无机材料学报 ›› 2017, Vol. 32 ›› Issue (8): 792-800.DOI: 10.15541/jim20160563
陈宇方1, 李宇杰2, 郑春满2, 谢 凯2, 陈重学3
收稿日期:2016-10-10
修回日期:2017-01-04
出版日期:2017-08-10
网络出版日期:2017-07-19
基金资助:CHEN Yu-Fang1, LI Yu-Jie2, ZHENG Chun-Man2, XIE Kai2, CHEN Zhong-Xue3
Received:2016-10-10
Revised:2017-01-04
Published:2017-08-10
Online:2017-07-19
Supported by:摘要:
富锂层状氧化物正极材料(xLi2MnO3·(1-x)LiMO2(M=NiyMnzCo1-y-z….)理论容量高、价格低廉, 是新一代锂离子电池正极材料的候选之一。本文概述了该正极材料的结构, 分析了其在电化学活化过程与循环过程中结构的演变, 探讨了结构变化对正极材料电化学性能的影响规律, 并概括了目前针对该类正极材料电化学性能提升所开展的离子掺杂和表面改性的研究工作, 展望了未来富锂层状氧化物正极材料的发展方向。
中图分类号:
陈宇方, 李宇杰, 郑春满, 谢 凯, 陈重学. 富锂层状氧化物正极材料研究进展[J]. 无机材料学报, 2017, 32(8): 792-800.
CHEN Yu-Fang, LI Yu-Jie, ZHENG Chun-Man, XIE Kai, CHEN Zhong-Xue. Research Development on Lithium Rich Layered Oxide Cathode Materials[J]. Journal of Inorganic Materials, 2017, 32(8): 792-800.
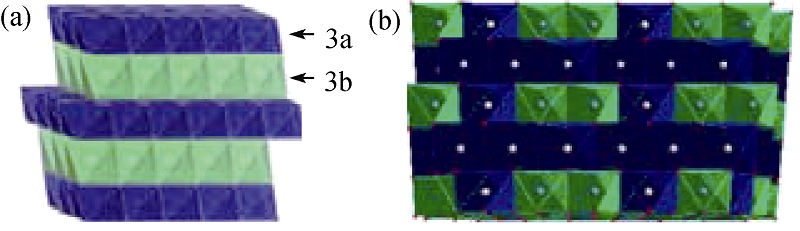
图1 层状六方晶相与单斜晶相的过渡金属离子和锂离子占位示意图((a)层状六方晶相; (b)单斜晶相)[7, 9]
Fig. 1 Scheme of crystal structure and cation occupancy of transition metal cation and lithium ions in layered rohomohedral phase and monoclinic structure (a) Layered rohomohedral phase; (b) Monoclinic phase[7, 9]

图2 Li2MnO3晶体结构的堆积位错示意图和不同位错对应的XRD图谱((a)离子堆积方式; (b)不同位错的XRD拟合图谱)[2, 11]
Fig. 2 Scheme of stacking fault and XRD patterns for various stacking fault in Li2MnO3 (a) Ionics stacking; (b) XRD simulation pattern of Li2MnO3 with various stacking fault)[2, 11]
| Surface modified materials | Initial culombical efficiency (%) | Capacity rentention(%) | |
|---|---|---|---|
| Unelectrochemical activity oxides | Al2O3[ | [87.4, (77.1)][ 96, 98(92)][ | [90, 84, 89, 84, 87(60)][ 94.9(87.5)[ |
| Electrochemical activity oxides | V2O5[ | 85(79)[ 85.1-100(79)[ | 90(50)[ 95(84.9)[ |
| Fluorides | AlF3[ | 90.8(82.8)[ | 91.6(73.4)[ |
| C Derivative | C [ | 96.7(84)[ | 92(44)[ 85.1(74.7) [ |
| Other materials | AlPO4[ Li2SiO3[ | 92.2(77.1)[ 76.4(69.6)[ | 73(65)[ |
表1 富锂层状氧化物正极材料表面包覆改性研究
Table 1 Studies on coating the lithium-rich cathode materials
| Surface modified materials | Initial culombical efficiency (%) | Capacity rentention(%) | |
|---|---|---|---|
| Unelectrochemical activity oxides | Al2O3[ | [87.4, (77.1)][ 96, 98(92)][ | [90, 84, 89, 84, 87(60)][ 94.9(87.5)[ |
| Electrochemical activity oxides | V2O5[ | 85(79)[ 85.1-100(79)[ | 90(50)[ 95(84.9)[ |
| Fluorides | AlF3[ | 90.8(82.8)[ | 91.6(73.4)[ |
| C Derivative | C [ | 96.7(84)[ | 92(44)[ 85.1(74.7) [ |
| Other materials | AlPO4[ Li2SiO3[ | 92.2(77.1)[ 76.4(69.6)[ | 73(65)[ |
| [1] | ARMAND M, TARASCON J M.Building better batteries.Nature, 2008, 451(7179): 652-657. |
| [2] | YU H, ZHOU H.High-energy cathode materials (Li2MnO3-LiMO2) for Lithium-ion batteries. The Journal of Physical Chemistry Letters, 2013, 4: 1268-1280. |
| [3] | GOODENOUGH J B.Cathode materials: a personal perspective.J. Power Sources, 2007, 174(2): 996-1000. |
| [4] | STROBEL P, LAMBERT-ANDRON B.Crystallographic and magnetic structure of Li2MnO3.J. Solid State Chem., 1988, 75(1): 90-98. |
