无机材料学报 ›› 2017, Vol. 32 ›› Issue (1): 107-112.DOI: 10.15541/jim20160143
• • 上一篇
马鹏飞1, 2, 李日红1, 张 龙1
收稿日期:2016-03-14
出版日期:2017-01-20
网络出版日期:2016-12-15
作者简介:马鹏飞. Email: mapf@shanghaitech.edu.cn
MA Peng-Fei1, 2, LI Ri-Hong1, ZHANG Long1
Received:2016-03-14
Published:2017-01-20
Online:2016-12-15
About author:MA Peng-Fei (1989–), male, candidate of Master degree. Email: mapf@shanghaitech.edu.cn
Supported by:摘要:
采用溶胶-凝胶法, 在不使用模板剂的情况下制备出高比表面积的介孔CaO-Al2O3-P2O5生物活性玻璃(MBGs), 用BET、XRD、DTA以及FTIR对MBGs的结构进行了表征, 并用生物模拟体液(SBF)在36.5℃对生物玻璃进行了体外活性测试, 测试时间为1 d、3 d、7 d和14 d。介孔玻璃的比表面积最高达到461.1 m2/g, 随着CaO的含量从5mol%增加到30mol%, 介孔玻璃的比表面积呈降低趋势。用XRD和FTIR验证了材料的玻璃结构。然而, 在生物模拟体液(SBF)实验中, 当CaO摩尔含量达到20mol%时, 介孔玻璃表现出较高生物活性。这种特殊的高比表面积的介孔铝磷钙生物活性玻璃在生物医药方面有潜在的应用价值。本文的实验结果对优化生物玻璃的介孔结构和CaO含量来提升玻璃的生物活性有一定的指导意义。
中图分类号:
马鹏飞, 李日红, 张 龙. 溶胶-凝胶法制备高比表面积铝磷钙生物活性玻璃[J]. 无机材料学报, 2017, 32(1): 107-112.
MA Peng-Fei, LI Ri-Hong, ZHANG Long. Sol-Gel-derived Mesoporous Calcium Aluminum Phosphate Bioactive Glasses with High Surface Area[J]. Journal of Inorganic Materials, 2017, 32(1): 107-112.
| Glass code | CaO/mol% | AlO3/2/mol% | PO5/2/mol% | Annealing, T/℃ | Tc(℃) (glass), T/℃ |
|---|---|---|---|---|---|
| 5%CaO-MBG | 5 | 45 | 50 | 600 | 806.75 |
| 10%CaO-MBG | 10 | 40 | 50 | 600 | 738.00 |
| 15%CaO-MBG | 15 | 35 | 50 | 600 | 728.08 |
| 20%CaO-MBG | 20 | 30 | 50 | 600 | 717.66 |
| 25%CaO-MBG | 25 | 25 | 50 | 600 | 709.58 |
| 30%CaO-MBG | 30 | 20 | 50 | 600 | 701.10 |
Table 1 The phosphate coding, corresponding molar composition, annealing temperature and Tc temperature
| Glass code | CaO/mol% | AlO3/2/mol% | PO5/2/mol% | Annealing, T/℃ | Tc(℃) (glass), T/℃ |
|---|---|---|---|---|---|
| 5%CaO-MBG | 5 | 45 | 50 | 600 | 806.75 |
| 10%CaO-MBG | 10 | 40 | 50 | 600 | 738.00 |
| 15%CaO-MBG | 15 | 35 | 50 | 600 | 728.08 |
| 20%CaO-MBG | 20 | 30 | 50 | 600 | 717.66 |
| 25%CaO-MBG | 25 | 25 | 50 | 600 | 709.58 |
| 30%CaO-MBG | 30 | 20 | 50 | 600 | 701.10 |
| Glass code | Specific surface area/ (m²·g-1) | Pore volume/ (mL·g-1) | Pore size/nm |
|---|---|---|---|
| 5%CaO-MBG | 461.1 | 0.697 | 4.3 |
| 10%CaO-MBG | 332.6 | 0.412 | 3.0 |
| 15%CaO-MBG | 251.3 | 0.421 | 2.9 |
| 20%CaO-MBG | 246.2 | 0.380 | 2.7 |
| 25%CaO-MBG | 125.7 | 0.195 | 2.3 |
| 30%CaO-MBG | 25.6 | 0.066 | 1.9 |
Table 2 Specific surface area, pore volume and pore size of the series sample of different samples
| Glass code | Specific surface area/ (m²·g-1) | Pore volume/ (mL·g-1) | Pore size/nm |
|---|---|---|---|
| 5%CaO-MBG | 461.1 | 0.697 | 4.3 |
| 10%CaO-MBG | 332.6 | 0.412 | 3.0 |
| 15%CaO-MBG | 251.3 | 0.421 | 2.9 |
| 20%CaO-MBG | 246.2 | 0.380 | 2.7 |
| 25%CaO-MBG | 125.7 | 0.195 | 2.3 |
| 30%CaO-MBG | 25.6 | 0.066 | 1.9 |

Fig. 3 shows the DTA traces collected for the different compositions with the increasing calcium oxide. And the Tc temperatures are presented in Table 1. Due to the Sol-Gel methods and the thermal treatment at 600℃, the Tg temperature can’t be reflected clearly from the tracts of DTA. As seen from the Fig. 3, a decrease in Tc temperature is obtained with increasing content of calcium oxide.

