无机材料学报 ›› 2017, Vol. 32 ›› Issue (1): 25-32.DOI: 10.15541/jim20160184
杨 英, 张 政, 高 菁, 林泽华, 严靖园, 郭学益
收稿日期:2016-03-28
修回日期:2016-06-02
出版日期:2017-01-20
网络出版日期:2016-12-15
作者简介:杨 英(1980–), 女, 副教授. E-mail: muyicaoyang@csu.edu.cn
基金资助:YANG Ying, ZHANG Zheng, GAO Jing, LIN Ze-Hua, YAN Jing-Yuan, GUO Xue-Yi
Received:2016-03-28
Revised:2016-06-02
Published:2017-01-20
Online:2016-12-15
About author:YANG Ying. E-mail: muyicaoyang@csu.edu.cn
Supported by:摘要:
以琼脂糖聚合物为基质, 1-甲基-2-吡咯烷酮(NMP)为溶剂, 由尿素、氯化胆碱合成的低共熔溶剂(DES)作为添加剂制备聚合物电解质用于制备准固态染料敏化太阳能电池(DSSC)。研究了尿素/氯化胆碱配比、低共熔溶剂添加量对聚合物电解质电化学性能及其DSSC光电性能的影响。研究结果表明: 随着尿素/氯化胆碱配比增加, 准固态电解质的导电性能增加, 尿素、氯化胆碱的配比为2:1时可获得最佳电导率7.24 mS/cm。对不同低共熔溶剂含量电解质进行离子电导率测试研究发现: 添加量为20wt%时, 电解质获得最佳电导率6.21 mS/cm。染料敏化太阳能电池的光电效率随低共熔溶剂含量的增加先增加后降低, 低共熔溶剂添加量为20wt%时, 获得最佳光电效率η=3.18%, 短路电流密度Jsc=10.28 mA/cm2, 开路电压Voc=0.59 V, 填充因子FF=0.51。
中图分类号:
杨 英, 张 政, 高 菁, 林泽华, 严靖园, 郭学益. 基于低共熔溶剂聚合物电解质染料敏化太阳能电池研究[J]. 无机材料学报, 2017, 32(1): 25-32.
YANG Ying, ZHANG Zheng, GAO Jing, LIN Ze-Hua, YAN Jing-Yuan, GUO Xue-Yi. Deep Eutectic Solvent Based Polymer Electrolyte for Dye-sensitized Solar Cells[J]. Journal of Inorganic Materials, 2017, 32(1): 25-32.
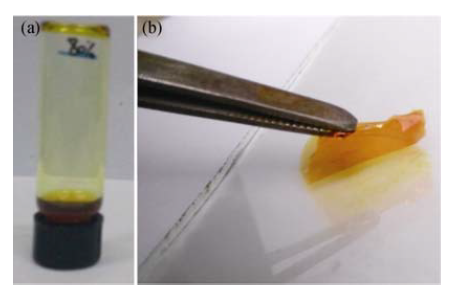
图1 (a)低共熔溶剂聚合物电解质溶液和(b)低共熔溶剂聚合物电解质烘干后实物图
Fig. 1 Pictures of (a) DES based polymer electrolyte solution and (b) DES based polymer electrolyte film after drying
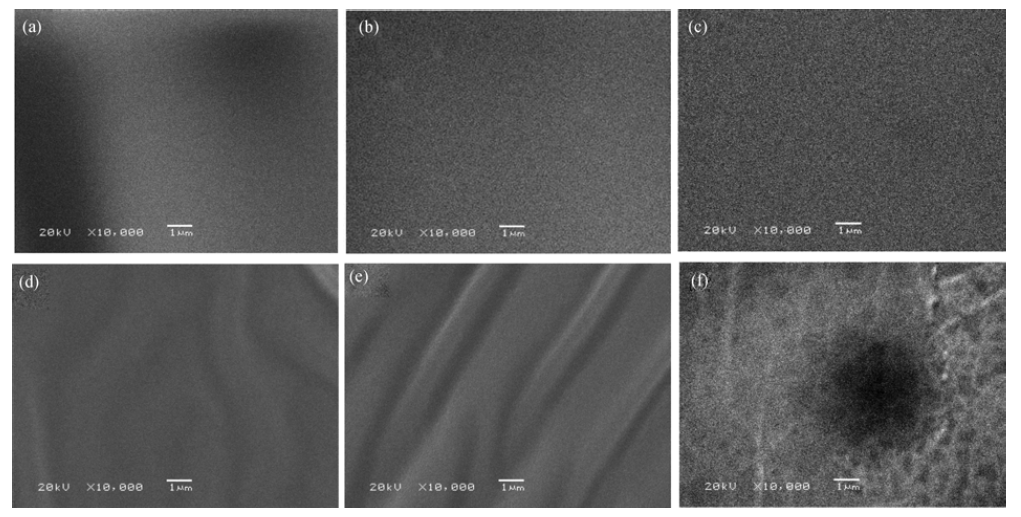
图5 不同含量低共熔溶剂改性的聚合物电解质的SEM照片
Fig. 5 SEM images of polymer electrolyte with different contents of DES(a) 0; (b) 10wt%; (c) 20wt%; (d) 40wt%; (e) 60wt%; (f) 80wt%
| ω/ wt% | Jsc/(mA·cm-2) | Voc/V | FF | η/% |
|---|---|---|---|---|
| 0 | 8.36 | 0.44 | 0.33 | 1.24 % |
| 10 | 10.32 | 0.40 | 0.33 | 1.40% |
| 20 | 10.28 | 0.59 | 0.51 | 3.18% |
| 40 | 6.04 | 0.45 | 0.50 | 1.41% |
| 60 | 3.46 | 0.47 | 0.54 | 0.91% |
| 80 | 2.40 | 0.71 | 0.54 | 0.93% |
表1 不同含量低共熔溶剂电解质电池光电性能关键参数
Table 1 Performance parameters of the DSSCs fabricated using electrolytes modified with different contents of DES
| ω/ wt% | Jsc/(mA·cm-2) | Voc/V | FF | η/% |
|---|---|---|---|---|
| 0 | 8.36 | 0.44 | 0.33 | 1.24 % |
| 10 | 10.32 | 0.40 | 0.33 | 1.40% |
| 20 | 10.28 | 0.59 | 0.51 | 3.18% |
| 40 | 6.04 | 0.45 | 0.50 | 1.41% |
| 60 | 3.46 | 0.47 | 0.54 | 0.91% |
| 80 | 2.40 | 0.71 | 0.54 | 0.93% |
| [1] | 戴松元, 刘伟庆, 闫金定. 染料敏化太阳能电池. 北京: 科学出版社, 2014: 176-178. |
| [2] | KOZYCZ L M, GAO D, SEFEROS D S.Compositional influence on the regioregularity and device parameters of a conjugated statistical copolymer.Macromolecules, 2013, 46(3): 613-621. |
| [3] | COCILOVO B, AMOOALI A, LOPEZ-SANTLAAGO A, et al. Effect of modular diffraction gratings on absorption in P3HT: PCBM layers.Applied optics, 2013, 52(5): 1025-1034. |