| [5] | MASSAROTTI V, CAPSONI D, BINI M, et al.Electric and Magnetic Properties of LiMn2O4- and Li2MnO3-type oxides.J. Solid State Chem., 1997, 131(1): 94-100. |
| [6] | ARMSTRONG A R, HOLZAPFEL M, NOVAK P, et al.Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2.J. Am. Chem. Soc., 2006, 128(26): 8694-8698. |
| [7] | BROUSSELY M, PERTON F, BIENSAN P, et al.LixNiO2, a promising cathode for rechargeable lithium batteries.J. Power Sources, 1995, 54(1): 109-114. |
| [8] | BOULINEAU A, CROGUENNEC L, DELMAS C, et al.Reinvestigation of Li2MnO3 structure: electron diffraction and High resolution TEM.Chem. Mater., 2009, 21(18): 4216-4222. |
| [9] | XIAO R, LI H, CHEN L.Density functional investigation on Li2MnO3.Chem. Mater., 2012, 24(21): 4242-4251. |
| [10] | BAREÑO J, BALASUBRAMANIAN M, KANG S H, et al. Long- range and local structure in the layered oxide Li1.2Co0.4Mn0.4O2.Chem. Mater., 2011, 23(8): 2039-2050. |
| [11] | BOULINEAU A, CROGUENNEC L, DELMAS C, et al.Structure of Li2MnO3 with different degrees of defects.Solid State Ionics, 2010, 180(40): 1652-1659. |
| [12] | CHEN Y F, CHEN Z X, XIE K.Effect of annealing on the first-cycle performance and reversible capabilities of lithium-rich layered oxide cathodes.The Journal of Physical Chemistry C, 2014, 118(22): 11505-11511. |
| [13] | ZHANG QIAN, LIU WEI-WEI, FANG GUO-QING, et al.Structural and electrochemical performances of Li1+2xMn0.3+xNi0.3-3xCr0.4O2 synthesized by spray-dry method.Journal of Inorganic Materials, 2013, 28(6): 616-622. |
| [14] | NUMATA K, SAKAKI C, YAMANAKA S.Synthesis and characterization of layer structured solid solutions in the system of LiCoO2-Li2MnO3.Solid State Ionics, 1999, 117(3/4): 257-263. |
| [15] | THACKERAY M M, JOHNSON C S, VAUGHEY J T, et al.Advances in manganese-oxide 'composite' electrodes for lithium-ion batteries.J. Mater. Chem., 2005, 15(23): 2257-2267. |
| [16] | LEE E, KORITALA R, MILLER D J, et al.Aluminum and gallium substitution into 0.5Li2MnO3·0.5Li(Ni0.375Mn0.375Co0.25)O2 layered composite and the voltage fade effect.J. Electrochem. Soc., 2015, 162(3): A322-A329. |
| [17] | JEONG J H, JIN B S, KIM W S, et al.The influence of compositional change of 0.3Li2MnO3·0.7LiMn1-xNiyCo0.1O2 (0.2≤x≤0.5, y=x-0.1) cathode materials prepared by co-precipitation.J. Power Sources, 2011, 196(7): 3439-3442. |
| [18] | ZHANG L, TAKADA K, OHTA N, et al.Synthesis and electrochemistry of layered 0.6LiNi0.5Mn0.5O2·xLi2MnO3·yLiCoO2 (x+y= 0.4) cathode materials.Mater. Lett., 2004, 58(25): 3197-3200. |
| [19] | BREGER J, JIANG M, DUPRE N, et al.High-resolution X-ray diffraction, DIFFaX, NMR and first principles study of disorder in the Li2MnO3-Li[Ni1/2Mn1/2]O2 solid solution.J. Solid State Chem., 2005, 178(9): 2575-2585. |
| [20] | LU Z, BEAULIEU L Y, DONABERGER R A, et al.Synthesis, structure, and electrochemical behavior of Li [ NixLi1/3-2x/3Mn2/3-x/3]O2.J. Electrochem. Soc., 2002, 149(6): A778-A791. |