Fig. 5 shows the FTIR spectra of the phosphate glass samples. Almost no change is seen in the samples with the increasing content of calcium oxide. Each FTIR spectra shows the characteristic 490 cm-1 which correspond to the flexural vibration of the O-P-O bond correspond to P=O at 1250 cm-1, which appears on the occasion of crystallization in phosphate glass, proving the amorphous form of phosphate glass. The result of FTIR spectra is in line with the conclusion of X-Ray diffraction patterns.

Fig. 6 shows the XRD patterns of the samples with the increasing content of calcium oxide before and after soaking in SBF solution. The presence of a broad band between 20° and 30° (2θ value) with no diffraction is observed, which confirms the amorphous structure of the phosphate matrix of glasses without dipping in SBF. After soaking, the major hydroxyapatite diffraction peaks [JCPDS 09-0432] (International Center for Diffraction Data, Swarthmore, PA, 2002) on the patterns at the different time periods, illustrating the change bioactive of the varying content glasses.
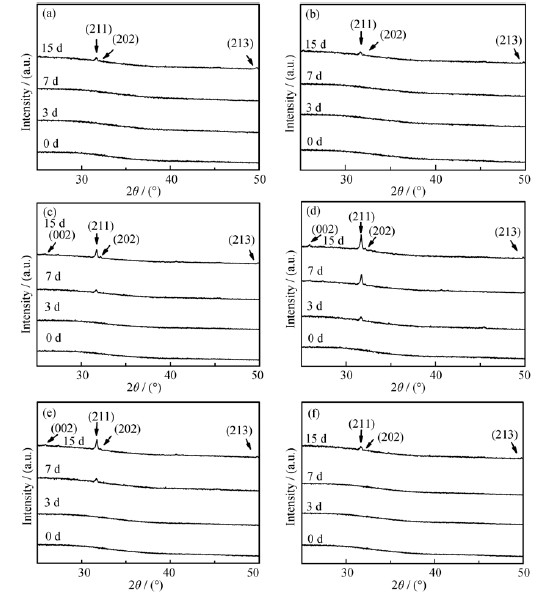
Fig. 6 XRD patterns of the samples before and after soaking in SBF solutiona) 5%CaO-MBG; b) 10%CaO-MBG; c) 15%CaO-MBG; d) 20%CaO-MBG; e) 25%CaO-MBG; f) 30%CaO-MBG
| [1] | QUINLAN E, PARTAP S, AZEVEDO M M,et al.Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair.Biomaterials, 2015, 52: 358-366. |
| [2] | LIU HUI, CHEN XIAO-FENG, LI XIAN,et al.Gene transfection of bioactive glass fibers.Journal of Inorganic Materials, 2014, 29(10): 1023-1028. |
| [3] | ZHU Y, SHANG F, LI B,et al.Magnetic mesoporous bioactive glass scaffolds: preparation, physicochemistry and biological properties.Journal of Materials Chemistry B, 2013, 1(9): 1279-1288. |
| [4] | ZHI-HONG D, ZHI-PING N, CHANG-CHUN Z.Bionic remineralization of acidic etched enamel induced by using mesoporous bioactive glass in natural oral saliva.Journal of Inorganic Materials, 2016, 31(1): 88-94. |
| [5] | AJITA J, SARAVANAN S, SELVAMURUGAN N.Effect of size of bioactive glass nanoparticles on mesenchymal stem cell proliferation for dental and orthopedic applications.Materials Science and Engineering: C, 2015, 53: 142-149. |
| [6] | LI Y, LIU Y Z, LONG T,et al.Mesoporous bioactive glass as a drug delivery system: fabrication, bactericidal properties and biocompatibility.Journal of Materials Science: Materials in Medicine, 2013, 24(8): 1951-1961. |
| [7] | HUANG S, KANG X, CHENG Z,et al.Electrospinning preparation and drug delivery properties of Eu3+/Tb3+ doped mesoporous bioactive glass nanofibers.Journal of Colloid and Interface Science, 2012, 387(1): 285-291. |
| [8] | WANG X, WEN C.Corrosion protection of mesoporous bioactive glass coating on biodegradable magnesium.Applied Surface Science, 2014, 303: 196-204. |
