无机材料学报 ›› 2017, Vol. 32 ›› Issue (9): 923-930.DOI: 10.15541/jim20160662
赵 晗1, 2, 周晓霞1, 潘琳钰1, 2, 陈航榕1
收稿日期:2016-11-28
修回日期:2017-01-16
出版日期:2017-09-30
网络出版日期:2017-08-29
作者简介:赵 晗(1991–), 男, 博士研究生. E-mail: zhaohan@student.sic.ac.cn
基金资助:ZHAO Han1, 2, ZHOU Xiao-Xia1, PAN Lin-Yu1, 2, CHEN Hang-Rong1
Received:2016-11-28
Revised:2017-01-16
Published:2017-09-30
Online:2017-08-29
About author:ZHAO Han. E-mail: zhaohan@student.sic.ac.cn
Supported by:摘要:
采用尿素均相沉淀法合成了CuCeZrOx(CCZ)三元复合氧化物催化剂。采用X射线衍射分析(XRD)、N2-吸脱附测试、X射线光电子能谱(XPS)、扫描透射电镜(STEM)、程序升温还原(脱附、氧化)等技术, 考察煅烧温度对催化剂的物化性质和催化碳烟燃烧活性的影响。结果表明: 350℃煅烧产物CCZ-350表现出最优的催化活性: 在空速为12000 mL/(gcatalyst·h), O2浓度为10vol%, NO浓度为500×10-6, 催化剂和碳烟以10︰1质量比松散接触条件下, 碳烟颗粒最大燃烧速率温度T50 = 407℃, 同时表现出极佳的抗水汽中毒和抗SO2中毒性能, 这与活性组分的高度分散以及催化剂表面大量高活性吸附氧物种的存在有关。此外, 催化剂材料具有疏松多孔的鸟巢状结构, 有利于催化剂和碳烟颗粒的充分接触。该催化剂在柴油车尾气排放温度范围内(150~400℃)还能完全催化氧化柴油车尾气中CO、C3H6和C3H8等其他污染物, 显示出优异的催化净化柴油车尾气的综合性能。
中图分类号:
赵 晗, 周晓霞, 潘琳钰, 陈航榕. 鸟巢状CuCeZrOx三元复合氧化物的合成及其对碳烟颗粒的催化氧化性能研[J]. 无机材料学报, 2017, 32(9): 923-930.
ZHAO Han, ZHOU Xiao-Xia, PAN Lin-Yu, CHEN Hang-Rong. Birdnest-like CuCeZr Mixed Oxides: Synthesis and Excellent Catalysts for Diesel Exhaust Oxidatio[J]. Journal of Inorganic Materials, 2017, 32(9): 923-930.
| Sample | Lattice parameter /nm a | SBET/ (m2·g-1) b | VBJH/ (cm3·g-1) c | Dp/nm c |
|---|---|---|---|---|
| CCZ-300 | 5.416 | 198.0 | 0.960 | 33.8 |
| CCZ-350 | 5.415 | 186.0 | 0.900 | 32.6 |
| CCZ-400 | 5.420 | 160.0 | 0.850 | 33.6 |
| CCZ-500 | 5.418 | 128.0 | 0.800 | 32.5 |
| CCZ-600 | 5.420 | 110.0 | 0.700 | 32.9 |
| CCZ-800 | 5.415 | 12.0 | 0.150 | 49.0 |
| CCZ-1000 | 5.389 | 0.1 | 0.001 | No data |
表1 不同温度煅烧产物CCZ-x的晶胞参数、比表面积、孔容和孔径(x = 300~1000)
Table 1 Structural properties of different CCZ-x catalysts (x = 300-1000)
| Sample | Lattice parameter /nm a | SBET/ (m2·g-1) b | VBJH/ (cm3·g-1) c | Dp/nm c |
|---|---|---|---|---|
| CCZ-300 | 5.416 | 198.0 | 0.960 | 33.8 |
| CCZ-350 | 5.415 | 186.0 | 0.900 | 32.6 |
| CCZ-400 | 5.420 | 160.0 | 0.850 | 33.6 |
