipitation process of insoluble Me-RBS, (B) the NIR-responsive NO release mechanism of Me-RBS, (C) comparison of UV absorption behaviors of Me-RBS and (D) comparison of NO release behaviors of Me-RBS[38] RBS: Rosen Black Salt
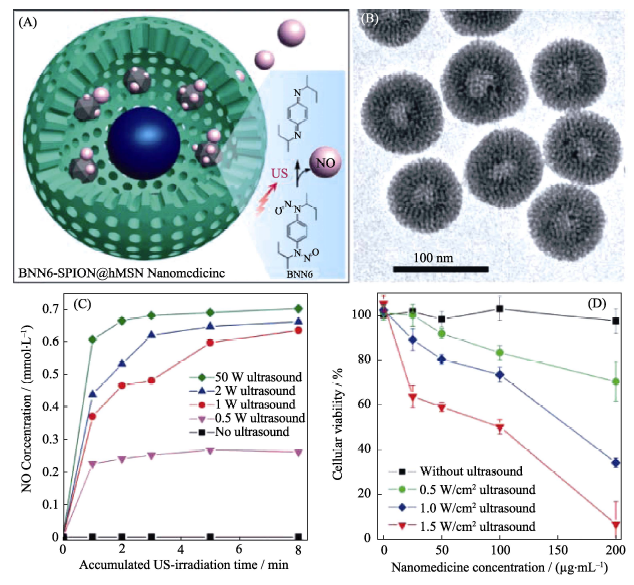
图4 (A)具有“铃铛”结构的BNN6-SPION@hMSN纳米药物的超声响应释放NO气体的原理示意图, (B)纳米药物的TEM照片, (C)纳米药物的超声可控释放NO行为和(D)超声诱导纳米药物细胞毒性行为[41]
Fig. 4 (A) The mechanism of ultrasound-responsive NO release from the rattle-structured BNN6-SPION@hMSN nanomedicine, (B) TEM image of the nanomedicine, (C) ultrasound-responsive NO release behavior of the nanomedicine and (D) ultrasound-induced cytotoxicity of the nanomedicine[41] BNN: bis-N-nitroso; SPION: Superparamagnetic Iron Oxide-encapsulated; hMSN: hollow Mesoporous Silica Nanoparticles
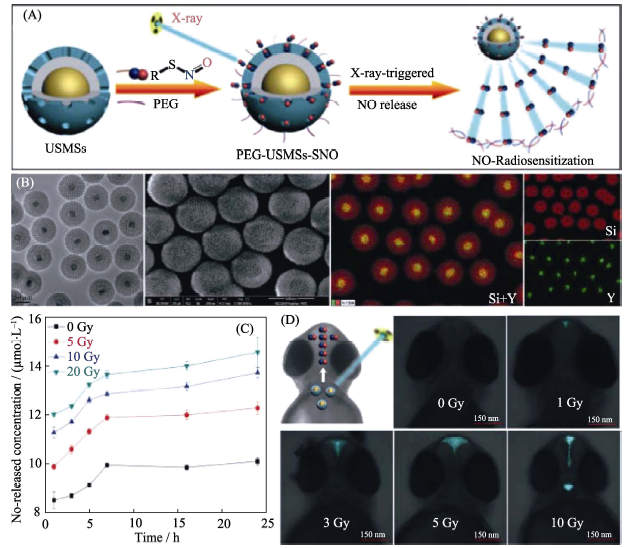
图5 (A)具有“核壳”结构的PEG-USMSs-SNO纳米药物的X射线响应性释放NO气体的原理示意图, (B)纳米药物的TEM照片和元素分布图, (C)纳米药物在体外的X射线可控释放NO行为和(D)纳米药物在斑马鱼体内的X射线可控释放NO行为[42]
Fig. 5 (A) The mechanism of X-ray responsive NO release from the PEG-USMSs-SNO nanomedicine with the core-shell structure, (B) TEM images and elementary mapping of the nanomedicine, (C) X-ray controlled NO release behavior of the nanomedicine in vitro, and (D) X-ray controlled NO release behavior of the nanomedicine on zebrafish[42] USMSs: Upconversion nano-theranostic system; SNO: S-nitrosothiol.