| [21] | LONG B R, CROY J R, DOGAN F, et al.Effect of cooling rates on phase separation in 0.5Li2MnO3·0.5LiCoO2 electrode materials for Li-ion batteries.Chem. Mater., 2014, 26(11): 3565-3572. |
| [22] | KIM J S, JOHNSON C S, VAUGHEY J T, et al.Electrochemical and structural properties of xLi2M‘O3·(1-x)LiMn0.5Ni0.5O2 electrodes for lithium batteries (M‘ = Ti, Mn, Zr; 0≤x≤0.3).Chem. Mater., 2004, 16(10): 1996-2006. |
| [23] | FRANCIS A S, MARKOVSKY B, SHARON D, et al.Study of the electrochemical behavior of the “inactive” Li2MnO3.Electrochim. Acta, 2012, 78: 32-39. |
| [24] | ROBERTSON A D, BRUCE P G.Mechanism of electrochemical activity in Li2MnO3.Chem. Mater., 2003, 15(10): 1984-1992. |
| [25] | KANG S H, KEMPGENS P, GREENBAUM S, et al.Interpreting the structural and electrochemical complexity of 0.5Li2MnO3·0.5LiMO2 electrodes for lithium batteries (M=Mn0.5-xNi0.5-xCo2x, 0≤x≤0.5).J. Mater. Chem., 2007, 17(20): 2069-2077. |
| [26] | WEI Z, XIA Y, QIU B, et al.Correlation between transition metal ion migration and the voltage ranges of electrochemical process for lithium-rich manganese-based material. J. Power Sources, 2015, 281: 7-10. |
| [27] | ITO A, LI D, OHSAWA Y, et al.A new approach to improve the high-voltage cyclic performance of Li-rich layered cathode material by electrochemical pre-treatment.J. Power Sources, 2008, 183(1): 344-346. |
| [28] | ITO A, LI D, SATO Y, et al.Cyclic deterioration and its improvement for Li-rich layered cathode material Li[Ni0.17Li0.2Co0.07Mn0.56]O2.J. Power Sources, 2010, 195(2): 567-573. |
| [29] | ITO A, SATO Y, SANADA T, et al.In situ X-ray absorption spectroscopic study of Li-rich layered cathode material Li[Ni0.17Li0.2Co0.07Mn0.56]O2.J. Power Sources, 2011, 196(16): 6828-6834. |
| [30] | ZHENG J, GU M, XIAO J et al. Corrosion/fragmentation of layered composite cathode and related capacity/voltage fading during cycling process.Nano Lett., 2013, 13(8): 3824-3830. |
| [31] | JOHNSON C S, LI N, LEFIEF C, et al.Anomalous capacity and cycling stability of xLi2MnO3·(1-x)LiMO2 electrodes (M=Mn, Ni, Co) in lithium batteries at 50℃.Electrochem. Commun., 2007, 9(4): 787-795. |
| [32] | LANZ P, VILLEVIEILLE C, NOVÁK P. Electrochemical activation of Li2MnO3 at elevated temperature investigated by in situ Raman microscopy.Electrochim. Acta, 2013, 109(0): 426-432. |
| [33] | JOHNSON C S, LI N, LEFIEF C, THACKERAY M M.Anomalous capacity and cycling stability of xLi2MnO3·(1-x)LiMO2 electrodes (M=Mn, Ni, Co) in lithium batteries at 50℃.Electrochem. Commun., 2007, 9(4): 787-795. |
| [34] | GREY C P, YOON W-S, REED J, et al.Electrochemical activity of li in the transition-metal sites of O3 Li [ Li(1-2x)/3Mn(2-x)/3Nix]O2. electrochem.Solid-State Lett., 2004, 7(9): A290-A293. |
| [35] | GU L, XIAO D, HU Y S, et al.Atomic-scale structure evolution in a quasi-equilibrated electrochemical process of electrode materials for rechargeable batteries.Adv. Mater., 2015, 27(13): 2134-2149. |