| CCZ-500 | 5.418 | 128.0 | 0.800 | 32.5 |
| CCZ-600 | 5.420 | 110.0 | 0.700 | 32.9 |
| CCZ-800 | 5.415 | 12.0 | 0.150 | 49.0 |
| CCZ-1000 | 5.389 | 0.1 | 0.001 | No data |
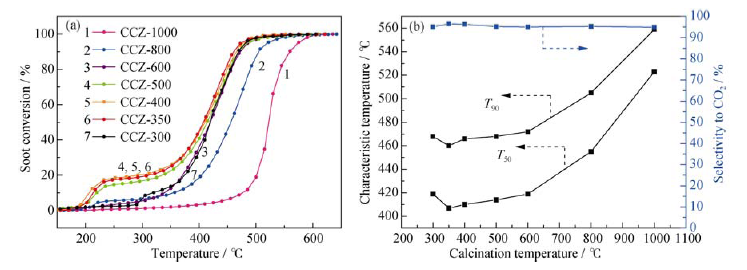
图2 CCZ-x(x=300-1000)催化碳烟氧化过程的(a)碳烟转化率曲线, (b)特征温度T90、T50及CO2选择性随煅烧温度的变化
Fig. 2 Temperature-programmed oxidation of soot catalyzed by different CCZ-x catalysts (x = 300-1000): soot conversion curves (a), and the change in characteristic temperatures (T90 and T50) and selectivity to CO2 () with increasing calcination temperature (b)
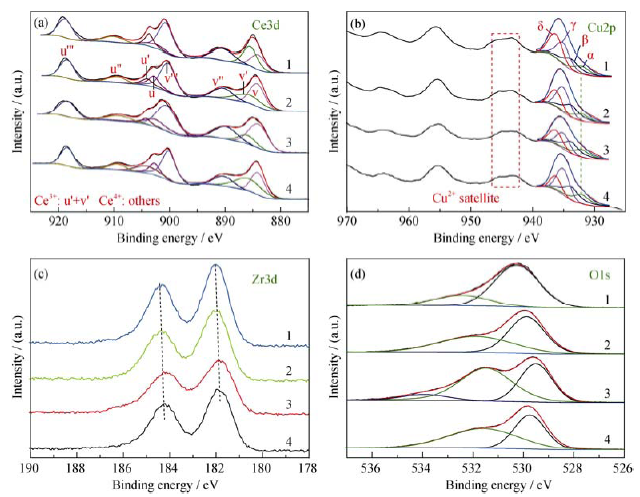
图3 CCZ-x (4: x = 300, 3: x = 350, 2: x = 500, 1: x = 800) 的XPS谱图: Ce 3d (a), Cu 2p (b), Zr 3d (c), O 1s (d)
Fig. 3 Ce 3d (a) , Cu 2p (b), Zr 3d (c), and O 1s (d) XPS patterns of CCZ-x (4: x = 300, 3: x = 350, 2: x = 500, 1: x = 800)
| Sample | Cu: Ce: Zr a | Oads/(Oads+Olatt) |
|---|---|---|
| CCZ-800 | 0.17: 0.60: 0.23 | 0.23 |
| CCZ-500 | 0.15: 0.65: 0.20 | 0.46 |
| CCZ-350 | 0.12: 0.67: 0.21 | 0.64 |
| CCZ-300 | 0.12: 0.69: 0.19 | 0.57 |
表2 不同煅烧温度产物CCZ-x(x = 800, 500, 350, 300)表面化学组分的XPS分析
Table 2 XPS surface analyses of different CCZ-x (x = 800, 500, 350, 300)
| Sample | Cu: Ce: Zr a | Oads/(Oads+Olatt) |
|---|---|---|
| CCZ-800 | 0.17: 0.60: 0.23 | 0.23 |
| CCZ-500 | 0.15: 0.65: 0.20 | 0.46 |
| CCZ-350 | 0.12: 0.67: 0.21 | 0.64 |
| CCZ-300 | 0.12: 0.69: 0.19 | 0.57 |
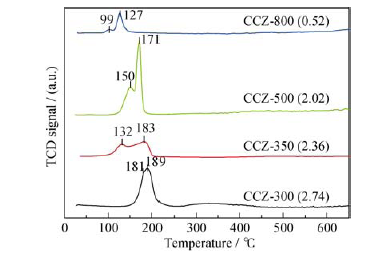
图4 不同煅烧温度产物CCZ-x (x = 300, 350, 500, 800)的H2-TPR 曲线 (括号中的数据为H2-TPR曲线的积分面积)
Fig. 4 H2-TPR profiles of the selected CCZ-x catalysts (x= 300, 350, 500, and 800) Data in the brackets indicate the integrated area of the H2-TPR curves

图5 不同煅烧温度产物CCZ-x (x = 300, 350, 500, 800)的O2-TPD曲线 (括号中的数据为O2-TPD曲线的积分面积)
Fig. 5 O2-TPD profiles of the selected CCZ-x catalysts (x= 300, 350, 500, and 800) Data in the brackets indicate the integrated area of the O2-TPD curves
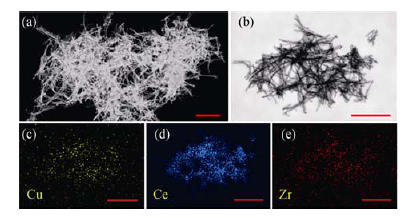
图6 CCZ-350的STEM (a, b) 图像和元素分布图(c-e)