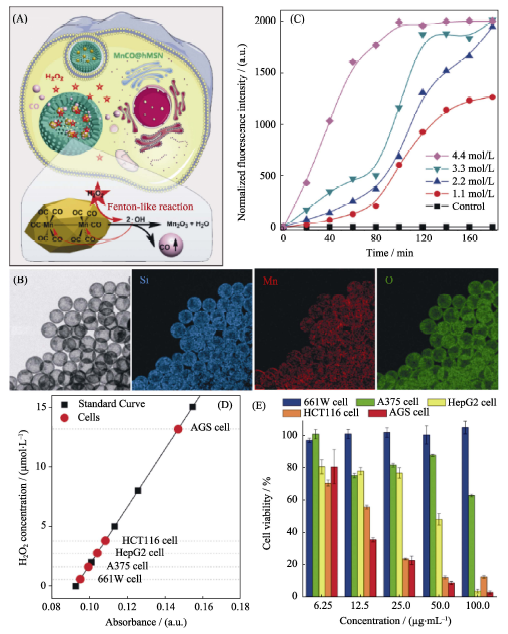
图6 (A)基于空心介孔二氧化硅纳米载体和羰基锰前药的MnCO@hMSN纳米药物的双氧水响应性释放CO气体的原理示意图, (B)纳米药物的TEM照片和元素分布图, (C)纳米药物在体外的双氧水可控释放CO行为, (D)各种细胞内的双氧水水平比较和(E)纳米药物对各种细胞的细胞毒性研究[43]
Fig. 6 (A) The H2O2-triggered CO release mechanism of the MnCO@hMSN nanomedicine constructed by hMSN and manganese carbonyl prodrug, (B) TEM image and elementary mapping of the nanomedicine, (C) H2O2-triggered CO release behavior of the nanomedicine in vitro, (D) comparison of H2O2 levels in various cells and (E) comparison of cytotoxicity against various cells[43]hMSN: hollow Mesoporous Silica Nanoparticles
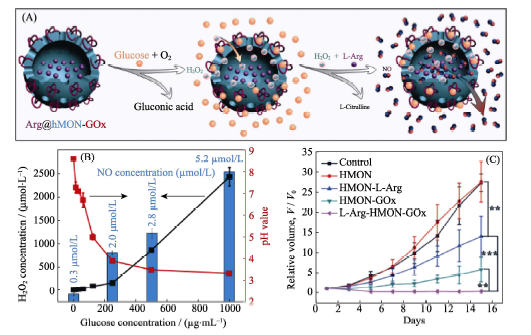
图7 (A)基于空心介孔二氧化硅纳米载体和葡萄糖氧化酶/L-精氨酸前药的Arg@hMON-GOx纳米药物的构建及其葡萄糖响应性释放NO气体的原理示意图, (B)葡萄糖浓度对双氧水浓度、pH和NO浓度的影响和(C)纳米药物对荷瘤鼠的治疗作用[44]
Fig. 7 (A) Construction and SEM image of the Arg@hMON-GOx nanomedicine based on the hMON carrier and the Arginie/GOx prodrugs, and the mechanism of glucose-responsive release of NO, (B) effects of glucose concentration on hydrogen peroxide concentration, pH and NO concentration, and (C) in vivo outcome of gas therapy by nanomedicine[44]hMON: hollow Mesoporous Organosilica Nanoparticle
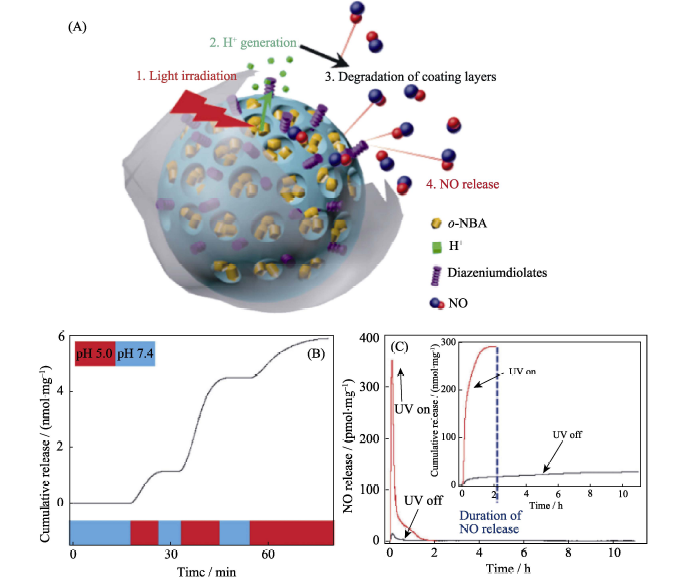
图8 (A)通过介孔二氧化硅纳米载体包裹NO气体前药并涂覆磷酸钙响应层构建的新型纳米药物MSN-CaP-NO及其控释原理示意图, (B)纳米药物的酸响应NO释放行为和(C)纳米药物的光控NO释放行为[32]
Fig. 8 (A) The construction of the MSN-CaP-NO nanomedicine and its controlled NO release mechanism, (B) acid-responsive NO release behavior of the nanomedicine, and (C) light-controlled NO release profile of the nanomedicine[32]MSN: Mesoporous Silica Nanoparticles
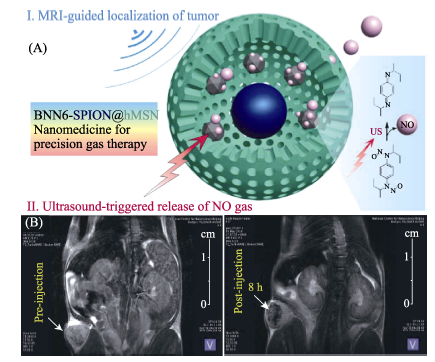
图9 (A)具有“铃铛”结构的BNN6-SPION@hMSN纳米药物的MRI成像引导超声可控NO气体释放的原理示意图, 和(B)纳米药物的肿瘤靶向行为和MRI成像照片[41]
Fig. 9 (A) The MRI-guided ultrasound-triggered NO release mechanism of BNN6-SPION@hMSN nanomedicine, and (B) tumor-targeting property and the corresponding MRI profile[41]BNN: bis-N-nitroso; SPION: Superparamagnetic Iron Oxide-encapsulated; hMSN: hollow Mesoporous Silica Nanoparticles
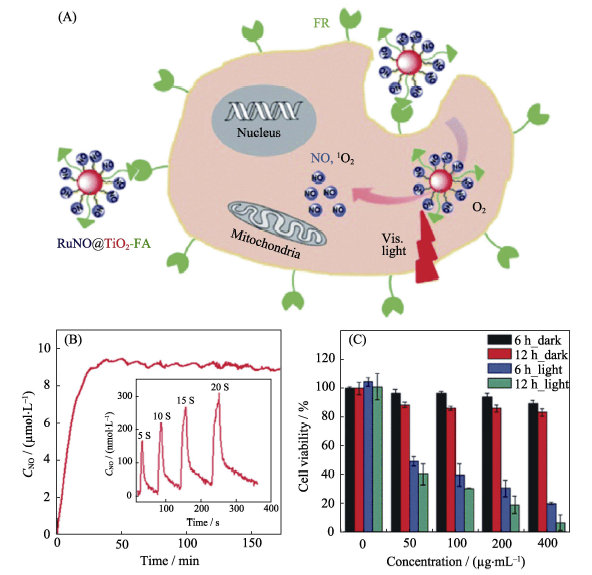
图10 (A)由二氧化钛纳米粒包裹NO前药构建的纳米药物RuNO@TiO2NPs及其光控ROS和NO共释放原理示意图, (B)纳米药物的光控NO释放行为和(C)纳米药物的细胞毒性研究[48]