| [4] | NAZEERUDDIN M K, BARANOFF E, GRÄTZEL M. Dye-sensitized solar cells: a brief overview.Solar Energy, 2011, 85(6): 1172-1178. |
| [5] | MATHEW S, YELLA A, GAO P, et al. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers.Nature Chemistry ,2014, 6(3): 242-247. |
| [6] | WU J H, HAO S C, LAN Z,et al. A thermoplastic gel electrolyte for stable quasi‐solid-state dye‐sensitized solar cells. Advanced Functional Materials, 2007, 17(15): 2645-2652. |
| [7] | BISQUERT J, CAHEN D, HODES G, et al. Physical chemical principles of photovoltaic conversion with nanoparticulate, mesoporous dye-sensitized solar cells. The Journal of Physical Chemistry B, 2004, 108(24): 8106-8118. |
| [8] | KUBO W, KITAMURA T, HANABUSA K, et al. Quasi-solid-state dye-sensitized solar cells using room temperature molten salts and a low molecular weight gelator.Chemical Communications,2002(4): 374-375. |
| [9] | KUBO W, KAMBE S, NAKADE S, et al. Photocurrent-determining processes in quasi-solid-state dye-sensitized solar cells using ionic gel electrolytes.The Journal of Physical Chemistry B, 2003, 107(18): 4374-4381. |
| [10] | CAI N, ZHANG J, ZHOU D, et al. N-methyl-N-allylpyrrolidinium based ionic liquids for solvent-free dye-sensitized solar cells.The Journal of Physical Chemistry C, 2009, 113(10): 4215-4221. |
| [11] | KUANG D, WANG P, ITO S,et al. Stable mesoscopic dye-sensitized solar cells based on tetracyanoborate ionic liquid electrolyte.Journal of the American Chemical Society, 2006, 128(24): 7732-7733. |
| [12] | KUANG D, KLEIN C, ZHANG Z,et al. Stable, high‐efficiency ionic‐liquid‐based mesoscopic dye‐sensitized solar cells.Small, 2007, 3(12): 2094-2102. |
| [13] | BAI Y, CAO Y, ZHANG HANG J,et al. High-performance dye-sensitized solar cells based on solvent-free electrolytes produced from eutectic melts.Nature materials, 2008, 7(8): 626-630. |
| [14] | WANG P, WENGER B, HUMPHR-BAKER R, et al. Charge separation and efficient light energy conversion in sensitized mesoscopic solar cells based on binary ionic liquids.Journal of the American Chemical Society, 2005, 127(18): 6850-6856. |
| [15] | LI D, WANG M, WU J,et al. Application of a new cyclic guanidinium ionic liquid on dye-sensitized solar cells (DSCs).Langmuir, 2009, 25(8): 4808-4814. |
| [16] | MAZILLE F, FEI Z, KUANG D, et al. Influence of ionic liquids bearing functional groups in dye-sensitized solar cells.Inorganic chemistry, 2006, 45(4): 1585-1590. |
| [17] | ABBOTT A P, CAPPER G, DAVIES D L, et al. Novel solvent properties of choline chloride/urea mixtures.Chemical Communications, 2003, 1: 70-71. |
| [18] | ABBOTT A P, BOOTHBY D, CAPPER G, et al. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids.Journal of the American Chemical Society, 2004, 126(29): 9142-9147. |