| [36] | CROY J R, GALLAGHER K G, BALASUBRAMANIAN M, et al.Quantifying hysteresis and voltage fade in xLi2MnO3·(1-x)LiMn0.5Ni0.5O2 electrodes as a function of Li2MnO3 content.J. Electrochem. Soc., 2014, 161(3): A318-A325. |
| [37] | CROY J R, GALLAGHER K G, BALASUBRAMANIAN M, et al.Examining hysteresis in composite xLi2MnO3·(1-x)LiMO2 cathode structures.The Journal of Physical Chemistry C, 2013, 117(13): 6525-6536. |
| [38] | DREYER W, GUKLKE C, HERRMANN M.Hysteresis and phase transition in many-particle storage systems.Continuum Mech. Thermodyn., 2011, 23(3): 211-231. |
| [39] | MOHANTY D, LI J, ABRAHAM D P, et al.Unraveling the voltage-fade mechanism in high-energy-density lithium-ion batteries: origin of the tetrahedral cations for spinel conversion.Chem. Mater., 2014, 26(21): 6272-6280. |
| [40] | DOGAN F, LONG B R, CROY J R, et al.Re-entrant lithium local environments and defect driven electrochemistry of Li-and Mn- rich Li-ion battery cathodes.J. Am. Chem. Soc., 2015, 137(6): 2328-2335. |
| [41] | LI J, CAMARDESE J, SHUNMUGASUNDARAM R, et al.Synthesis and characterization of the lithium-rich core-shell cathodes with low irreversible capacity and mitigated voltage fade. Chem. Mater., 2015, 27(9): 3366-3377. |
| [42] | MOHANTY D, LI J, ABRAHAM D P, et al.Unraveling the voltage-fade mechanism in high-energy-density lithium-ion batteries: origin of the tetrahedral cations for spinel conversion.Chem. Mater., 2014, 26(21): 6272-6280. |
| [43] | JOHNSON C S, LI N, LEFIEF C, et al.Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3·(1-x)LiMn0.333Ni0.333Co0.333O2 (0≤x≤0.7).Chem. Mater., 2008, 20(19): 6095-6106. |
| [44] | LI Y, BARENO J, BETTGE M, et al.Unexpected voltage fade in LMR-NMC oxides cycled below the “Activation” plateau.J. Electrochem. Soc., 2015, 162(1): A155-A161. |
| [45] | GALLAGHER K G, CROY J R, BALASUBRAMANIAN M, et al.Correlating hysteresis and voltage fade in lithium- and manganese-rich layered transition-metal oxide electrodes.Electrochem. Commun., 2013, 33(0): 96-98. |
| [46] | ZHANG X, MENG X, ELAM J W, et al. Electrochemical characterization of voltage fade of Li1.2Ni0.2Mn0.6O2 cathode. Solid State Ionics, 2014, 268, Part B(0): 231-235. |
| [47] | KASAI M, NISHIMURA S, GUNJI A, et al.Electrochemical study on xLi2MnO3·(1-x)LiNi1/3Co1/3Mn1/3O2 (x=0.5) layered complex cathode showing voltage hysteresis. Electrochim. Acta, 2014, 146(0): 79-88. |
| [48] | KAN Y, HU Y, LIN C-K, et al.Migration of Mn cations in delithiated lithium manganese oxides.PCCP, 2014, 16(38): 20697-20702. |
| [49] | JEONG J H, JIN B S, KIM W S, et al.The influence of compositional change of 0.3Li2MnO3·0.7LiMn1-xNiyCo0.1O2 (0.2≤x≤0.5, y=x-0.1) cathode materials prepared by co-precipitation.J. Power Sources, 2011, 196(7): 3439-3442. |
| [50] | HY S, CHENG J H, LIU J Y, et al.Understanding the role of Ni in stabilizing the lithium-rich high-capacity cathode material Li[NixLi(1-2x)/3Mn(2-x)/3]O2 (0≤x≤0.5).Chem. Mater., 2014, 26(24): 6919-6927. |