Fig. 10 (A) The construction of the RuNO@TiO2NPs nanomedicine and its light-controlled ROS/NO co-release mechanism, (B) behavior of light-responsive release of NO and (C) cytotoxicity profile of the nanmedicine[48]
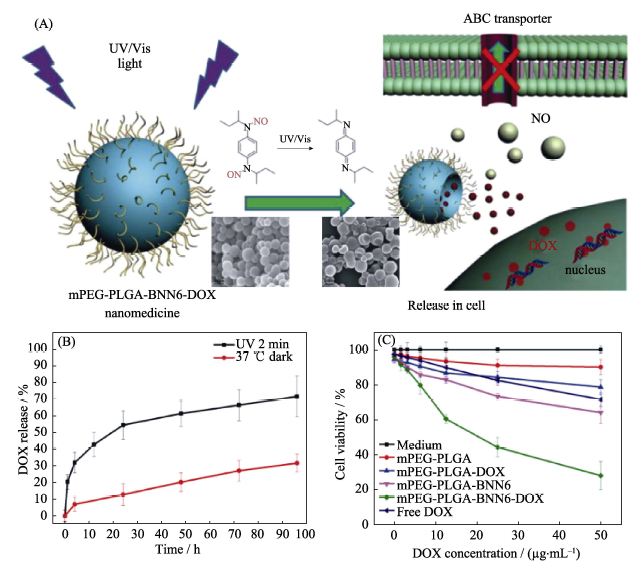
图11 (A)mPEG-PLGA载体共担载化疗药物DOX和气体前药BNN6构建的纳米药物的光控多药共释原理示意图, (B)纳米药物的光控释放NO行为和(C)纳米药物的细胞毒性研究[31]
Fig. 11 (A) The mechanism of light-controlled multidrug co-release from the mPEG-BNN6-DOX nanomedicine, (B) behavior of UV-controlled NO release of the nanomedicine, and (C) cytotobxicity profile of the nanomedicine[31]BNN: bis-N-nitroso; DOX: Doxorubicin
| [1] | SZABÓ C.Gasotransmitters in cancer: from pathophysiology to experimental therapy.Nat. Rev. Drug Discov., 2016, 15(3): 185-203. |
| [2] | SZABÓ C.Hydrogen sulphide and its therapeutic potential.Nat. Rev. Drug Discov., 2007, 6(11): 917-935. |
| [3] | FUKUMURA D, KASHIWAGI S, JAIN R K.The role of nitric oxide in tumour progression.Nat. Rev. Cancer., 2006, 6(7): 521-534. |
| [4] | MOTTERLINI R, OTTERBEIN L E.The therapeutic potential of carbon monoxide. Nat. Rev. Drug. Discov., 2010, 9(9): 728-743. |
| [5] | GALLEGO S G,BERNARDES G J L. Carbon-monoxide-releasing molecules for the delivery of therapeutic CO in vivo. Angew. Chem. Int. Ed., 2014, 53(37): 9712-9721. |
| [6] | BIBHUTI B M, VIJAY A K R, GREGORY W M,et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β Christian Bogdan. .Nat. Immunol., 2013, 14(1): 52-60. |
| [7] | LALA P K, CHAKRABORTY C.Role of nitric oxide in carcinogenesis and tumour progression.Lancet Oncol., 2001, 2(3): 149-156. |
| [8] | CHIN B Y, JIANG G, WEGIEL B,et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning.Proc. Natl. Acad. Sci. USA, 2007, 104(12): 5109-5114. |
| [9] | OTTERBEIN L E, ZUCKERBRAUN B S, HAGA M,et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury.Nat. Med., 2003, 9(2): 183-190. |
| [10] | OTTERBEIN L E, BACH F H, ALAM J,et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med., 2000, 6(4): 422-428. |
| [11] | NASSOUR I, KAUTZA B, RUBIN M,et al. Carbon monoxide protects against hemorrhagic shock and resuscitation-induced microcirculatory injury and tissue injury.Shock, 2015, 43(2): 166-171. |