| [9] | AL-NOAMAN A,RAWLINSON S C F,HILL R G. The role of MgO on thermal properties, structure and bioactivity of bioactive glass coating for dental implants.Journal of Non-Crystalline Solids, 2012, 358(22): 3019-3027. |
| [10] | JONES J R.Review of bioactive glass: from Hench to hybrids.Acta Biomaterialia, 2013, 9(1): 4457-4486. |
| [11] | YANG G J, LIN M, ZHANG L,et al.Progress of calcium sulfate and inorganic composites for bone defect repair.Journal of Inorganic Materials, 2013, 28(8): 795-803. |
| [12] | SHRUTI S, SALINAS A J, MALAVASI G,et al.Structural and in vitro study of cerium, gallium and zinc containing Sol-Gel bioactive glasses.Journal of Materials Chemistry, 2012, 22(27): 13698-13706. |
| [13] | CHEN J, QUE W, XING Y,et al.Molecular level-based bioactive glass-poly (caprolactone) hybrids monoliths with porous structure for bone tissue repair.Ceramics International, 2015, 41(2): 3330-3334. |
| [14] | HENDRIKX S, KASCHOLKE C, FLATH T,et al.Indirect rapid prototyping of sol-gel hybrid glass scaffolds for bone regeneration- effects of organic crosslinker valence, content and molecular weight on mechanical properties.Acta Biomaterialia, 2016, 35: 318-329. |
| [15] | TAI H, MATHER M L, HOWARD D,et al.Control of pore size and structure of tissue engineering scaffolds produced by supercritical fluid processing.Eur. Cell Mater., 2007, 14: 64-77. |
| [16] | OKII N, NISHIMURA S, KURISU K,et al.In vivo histological changes occurring in hydroxyapatite cranial reconstruction.Neurologia Medico-Chirurgica, 2001, 41(2): 100-104. |
| [17] | EMADI R, TAVANGARIAN F, ESFAHANI S I R,et al.Nanostructured forsterite coating strengthens porous hydroxyapatite for bone tissue engineering. Journal of the American Ceramic Society, 2010, 93(9): 2679-2683. |
| [18] | FATHI M H, DOOSTMOHAMMADI A.Bioactive glass nanopowder and bioglass coating for biocompatibility improvement of metallic implant.Journal of Materials Processing Technology, 2009, 209(3): 1385-1391. |
| [19] | HENCH L L.Sol-Gel materials for bioceramic applications.Current Opinion in Solid State and Materials Science, 1997, 2(5): 604-610. |
| [20] | WANG X, LI X, ITO A,et al.Synthesis and characterization of hierarchically macroporous and mesoporous CaO-MO-SiO2-P2O5(M= Mg, Zn, Sr) bioactive glass scaffolds.Acta Biomaterialia, 2011, 7(10): 3638-3644. |
| [21] | MA Z, DONG G, LV C,et al.Core-shell glass fibers with high bioactivity and good flexibility.Materials Letters, 2012, 88: 136-139. |
| [22] | POOLOGASUNDARAMPILLAI G, WANG D, LI S,et al.Cotton-wool-like bioactive glasses for bone regeneration.Acta Biomaterialia, 2014, 10(8): 3733-3746. |
| [23] | CUI Y, LI H, YI K,et al.Moisture absorption characteristics of a SiO2 film from 2 to 3 μm.Chinese Optics Letters, 2015, 13(2): 023101. |