| [51] | LIU J, LIU J, WANG R, et al.Degradation and structural evolution of xLi2MnO3·(1-x)LiMn1/3Ni1/3Co1/3O2 during cycling.J. Electrochem. Soc., 2014, 161(1): A160-A167. |
| [52] | ZHENG J, XU P, GU M, et al.Structural and chemical evolution of Li- and Mn-rich layered cathode material.Chem. Mater., 2015, 27(4): 1381-1390. |
| [53] | JOHNSON C S, KIM J S, LEFIEF C, et al.The significance of the Li2MnO3 component in ‘composite’ xLi2MnO3·(1-x)LiMn0.5Ni0.5O2 electrodes.Electrochem. Commun., 2004, 6(10): 1085-1091. |
| [54] | JOHNSON C S, KIM J S, KROPF A J, et al. Structural and electrochemical evaluation of (1-x)Li2TiO3·xLiMn0.5Ni0.5O2 electrodes for lithium batteries. J. Power Sources, 2003, 119- 121(0): 139-144. |
| [55] | LIU J, CHEN H, XIE J, et al.Electrochemical performance studies of Li-rich cathode materials with different primary particle sizes.J. Power Sources, 2014, 251(0): 208-214. |
| [56] | ITO A, SHODA K, SATO Y, et al.Direct observation of the partial formation of a framework structure for Li-rich layered cathode material Li[Ni0.17Li0.2Co0.07Mn0.56]O2 upon the first charge and discharge. J. Power Sources, 2011, 196(10): 4785-4790. |
| [57] | YU S-H, YOON T, MUN J, et al.Continuous activation of Li2MnO3 component upon cycling in Li1.167Ni0.233Co0.100Mn0.467Mo0.033O2 cathode material for lithium ion batteries.Journal of Materials Chemistry A, 2013, 1(8): 2833-2839. |
| [58] | YABUUCHI N, LU Y C, MANSOUR A N, et al.The influence of heat-treatment temperature on the cation distribution of LiNi0.5Mn0.5O2 and its rate capability in lithium rechargeable batteries.J. Electrochem. Soc., 2011, 158(2): A192-A200. |
| [59] | YU C, WANG H, GUAN X, et al.Conductivity and electrochemical performance of cathode xLi2MnO3·(1-x)LiMn1/3Ni1/3Co1/3O2 (x=0.1, 0.2, 0.3, 0.4) at different temperatures.J. Alloys Compd., 2013, 546(0): 239-245. |
| [60] | CROY J R, KIM D, BALASUBRAMANIAN M, et al.Countering the Voltage decay in high capacity xLi2MnO3·(1-x)LiMO2 electrodes (M=Mn, Ni, Co) for Li+-Ion batteries.J. Electrochem. Soc., 2012, 159(6): A781-A790. |
| [61] | VU A, QIN Y, LIN C K, et al.Effect of composition on the voltage fade phenomenon in lithium-, manganese-rich xLi2MnO3·(1-x)LiNiaMnbCocO2: A combinatorial synthesis approach.J. Power Sources, 2015, 294: 711-718. |
| [62] | VERDE M G, LIU H, CARROLL K J, et al.Effect of morphology and manganese valence on the voltage fade and capacity retention of Li[Li2/12Ni3/12Mn7/12]O2.ACS Applied Materials & Interfaces, 2014, 6(21): 18868-18877. |
| [63] | YANG X, WANG D, YU R, et al.Suppressed capacity/voltage fading of high-capacity lithium-rich layered materials via the design of heterogeneous distribution in the composition.Journal of Materials Chemistry A, 2014, 2(11): 3899-3911. |
| [64] | PERALTA D, COLIN J F, BOULINEAU A, et al.Role of the composition of lithium-rich layered oxide materials on the voltage decay.J. Power Sources, 2015, 280(0): 687-694. |