| [12] | CARPENTER A W, SCHOENFISCH M H.Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev., 2012, 41(10): 3742-3752. |
| [13] | DIRING S, WANG D O, KIM C,et al. Localized cell stimulation by nitric oxide using a photoactive porous coordination polymer platform. Nat. Commun., 2013, 4(10): 2684. |
| [14] | KIM J, SARAVANAKUMAR G, CHOI H W,et al. A platform for nitric oxide delivery.J. Mater. Chem., 2014, 2(4): 341-356. |
| [15] | RAJU T N.The Nobel chronicles. 1998: Robert Francis Furchgott (b 1911), Louis J Ignarro (b 1941), and Ferid Murad (b 1936).Lancet, 2000, 356(9226): 346. |
| [16] | WEGIEL B, GALLO D, CSIZMADIA E,et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res., 2013, 73(23): 7009-7021. |
| [17] | MONCADA S, ERUSALIMSKY J D.Does nitric oxide modulate mitochondrial energy generation and apoptosis?.Nat. Rev. Mol. Cell Biol., 2002, 3(4): 214-220. |
| [18] | MÓDIS K, BOS E M, CALZIA E,et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects.Br. J. Pharmacol., 2014, 171(8): 2123-2146. |
| [19] | HE Q J.Precision gas therapy using intelligent nanomedicine.Biomater. Sci., 2017, 5(11): 2226-2230. |
| [20] | ERNST A, ZIBRAK J D.Carbon monoxide poisoning.N. Engl. J. Med., 1998, 339(22): 1603-1608. |
| [21] | SULLIVAN J B, KRIEGER G B, THOMAS R J.Methemoglobin-forming chemicals in hazardous materials toxicology: clinical principals of environmental health.J. Occup. Environ. Med., 1992, 34(4): 365-371. |
| [22] | RIDNOUR L A, THOMAS D D, DONZELLI S,et al. The biphasic nature of nitric oxide responses in tumor biology.Antioxid. Redox. Sign., 2006, 8(7/8): 1329-1337. |
| [23] | WANG L Z, SHI J L, YUN J,et al. Research progress of mesoporous silicon materials.J. Inorg. Mater., 1999, 14(3): 333-342. |
| [24] | SHI J L, CHEN Y, CHEN H R.Research progress of multifunctional mesoporous silica based nanoparticles for diagnosis and treatment.J. Inorg. Mater., 2013, 28(1): 1-11. |
| [25] | HE Q J, SHI J L.MSN anti-cancer nanomedicines: chemotherapy enhancement, overcoming of drug resistance and metastasis inhibition.Adv. Mater., 2014, 26(3): 391-411. |
| [26] | ZHOU Y L, FENG X X, ZHAI W Y.Research on loading and release of Epirubicin with mesoporous bioactive glass.J. Inorg. Mater., 2011, 26(1): 68-72. |