| [24] | KNOWLES J C, FRANKS K, ABRAHAMS I.Investigation of the solubility and ion release in the glass system K2O-Na2O-CaO-P2O5.Biomaterials, 2001, 22(23): 3091-3096. |
| [25] | KOKUBO T, TAKADAMA H.How useful is SBF in predicting in vivo bone bioactivity.Biomaterials, 2006, 27(15): 2907-2915. |
| [26] | AHMED I, LEWIS M, OLSEN I,et al.Phosphate glasses for tissue engineering: part 2. Processing and characterisation of a ternary-based P2O5-CaO-Na2O glass fibre system.Biomaterials, 2004, 25(3): 501-507. |
| [27] | FRANKS K, ABRAHAMS I, GEORGIOU G,et al.Investigation of thermal parameters and crytallisation in a ternary CaO-Na2O- P2O5-based glass system.Biomaterials, 2001, 22(5): 497-501. |
| [28] | SMITH J M, KING S P, BARNEY E R,et al.Structural study of Al2O3-Na2O-CaO-P2O5 bioactive glasses as a function of aluminium content.The Journal of Chemical Physics, 2013, 138(3): 034501. |
| [29] | EL-KHESHEN A A, KHALIAFA F A, SAAD E A, et al.Effect of Al2O3 addition on bioactivity, thermal and mechanical properties of some bioactive glasses.Ceramics International, 2008, 34(7): 1667-1673. |
| [30] | THIND K S, SINGH K, SHARMA G,et al.Influence of addition of Al2O3 on physical, structural, acoustical and in-vitro bioactive properties of phosphate glasses.Physica Status Solidi (a), 2009, 206(7): 1447-1455. |
| [1] | 吴锐, 张敏慧, 金成韵, 林健, 王德平. 光热核壳TiN@硼硅酸盐生物玻璃纳米颗粒的降解和矿化性能[J]. 无机材料学报, 2023, 38(6): 708-716. |
| [2] | 庞力斌, 王德平. 介孔硼硅酸盐玻璃微球药物载体的制备及其性能表征[J]. 无机材料学报, 2022, 37(7): 780-786. |
| [3] | 魏子钦, 夏翔, 李勤, 李国荣, 常江. 钛酸钡/硅酸钙复合生物活性压电陶瓷的制备及性能研究[J]. 无机材料学报, 2022, 37(6): 617-622. |
| [4] | 施吉翔, 翟东, 朱敏, 朱钰方. 生物活性玻璃-二氧化锰复合支架的制备与表征[J]. 无机材料学报, 2022, 37(4): 427-435. |
| [5] | 唐洁吟, 王刚, 刘聪, 邹学农, 陈晓峰. 微纳米生物活性玻璃诱导牙本质再矿化研究[J]. 无机材料学报, 2022, 37(4): 436-444. |
| [6] | 舒朝琴, 朱敏, 朱钰方. 熔盐法制备含钴氯磷灰石及其抗氧化性能和细胞相容性研究[J]. 无机材料学报, 2022, 37(11): 1225-1235. |
| [7] | 陈铖, 丁晶鑫, 王会, 王德平. 掺钕介孔硼硅酸盐生物活性玻璃陶瓷骨水泥的制备与性能表征[J]. 无机材料学报, 2022, 37(11): 1245-1258. |
| [8] | 朱子旻, 张敏慧, 张轩宇, 姚爱华, 林健, 王德平. 硼硅酸盐生物活性玻璃在直流电场下的体外矿化性能[J]. 无机材料学报, 2021, 36(9): 1006-1012. |
| [9] | 林子扬, 常宇辰, 吴章凡, 包荣, 林文庆, 王德平. 不同模拟体液对硼硅酸盐生物活性玻璃基骨水泥矿化性能的影响[J]. 无机材料学报, 2021, 36(7): 745-752. |
| [10] | 满鑫, 吴南, 张牧, 贺红亮, 孙旭东, 李晓东. Lu2O3-MgO纳米粉体合成及其复相红外透明陶瓷制备[J]. 无机材料学报, 2021, 36(12): 1263-1269. |
| [11] | 杨丛纲, 米乐, 冯爱虎, 于洋, 孙大志, 于云. KH-560改性SiO2绝缘薄膜的制备及性能研究[J]. 无机材料学报, 2021, 36(12): 1343-1348. |
| [12] | 常宇辰, 林子扬, 谢昕, 吴章凡, 姚爱华, 叶松, 林健, 王德平, 崔旭. 基于介孔硼硅酸盐生物活性玻璃微球的可注射复合骨水泥[J]. 无机材料学报, 2020, 35(12): 1398-1406. |
| [13] | 朱本必,张旺,张志坚,章建忠,IMRAN Zada,张荻. 光热增强光催化性能二氧化钛(B)/玻纤布复合研究[J]. 无机材料学报, 2019, 34(9): 961-966. |
| [14] | 张少丹, 包维维, 马海萍. Cu 2+、Tb 3+共掺杂BaZrO3高近红外反射颜料的制备及其性能研究[J]. 无机材料学报, 2019, 34(6): 599-604. |
| [15] | 吴金结, 李艳, 魏仁初, 汪建新, 屈树新, 翁杰, 智伟. 微振动应力环境影响羟基磷灰石陶瓷生物活性及力学稳定性的体外评价[J]. 无机材料学报, 2019, 34(4): 417-424. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||