Fig. 6 Dark-field (a) and bright-field (b) STEM images and elements mapping (c-e) (corresponding to TEM image in b) of CCZ-350 Scale bars: 100 nm
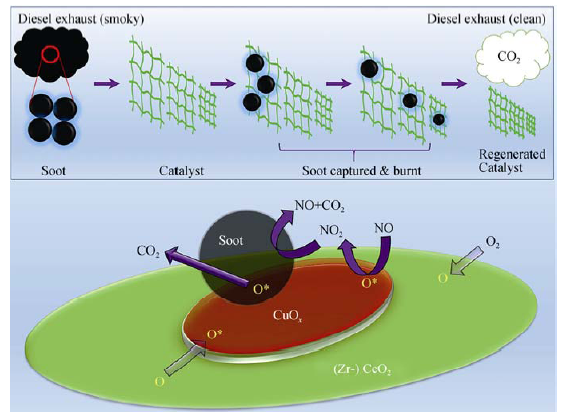
图7 CCZ-350催化碳烟燃烧过程中鸟巢结构“过滤+催化”作用示意图(a), 活性氧物种对碳烟氧化的催化作用机制(b)
Fig. 7 Schematic representation of (a) the role of the “nest structure” of CCZ-350 in soot combustion process, and (b) the soot combustion in NOx/O2 over CCZ-350 catalyst
| [1] | HORVATH H.Atmospheric light absorption—a revie.Atmospheric Environment, 1993, 27(3): 293-317. |
| [2] | VAN SETTEN B A A L, MAKKEE M, MOULIJN J A. Science and technology of catalytic diesel particulate filter.Catalysis Reviews-Science and Engineering, 2001, 43(4): 489-564. |
| [3] | YU X H, ZHAO Z, WEI Y C,et al.Synthesis of K-doped three-dimensionally ordered macroporous Mn0.5Ce0.5Oδ catalysts and their catalytic performance for soot oxidatio. Chinese Journal of Catalysis, 2015, 36(11): 1957-1967. |
| [4] | OBEID E, LIZARRAGA L, TSAMPAS M N,et al. Continuously regenerating diesel particulate filters based on ionically conducting ceramics. Journal of Catalysis, 2014, 309: 87-96. |
| [5] | UCHISAWA J O, OBUCHI A, ZHAO Z,et al. Carbon oxidation with platinum supported catalyst. Applied Catalysis B: Environmental, 1998, 18(3/4): L183-L187. |
| [6] | LIU J, ZHAO Z, WANG J Q,et al.The highly active catalysts of nanometric CeO2-supported cobalt oxides for soot combustio. Applied Catalysis B: Environmental, 2008, 84(1/2): 185-195. |
| [7] | WU X D, LIU S, LIN F,et al.Nitrate storage behavior of Ba/MnOx-CeO2 catalyst and its activity for soot oxidation with heat transfer limitation. Journal of Hazardous Materials, 2010, 181(1/2/3): 722-728. |
| [8] | XU J F, LIU J, ZHAO Z,et al.Easy synthesis of three-dimensionally ordered macroporous La1-xKxCoO3 catalysts and their high activities for the catalytic combustion of soo. Journal of Catalysis, 2011, 282(1): 1-12. |
| [9] | CAO C M, ZHANG Y X, LIU D S,et al. Gravity-driven multiple collision-enhanced catalytic soot combustion over a space-open array catalyst consisting of ultrathin ceria nanobelt. Small, 2015, 11(30): 3659-3664. |