| [19] | ABBOTT A P, CULLIS P M, GIBSONI M J,et al. Extraction of glycerol from biodiesel into a eutectic based ionic liquid.Green Chemistry, 2007, 9(8): 868-872. |
| [20] | JHONG H R,WONG D S H, WAN C C,et al. A novel deep eutectic solvent-based ionic liquid used as electrolyte for dye-sensitized solar cells.Electrochemistry Communications, 2009, 11(1): 209-211. |
| [21] | RAMESH S, SHANTI R, MORRIS E.Studies on the plasticization efficiency of deep eutectic solvent in suppressing the crystallinity of corn starch based polymer electrolytes.Carbohydrate Polymers 2012, 87(1): 701-706. |
| [22] | KEBEDE Z, LINDQUIST S E.Donor-acceptor interaction between non-aqueous solvents and I2 to generate I3- and its implication in dye sensitized solar cells. Solar Energy Materials and Solar Cells, 1999, 57(3): 259-275. |
| [23] | ABBOTT A P, HARRIS R C, RYDER K S.Application of hole theory to define ionic liquids by their transport properties.The Journal of Physical Chemistry B, 2007, 111(18): 4910-4913. |
| [24] | SUN H, LI Y, WU X, et al. Theoretical study on the structures and properties of mixtures of urea and choline chloride.Journal of Molecular Modeling, 2013, 19(6): 2433-2441. |
| [25] | ZHANG YING-YING, JI XIAO-YANG, LU XIAO-HUA.Properties and applications of choline chloride/urea and choline chloride/glycerol.SCIENTIA SINICA Chimica, 2014, 44(6): 927-941. |
| [26] | ZHANG Q, VIGIER K D O, ROYER S,et al. Deep eutectic solvents: syntheses, properties and applications.Chemical Society Reviews, 2012, 41(21): 7108-7146. |
| [27] | RAMESH S, LU S C.Effect of nanosized silica in poly (methyl methacrylate)-lithium bis (trifluoromethanesulfonyl) imide based polymer electrolytes.Journal of Power Sources, 2008, 185(2): 1439-1443. |
| [28] | ANATHA P S, HARIHARAN K.Physical and ionic transport studies on poly (ethylene oxide)-NaNO3 polymer electrolyte system.Solid State Ionics, 2005, 176(1): 155-162. |
| [29] | WINIE T, RAMESH S, AROF A K.Studies on the structure and transport properties of hexanoyl chitosan-based polymer electrolytes.Physica B: Condensed Matter, 2009, 404(21): 4308-4311. |
| [30] | GORDON C M, MCLEAN A J.Photoelectron transfer from excited-state ruthenium (II) tris (bipyridyl) to methylviologen in an ionic liquid. Chemical Communications, 2000, 15: 1395-1396. |
| [31] | SCHLICHTHŐRL G, HUANG S Y, SPRAGUE J, et al. Band edge movement and recombination kinetics in dye-sensitized nanocrystalline TiO2 solar cells: a study by intensity modulated photovoltage spectroscopy.The Journal of Physical Chemistry B, 1997, 101(41): 8141-8155. |