| [65] | KANG S H, JOHNSON C S, VAUGHEY J T, et al.The Effects of acid treatment on the electrochemical properties of 0.5 Li2MnO3 ∙ 0.5 LiNi0.44Co0.25Mn0.31O2 electrodes in lithium cells.J. Electrochem. Soc., 2006, 153(6): A1186-A1192. |
| [66] | KANG S H, THACKERAY M M.Enhancing the rate capability of high capacity xLi2MnO3·(1-x)LiMO2 (M=Mn, Ni, Co) electrodes by Li-Ni-PO4 treatment.Electrochem. Commun., 2009, 11(4): 748-751. |
| [67] | WU Y, MANTHIRAM A.Effect of surface modifications on the layered solid solution cathodes (1-z)Li[Li1/3Mn2/3]O2-zLi[Mn0.5-yNi0.5-yCo2y]O2.Solid State Ionics, 2009, 180(1): 50-56. |
| [68] | MYUNG S T, IZUMI K, KOMABA S, et al.Functionality of oxide coating for Li[Li0.05Ni0.4Co0.15Mn0.4]O2 as positive electrode materials for lithium-ion secondary batteries.The Journal of Physical Chemistry C, 2007, 111(10): 4061-4067. |
| [69] | WANG Z, LIU E, GUO L, et al.Cycle performance improvement of Li-rich layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by ZrO2 coating.Surf. Coat. Technol., 2013, 235(0): 570-576. |
| [70] | GAO J, KIM J, MANTHIRAM A.High capacity Li[Li0.2Mn0.54Ni0.13Co0.13]O2-V2O5 composite cathodes with low irreversible capacity loss for lithium ion batteries.Electrochem. Commun., 2009, 11(1): 84-86. |
| [71] | GUO S, YU H, LIU P, et al.Surface coating of lithium-manganese-rich layered oxides with delaminated MnO2 nanosheets as cathode materials for Li-ion batteries.Journal of Materials Chemistry A, 2014, 2(12): 4422-4428. |
| [72] | SUN Y K, LEE M J, YOON C S, et al.The role of AlF3 coatings in improving electrochemical cycling of Li-enriched nickel-manganese oxide electrodes for Li-ion batteries.Adv. Mater., 2012, 24(9): 1192-1196. |
| [73] | LI ZHONG, HONG JIAN-HE, HE GANG, et al.Effect of FePO4 coating on performance of Li1.2Mn0.54Ni0.13Co0.13O2 as cathode material for Li-ion battery.Journal of Inorganic Materials, 2015, 30(2): 129-134. |
| [74] | ZHENG J M, ZHANG Z R, WU X B, et al.The effects of AlF3 coating on the performance of Li [ Li0.2Mn0.54Ni0.13Co0.13 ] O2 positive electrode material for lithium-ion battery.J. Electrochem. Soc., 2008, 155(10): A775-A782. |
| [75] | SONG B, ZHOU C, CHEN Y, et al.Role of carbon coating in improving electrochemical performance of Li-rich Li(Li0.2Mn0.54Ni0.13Co0.13)O2 cathode.RSC Advances, 2014, 4(83): 44244-44252. |
| [76] | CHEN J J, LI Z D, XIANG H F, et al.Bifunctional effects of carbon coating on high-capacity Li1.2Ni0.13Co0.13Mn0.54O2 cathode for lithium-ion batteries.J. Solid State Electrochem., 2015, 19(4): 1027-1035. |
| [77] | WU F, ZHANG X, ZHAO T, et al.Multifunctional AlPO4 coating for improving electrochemical properties of low-cost Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 cathode materials for lithium-ion batteries.ACS Applied Materials & Interfaces, 2015, 7(6): 3773-3781. |