| [27] | HE Q, KIESEWETTER D O, QU Y,et al. NIR-responsive on-demand release of CO from metal carbonyl-caged graphene oxide nanomedicine.Adv. Mater., 2015, 27(42): 6741-6746. |
| [28] | FAN J, HE N Y, HE Q J,et al. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO.Nanoscale, 2015, 7(47): 20055-20062. |
| [29] | GARCIA J V, YANG J, SHEN D,et al. NIR-triggered release of caged nitric oxide using upconverting nanostructured materials.Small, 2012, 8(24): 3800-3805. |
| [30] | ZHANG X, TIAN G, YIN W,et al. Controllable generation of nitric oxide by near-infrared-sensitized upconversion nanoparticles for tumor therapy. Adv. Funct. Mater., 2015, 25(20): 3049-3055. |
| [31] | FAN J, HE Q, LIU Y,et al. Light-responsive biodegradable nanomedicine overcomes multidrug resistanc.via NO-enhanced chemosensitization. ACS Appl. Mater. Interfaces, 2016, 8(22): 13804-1381. |
| [32] | WOO C H, JIHOON K, JINHWAN K,et al. Light-induced acid generation on a gatekeeper for smart nitric oxide delivery.ACS Nano, 2016, 10(4): 4199-4208. |
| [33] | OSTROWSKI D, LIN B F, TIRRELL M V,et al. Liposome encapsulation of a photochemical NO precursor for controlled nitric oxide release and simultaneous fluorescence imaging.Mol. Pharm., 2012, 9(10): 2950-2955. |
| [34] | WANG P G, XIAN M, TANG X P,et al. Nitric oxide donors: Chemical activities and biological applications.Chem. Rev., 2002, 102(4): 1091-1134. |
| [35] | RIMMER R D, PIERRI A E, FORD P C.Photochemically activated carbon monoxide release for biological targets. toward developing air-stable photoCORMs labilized by visible light.Coordin. Chem. Rev., 2012, 256(15/16): 1509-1519. |
| [36] | ZHENG D W, LI B, LI C X, et al. Photocatalyzing CO2 to CO for enhanced cancer therapy.Adv. Mater., 2017, 29(44): 1703822-1-8. |
| [37] | Reddy G U, AXTHELM J, HOFFMANN P,et al. Co-registered molecular logic gate with a CO-releasing molecule triggered by light and peroxide.J. Am. Chem. Soc., 2017, 139(14): 4991-4994. |
| [38] | CHEN L J, HE Q J, LEI M Y,et al. Facile coordination-precipitation route to insoluble metal roussin’s black salts for NIR-responsive release of NO for anti-metastasis.ACS Appl. Mater. Interfaces., 2017, 9(42): 36473-36477. |