| [10] | AMADINE O, MATTI H, ABDELOUHADI K,et al.Ceria-supported copper nanoparticles: a highly efficient and recyclable catalyst for N-arylation of indol. Journal of Molecular Catalysis A: Chemical, 2014, 395: 409-419. |
| [11] | LIU L J, YAO Z J, LIU B,et al.Correlation of structural characteristics with catalytic performance of CuO/CexZr1-xO2 catalysts for NO reduction by C. Journal of Catalysis, 2010, 275(1): 45-60. |
| [12] | JIANG D, WANG W Z, ZHANG L,et al.A strategy for improving deactivation of catalytic combustion at low temperature via synergistic photo catalysi. Applied Catalysis B: Environmental, 2015, 165: 399-407. |
| [13] | KONSOLAKIS M.The role of copper-ceria interactions in catalysis science: recent theoretical and experimental advance.Applied Catalysis B: Environmental, 2016, 198: 49-66. |
| [14] | SAINZ-VIDAL A, BALMASEDA J, LARTUNDO-ROJAS L,et al. Preparation of Cu-mordenite by ionic exchange reaction under milling: a favorable route to form the mono-(μ-oxo) dicopper active specie. Microporous and Mesoporous Materials, 2014, 185: 113-120. |
| [15] | VERHELST J, DECROUPET D, DE VOS D.Catalytic self-cleaning coatings for thermal oxidation of organic deposits on glas.Catalysis Science & Technology, 2013, 3(6): 1579-1590. |
| [16] | DUPIN J C, GONBEAU D, VINATIER P,et al. Systematic XPS studies of metal oxides, hydroxides and peroxide. Physical Chemistry Chemical Physics, 2000, 2(6): 1319-1324. |
| [17] | HUO C L, OUYANG J, YANG H M.CuO nanoparticles encapsulated inside Al-MCM-41 mesoporous material.via direct synthetic route. Scientific Reports, 2014, 4: 3682. |
| [18] | FEI Z Y, LU P, FENG X Z,et al. Geometrical effect of CuO nanostructures on catalytic benzene combustio. Catalysis Science & Technology, 2012, 2(8): 1705-1710. |
| [19] | BUENO-LOPEZ A.Diesel soot combustion ceria catalyst.Applied Catalysis B: Environmental, 2014, 146: 1-11. |