| [78] | CHEN Y F, XIE K, ZHENG C M, et al.Enhanced Li storage performance of LiNi0.5Mn1.5O4-coated 0.4Li2MnO3·0.6LiNi1/3Co1/3Mn1/3O2 cathode materials for Li-ion batteries.ACS Applied Materials & Interfaces, 2014, 6(19): 16888-16894. |
| [79] | ZHAO J, WANG Z, GUO H, et al.Synthesis and electrochemical characterization of Zn-doped Li-rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material.Ceram. Int., 2015, 41(9, Part A): 11396-11401. |
| [80] | LI Q, LI G, FU C, et al.K+-doped Li1.2Mn0.54Co0.13Ni0.13O2: a novel cathode material with an enhanced cycling stability for lithium-ion batteries.ACS Applied Materials & Interfaces, 2014, 6(13): 10330-10341. |
| [81] | JIN X, XU Q, LIU H, et al.Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery.Electrochim. Acta, 2014, 136(0): 19-26. |
| [82] | CHEN Y F, ZHENG C M, CHEN Z X.The significance of the stable Rhombohedral structure in Li-rich cathodes for lithium-ion batteries.Ionics, 2017, 23(2): 367-375. |
| [83] | JOHNSON C S, KORTE S D, VAUGHEY J T, et al. Structural and electrochemical analysis of layered compounds from Li2MnO3. J. Power Sources, 1999, 81-82: 491-495. |
| [84] | DONG X, XU Y, XIONG L, et al.Sodium substitution for partial lithium to significantly enhance the cycling stability of Li2MnO3 cathode material.J. Power Sources, 2013, 243: 78-87. |
| [85] | XU J, LEE D H, CLEMENT R J, et al.Identifying the critical role of li substitution in P2-Nax[LiyNizMn1-y-z]O2 (0<x, y, z<1) intercalation cathode materials for high-energy Na-ion batteries.Chem. Mater., 2014, 26(2): 1260-1269. |
| [86] | YABUUCHI N, HARA R, KAJIYAMA M, et al. New O2/P2-type Li-excess layered manganese oxides as promising multi-functional electrode materials for rechargeable Li/Na batteries. Advanced Energy Materials, 2015, 4(13): 13072-1-3. |
| [87] | LEE K S, MYUNG S T, BANG H J, et al.Co-precipitation synthesis of spherical Li1.05M0.05Mn1.9O4 (M=Ni, Mg, Al) spinel and its application for lithium secondary battery cathode.Electrochim. Acta, 2007, 52(16): 5201-5206. |
| [88] | KARTHIKEYAN K, AMARESH S, LEE G W, et al.Electrochemical performance of cobalt free, Li1.2(Mn0.32Ni0.32Fe0.16)O2 cathodes for lithium batteries.Electrochim. Acta, 2012, 68: 246-253. |
| [89] | TABUCHI M, NAKASHIMA A, ADO K, et al.The effects of preparation condition and dopant on the electrochemical property for Fe-substituted Li2MnO3. J. Power Sources, 2005, 146(1/2): 287-293. |
| [90] | TABUCHI M, NAKASHIMA A, TAKEUCHI T, et al.Synthesis and electrochemical characterization of Fe and Ni substituted Li2MnO3—An effective means to use Fe for constructing “Co-free” Li2MnO3 based positive electrode material. J. Power Sources, 2011, 196(7): 3611-3622. |
| [91] | LU Z, MACNEIL D D, DAHN J R.Layered cathode materials Li[NixLi(1/3-2x/3)Mn(2/3-x/3)]O2 for lithium-ion batteries. Electrochem. Solid-State Lett., 2001, 4(11): A191-A194. |
| [92] | KALATHIL A K, ARUNKUMAR P, KIM D H, et al.Influence of Ti4+ on the electrochemical performance of Li-rich layered oxides - high power and long cycle life of Li2Ru1-xTixO3 Cathodes.ACS Applied Materials & Interfaces, 2015, 7(13): 7118-7128. |