| [39] | MARIN A, MUNIRUZZAMAN M, RAPOPORT N.Mechanism of the ultrasonic activation of micellar drug delivery. J. Control. Release, 2001, 75(1/2): 69-81. |
| [40] | POSTEMA M, BOUAKAZ A,CATE F J T,et al. Nitric oxide delivery by ultrasonic cracking: some limitations.Ultrasonics, 2006, 44(Suppl.): e109-e113. |
| [41] | JIN Z, WEN Y, HU Y,et al. MRI-guided and ultrasound-triggered release of NO by advanced nano-medicine.Nanoscale, 2017, 9(10): 3637-3645. |
| [42] | FAN W, BU W, HE Q,et al. X-ray radiation-controlled NO-release for on-demand depth-independent hypoxic radiosensitization.Angew. Chem. Int. Ed., 2015, 54(47): 14026-14030. |
| [43] | JIN Z, WEN Y, XIONG L,et al. Intratumoral H2O2-triggered release of CO from a metal carbonyl-based nanomedicine for efficient CO therapy. Chem. Commun., 2017, 53(40): 5557-5560. |
| [44] | FAN W, LU N, HUANG P,et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy.Angew. Chem. Int. Ed., 2016, 55(1): 1-6. |
| [45] | HE Q J, GUO S R, QIAN Z Y,et al. Development of individualized anti-metastasis strategies by engineering nanomedicines. Chem. Soc. Rev., 2015, 44(17): 6258-6286. |
| [46] | GUI R J, WAN A J, ZHANG Y L,et al. Light-triggered nitric oxide release and targeted fluorescence imaging in tumor cells developed from folic acid-graft-carboxymethyl chitosan nanospheres.RSC Adv., 2014, 4(57): 30129-30136. |
| [47] | ZHANG X F, MANSOURI S, MBEH D A,et al. Nitric oxide delivery by core/shell superparamagnetic nanoparticle vehicles with enhanced biocompatibility.Langmuir, 2012, 28(35): 12879-12885. |
| [48] | XIANG J, AN L, TANG W W,et al. Photo-controlled targeted intracellular delivery of both nitric oxide and singlet oxygen using a fluorescencence trackable ruthenium nitrosyl functional nanoplatform.Chem. Commun., 2015, 51(13): 2555-2558. |
| [49] | CHAKRABORTY I, JIMENEZ J, SAMEERA W M,et al. Luminescent Re(I) carbonyl complexes as trackable PhotoCORMs for CO delivery to cellular targets. Inorg. Chem., 2017, 56(5): 2863-2873. |