| [32] | FUKE N, FUKUI A, KOMIYA R,et al. New approach to low-cost dye-sensitized solar cells with back contact electrodes . Chemistry of Materials, 2008, 20(15): 4974-4979. |
| [33] | WANG M, LIN Y, ZHOU X,et al. Solidification of liquid electrolyte with imidazole polymers for quasi-solid-state dye-sensitized solar cells. Materials Chemistry and Physics, 2008, 107(1): 61-66. |
| [34] | WANG W, GUO X, YANG Y.Lithium iodide effect on the electrochemical behavior of agarose based polymer electrolyte for dye-sensitized solar cell. Electrochimica Acta,2011, 56(21): 7347-7351. |
| [35] | KERN R, SASTRAWAN R, FERBER J, et al. Modeling and interpretation of electrical impedance spectra of dye solar cells operated under open-circuit conditions.Electrochimica Acta, 2002, 47(26): 4213-4225. |
| [36] | HE J, BENKŐ G, KORODI F, et al. Modified phthalocyanines for efficient near-IR sensitization of nanostructured TiO2 electrode.Journal of the American Chemical Society, 2002, 124(17): 4922-4932. |
| [37] | CHEN ANG-RAN, ZHAO WEI, CUI HOU-LEi,et al. TiO2 nanowires infiltrated with graphene-decorated mesoporous TiO2 for enhanced dye-sensitized solar cell. Journal of Inorganic Materials, 2015, 30(8): 891-896. |
| [1] | 吕喜庆, 张环宇, 李瑞, 张梅, 郭敏. Nb2O5包覆对TiO2纳米阵列/上转换发光复合结构柔性染料敏化太阳能电池性能的影响[J]. 无机材料学报, 2019, 34(6): 590-598. |
| [2] | 杨英,潘德群,张政,陈甜,韩晓敏,张力松,郭学益. Ag2Se量子点共敏化固态染料敏化太阳能电池光电性能研究[J]. 无机材料学报, 2019, 34(2): 137-144. |
| [3] | 程厚燕, 罗俊, 黄丽群, 李家科, 杨志胜, 郭平春, 王艳香, 张琦锋. 基于ZnO纳米片多级结构的柔性染料敏化太阳能电池的制备[J]. 无机材料学报, 2018, 33(5): 507-514. |
| [4] | 冉慧丽, 黄 浩, 马梦君, 翟进生, 范佳杰. 性能增强的双层TiO2复合膜染料敏化太阳能电池[J]. 无机材料学报, 2017, 32(10): 1049-1054. |
| [5] | 尹月锋, 梁桂杰, 张 强, 潘 峥, 李望南, 李在房. 基于Pechini溶胶-凝胶法的染料敏化太阳能电池的优化[J]. 无机材料学报, 2016, 31(7): 739-744. |
| [6] | 张陈乐, 张培新, 云斯宁, 李永亮, 何挺树. 染料敏化太阳能电池过渡金属化合物对电极制备方法研究进展[J]. 无机材料学报, 2016, 31(2): 113-122. |
| [7] | 谢寿东, 王 刚, 陈慧媛, 林 红, 闫志男, 张 慧. 黑枸杞与石墨烯纳米片在染料敏化太阳能电池中的应用[J]. 无机材料学报, 2016, 31(10): 1117-1122. |
| [8] | 陈盎然,赵 伟,崔厚磊,支 键,黄富强. 基于介孔TiO2/石墨烯修饰的TiO2纳米线光阳极的染料敏化太阳能电池[J]. 无机材料学报, 2015, 30(8): 891-896. |
| [9] | 马西飞, 康 庄, 黄 晓, 张国军. 尿素法制备纳米氮化锆粉体[J]. 无机材料学报, 2015, 30(1): 77-80. |
| [10] | 陈 蔚, 刘阳桥, 罗建强, 靳喜海, 孙 静, 高 濂. 柔性染料敏化太阳能电池TiO2光阳极的制备[J]. 无机材料学报, 2014, 29(6): 561-570. |
| [11] | 唐蓓蓓, 郭明星, 姜 维, 尹淑慧, 张曼霞, 王 亮, 张艺鸣, 郑 磊, 孟悦琦. 钼酸锌-碳复合材料在染料敏化太阳能电池对电极中的应用[J]. 无机材料学报, 2014, 29(11): 1133-1138. |
| [12] | 王桂强, 王德龙, 况 帅, 禚淑萍. 染料敏化太阳能电池用过渡金属化合物对电极的研究进展[J]. 无机材料学报, 2013, 28(9): 907-915. |
| [13] | 罗华明, 刘志勇, 白传易, 鲁玉明, 蔡传兵. 基于二氧化钛纳米管的染料敏化电池光阳极研究[J]. 无机材料学报, 2013, 28(5): 521-526. |
| [14] | 孟凡宁, 王春雷, 武明星, 林 逍, 王同华, 马廷丽. 染料敏化太阳能电池无FTO煤基炭对电极的研究[J]. 无机材料学报, 2013, 28(3): 273-277. |
| [15] | 崔旭梅, 左承阳, 蓝德均, 王 军. TiO2/SnO2纳米晶膜的制备及其电学性能研究[J]. 无机材料学报, 2013, 28(11): 1233-1236. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||