| [93] | KNIGHT J C, NANDAKUMAR P, KAN W H, et al.Effect of Ru substitution on the first charge-discharge cycle of lithium-rich layered oxides. Journal of Materials Chemistry A, 2015, 3(5): 2006-2011. |
| [94] | LI X, XIN H, LIU Y, et al.Effect of niobium doping on the microstructure and electrochemical properties of lithium-rich layered Li[Li0.2Ni0.2Mn0.6]O2 as cathode materials for lithium ion batteries.RSC Advances, 2015, 5(56): 45351-45358. |
| [95] | LIU X, HUANG T, YU A.Fe doped Li1.2Mn0.6-x/2Ni0.2-x/2FexO2 (x≤0.1) as cathode materials for lithium-ion batteries.Electrochim. Acta, 2014, 133(0): 555-563. |
| [96] | KANG S H, AMINE K. Layered Li(Li0.2Ni0.15+0.5zCo0.10Mn0.55-0.5z)O2-zFz cathode materials for Li-ion secondary batteries.J. Power Sources, 2005, 146(1-2): 654-657. |
| [97] | ZHANG H Z, LI F, Pan, GUI L, et al.The effect of polyanion- doping on the structure and electrochemical performance of Li-rich layered oxides as cathode for lithium-ion batteries.Journal of The Electrochemical Society, 2015, 162(9): A1899-A1904. |
| [98] | ZHAO Y, LIU J T, WANG S B, et al.Surface structural transition induced by gradient polyanion-doping in Li-rich layered oxides: implications for enhanced electrochemical performance.Advanced Functional Materials, 2016, 26(26): 4760-4767. |
| [99] | LIM S N, SEO J Y, JUNG D S, et al.The crystal structure and electrochemical performance of Li1.167Mn0.548Ni0.18Co0.105O2 composite cathodes doped and co-doped with Mg and F.J. Electroanal. Chem., 2015, 740(0): 88-94. |
| [1] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| [2] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [3] | 陈强, 白书欣, 叶益聪. 热管理用高导热碳化硅陶瓷基复合材料研究进展[J]. 无机材料学报, 2023, 38(6): 634-646. |
| [4] | 林俊良, 王占杰. 铁电超晶格的研究进展[J]. 无机材料学报, 2023, 38(6): 606-618. |
| [5] | 牛嘉雪, 孙思, 柳鹏飞, 张晓东, 穆晓宇. 铜基纳米酶的特性及其生物医学应用[J]. 无机材料学报, 2023, 38(5): 489-502. |
| [6] | 苑景坤, 熊书锋, 陈张伟. 聚合物前驱体转化陶瓷增材制造技术研究趋势与挑战[J]. 无机材料学报, 2023, 38(5): 477-488. |
| [7] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [8] | 杨洋, 崔航源, 祝影, 万昌锦, 万青. 柔性神经形态晶体管研究进展[J]. 无机材料学报, 2023, 38(4): 367-377. |
| [9] | 游钧淇, 李策, 杨栋梁, 孙林锋. 氧化物双介质层忆阻器的设计及应用[J]. 无机材料学报, 2023, 38(4): 387-398. |
| [10] | 张超逸, 唐慧丽, 李宪珂, 王庆国, 罗平, 吴锋, 张晨波, 薛艳艳, 徐军, 韩建峰, 逯占文. 新型GaN与ZnO衬底ScAlMgO4晶体的研究进展[J]. 无机材料学报, 2023, 38(3): 228-242. |
| [11] | 陈昆峰, 胡乾宇, 刘锋, 薛冬峰. 多尺度晶体材料的原位表征技术与计算模拟研究进展[J]. 无机材料学报, 2023, 38(3): 256-269. |
| [12] | 齐占国, 刘磊, 王守志, 王国栋, 俞娇仙, 王忠新, 段秀兰, 徐现刚, 张雷. GaN单晶的HVPE生长与掺杂进展[J]. 无机材料学报, 2023, 38(3): 243-255. |
| [13] | 林思琪, 李艾燃, 付晨光, 李荣斌, 金敏. Zintl相Mg3X2(X=Sb, Bi)基晶体生长及热电性能研究进展[J]. 无机材料学报, 2023, 38(3): 270-279. |
| [14] | 刘岩, 张珂颖, 李天宇, 周菠, 刘学建, 黄政仁. 陶瓷材料电场辅助连接技术研究现状及发展趋势[J]. 无机材料学报, 2023, 38(2): 113-124. |
| [15] | 谢兵, 蔡金峡, 王铜铜, 刘智勇, 姜胜林, 张海波. 高储能密度聚合物基多层复合电介质的研究进展[J]. 无机材料学报, 2023, 38(2): 137-147. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||