| [50] | JI X, ZHOU C, JI K,et al. Click and release: a chemical strategy toward developing gasotransmitter prodrugs by using an intramolecular Diels-Alder reaction.Angew. Chem. Int. Ed., 2016, 55(1): 1-7. |
| [51] | CARRINGTON S J, CHAKRABORTY I, BERNARD J M L,et al. A theranostic two-tone luminescent PhotoCORM derived from Re(I) and (2-pyridyl)-benzothiazole: trackable CO delivery to malignant cells.Inorg. Chem., 2016, 55(16): 7852-7858. |
| [52] | CHAKRABORTY I, CARRINGTON S J, HAUSER J,et al. Rapid eradication of human breast cancer cells through trackable light- triggered CO delivery by mesoporous silica nanoparticles packed with a designed photoCORM.Chem. Mater., 2015, 27(24): 8387-8397. |
| [53] | CARRINGTON S, CHAKRABORTY I, BERNARD J M L,et al. Synthesis and characterization of a “turn-on” photoCORM for trackable CO delivery to biological targets.ACS Med. Chem. Lett., 2014, 5(12): 1324-1328. |
| [1] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| [2] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [3] | 陈强, 白书欣, 叶益聪. 热管理用高导热碳化硅陶瓷基复合材料研究进展[J]. 无机材料学报, 2023, 38(6): 634-646. |
| [4] | 林俊良, 王占杰. 铁电超晶格的研究进展[J]. 无机材料学报, 2023, 38(6): 606-618. |
| [5] | 牛嘉雪, 孙思, 柳鹏飞, 张晓东, 穆晓宇. 铜基纳米酶的特性及其生物医学应用[J]. 无机材料学报, 2023, 38(5): 489-502. |
| [6] | 苑景坤, 熊书锋, 陈张伟. 聚合物前驱体转化陶瓷增材制造技术研究趋势与挑战[J]. 无机材料学报, 2023, 38(5): 477-488. |
| [7] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [8] | 杨洋, 崔航源, 祝影, 万昌锦, 万青. 柔性神经形态晶体管研究进展[J]. 无机材料学报, 2023, 38(4): 367-377. |
| [9] | 游钧淇, 李策, 杨栋梁, 孙林锋. 氧化物双介质层忆阻器的设计及应用[J]. 无机材料学报, 2023, 38(4): 387-398. |
| [10] | 陈昆峰, 胡乾宇, 刘锋, 薛冬峰. 多尺度晶体材料的原位表征技术与计算模拟研究进展[J]. 无机材料学报, 2023, 38(3): 256-269. |
| [11] | 张超逸, 唐慧丽, 李宪珂, 王庆国, 罗平, 吴锋, 张晨波, 薛艳艳, 徐军, 韩建峰, 逯占文. 新型GaN与ZnO衬底ScAlMgO4晶体的研究进展[J]. 无机材料学报, 2023, 38(3): 228-242. |
| [12] | 齐占国, 刘磊, 王守志, 王国栋, 俞娇仙, 王忠新, 段秀兰, 徐现刚, 张雷. GaN单晶的HVPE生长与掺杂进展[J]. 无机材料学报, 2023, 38(3): 243-255. |
| [13] | 林思琪, 李艾燃, 付晨光, 李荣斌, 金敏. Zintl相Mg3X2(X=Sb, Bi)基晶体生长及热电性能研究进展[J]. 无机材料学报, 2023, 38(3): 270-279. |
| [14] | 刘岩, 张珂颖, 李天宇, 周菠, 刘学建, 黄政仁. 陶瓷材料电场辅助连接技术研究现状及发展趋势[J]. 无机材料学报, 2023, 38(2): 113-124. |
| [15] | 谢兵, 蔡金峡, 王铜铜, 刘智勇, 姜胜林, 张海波. 高储能密度聚合物基多层复合电介质的研究进展[J]. 无机材料学报, 2023, 38(2): 137-147. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||