| [20] | ANEGGI E, DE LEITENBURG C, DOLCETTI G, et al. Promotional effect of rare earths and transition metals in the combustion of diesel soot over CeO2 and CeO2-ZrO2.Catalysis Today, 114(1): 40-47. |
| [21] | KASPAR J, FORNASIERO P, GRAZIANI M.Use of CeO2-based oxides in the three-way catalysi.Catalysis Today, 1999, 50(2): 285-298. |
| [22] | SETIABUDI A, MAKKEE M, MOULIJN J A.The role of NO2 and O2 in the accelerated combustion of soot in diesel exhaust gase.Applied Catalysis B: Environmental, 2004, 50(3): 185-194. |
| [1] | 顾薛苏, 殷杰, 王康龙, 崔崇, 梅辉, 陈忠明, 刘学建, 黄政仁. 颗粒级配对黏结剂喷射打印碳化硅陶瓷性能的影响[J]. 无机材料学报, 0, (): 216-. |
| [2] | 陈雨, 林埔安, 蔡冰, 张文华. 钙钛矿太阳能电池无机空穴传输材料的研究进展[J]. 无机材料学报, 0, (): 105-. |
| [3] | 田煜彬, 田超凡, 李森, 赵永鑫, 邢涛, 李智, 陈萧如, 向帅蓉, 代鹏程. 高导电性生物质碳布的制备及其燃料电池气体扩散层性能[J]. 无机材料学报, 0, (): 127-. |
| [4] | 江润璐, 吴鑫, 郭昊骋, 郑琦, 王连军, 江莞. UiO-67基导电复合材料的制备及其热电性能研究[J]. 无机材料学报, 0, (): 197-. |
| [5] | 李海燕, 旷峰华, 吴昊龙, 刘小根, 包亦望, 万德田. 残余拉应力的温度依赖性及其对裂纹扩展行为的影响[J]. 无机材料学报, 0, (): 214-. |
| [6] | 方万丽, 沈黎丽, 李海燕, 陈薪羽, 陈宗琦, 寿春晖, 赵斌, 杨松旺. NiOx介孔层的成膜过程对碳电极钙钛矿太阳能电池性能的影响[J]. 无机材料学报, 0, (): 2-. |
| [7] | 丁统顺, 丰平, 孙学文, 单沪生, 李琪, 宋健. Fmoc-FF-OH钝化钙钛矿薄膜及其太阳能电池性能研究[J]. 无机材料学报, 0, (): 50-. |
| [8] | 徐昊, 钱伟, 花银群, 叶云霞, 戴峰泽, 蔡杰. 皮秒激光加工的微织构对碳化硅润湿性的影响[J]. 无机材料学报, 0, (): 73-. |
| [9] | 邱海洋, 苗广潭, 李辉, 栾奇, 刘国侠, 单福凯. 等离子体处理对突触晶体管长程塑性的影响[J]. 无机材料学报, 2023, 38(4): 406-412. |
| [10] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [11] | 杨洋, 崔航源, 祝影, 万昌锦, 万青. 柔性神经形态晶体管研究进展[J]. 无机材料学报, 2023, 38(4): 367-377. |
| [12] | 吴俊林, 丁继扬, 黄新友, 朱丹阳, 黄东, 代正发, 杨文钦, 蒋兴奋, 周健荣, 孙志嘉, 李江. Gd2O2S:Tb闪烁陶瓷的制备与结构: 水浴合成中H2SO4/Gd2O3摩尔比的影响[J]. 无机材料学报, 2023, 38(4): 452-460. |
| [13] | 陈鑫力, 李岩, 王伟胜, 石智文, 竺立强. 明胶/羧化壳聚糖栅控氧化物神经形态晶体管[J]. 无机材料学报, 2023, 38(4): 421-428. |
| [14] | 游钧淇, 李策, 杨栋梁, 孙林锋. 氧化物双介质层忆阻器的设计及应用[J]. 无机材料学报, 2023, 38(4): 387-398. |
| [15] | 方仁瑞, 任宽, 郭泽钰, 徐晗, 张握瑜, 王菲, 张培文, 李悦, 尚大山. 基于氧化物基电解质栅控晶体管突触的关联学习[J]. 无机材料学报, 2023, 38(4): 399-405. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||