无机材料学报 ›› 2016, Vol. 31 ›› Issue (11): 1198-1204.DOI: 10.15541/jim20160069
李力成1, 何甜甜1, 赵学娟2, 钱 祺1, 王 磊1, 李小保1
收稿日期:2016-01-28
修回日期:2016-03-25
出版日期:2016-11-10
网络出版日期:2016-10-25
基金资助:LI Li-Cheng1, HE Tian-Tian1, ZHAO Xue-Juan2, QIAN Qi1, WANG Lei1, LI Xiao-Bao1
Received:2016-01-28
Revised:2016-03-25
Published:2016-11-10
Online:2016-10-25
Supported by:摘要:
通过MoO3与TiO2相互支撑的方法制备了一系列多孔钼钛氧化物, 并在此基础上研究了该材料结构在随焙烧温度变化过程中的转变机制, 通过XRD、BET、FESEM、TG/DTG等表征分析, 当焙烧温度低于600℃时, MoO3呈固体状态, 通过MoO3与TiO2相互支撑可以制备出比表面积高达182 m2/g的介孔钼钛氧化物, 可负载更多分散良好的MoO3, 其加氢脱硫性能显著优于常规浸渍法制备的催化材料; 当焙烧温度高于600℃时, MoO3呈熔融状态, “自支撑效应”消失, 钼钛氧化物孔结构发生坍塌。
中图分类号:
李力成, 何甜甜, 赵学娟, 钱 祺, 王 磊, 李小保. 多孔钼钛氧化物的自支撑制备及其结构转变[J]. 无机材料学报, 2016, 31(11): 1198-1204.
LI Li-Cheng, HE Tian-Tian, ZHAO Xue-Juan, QIAN Qi, WANG Lei, LI Xiao-Bao. Self Supported Synthesis of Porous Molybdenum-titanium Oxide and the Resulting Structural Transformation[J]. Journal of Inorganic Materials, 2016, 31(11): 1198-1204.
| Samples | Crystalline phase | Crystalline particle size / nmαα | Surface area / (m2·g-1) | Pore volume / (cm3·g-1) | Average pore size / nm | MoO3 content / % |
|---|---|---|---|---|---|---|
| Titanate derivate | Amorphous | / | 234 | 0.17 | 3.2 | / |
| TiO2 | Anatase | 8.6 A | 103 | 0.20 | 5.9 | / |
| Mo-TiO2(400) | Anatase | 4.7 A | 182 | 0.14 | 3.7 | 16.1 |
| Mo-TiO2(500) | Anatase | 6.2 A | 173 | 0.21 | 4.9 | 14.0 |
| Mo-TiO2(600) | Anatase/rutile | 14.1 A, 19.3 R | 39 | 0.16 | 14.1 | 9.2 |
| Mo-TiO2(700) | Rutile | 19.0 R | 7.0 | 0.03 | / | 2.9 |
| Mo-TiO2(800) | Rutile | 21.5 R | 3.2 | <0.01 | / | 2.5 |
表1 不同钼钛氧化物的结构数据
Table 1 Structural data of various porous molybdenum-titanium oxides
| Samples | Crystalline phase | Crystalline particle size / nmαα | Surface area / (m2·g-1) | Pore volume / (cm3·g-1) | Average pore size / nm | MoO3 content / % |
|---|---|---|---|---|---|---|
| Titanate derivate | Amorphous | / | 234 | 0.17 | 3.2 | / |
| TiO2 | Anatase | 8.6 A | 103 | 0.20 | 5.9 | / |
| Mo-TiO2(400) | Anatase | 4.7 A | 182 | 0.14 | 3.7 | 16.1 |
| Mo-TiO2(500) | Anatase | 6.2 A | 173 | 0.21 | 4.9 | 14.0 |
| Mo-TiO2(600) | Anatase/rutile | 14.1 A, 19.3 R | 39 | 0.16 | 14.1 | 9.2 |
| Mo-TiO2(700) | Rutile | 19.0 R | 7.0 | 0.03 | / | 2.9 |
| Mo-TiO2(800) | Rutile | 21.5 R | 3.2 | <0.01 | / | 2.5 |
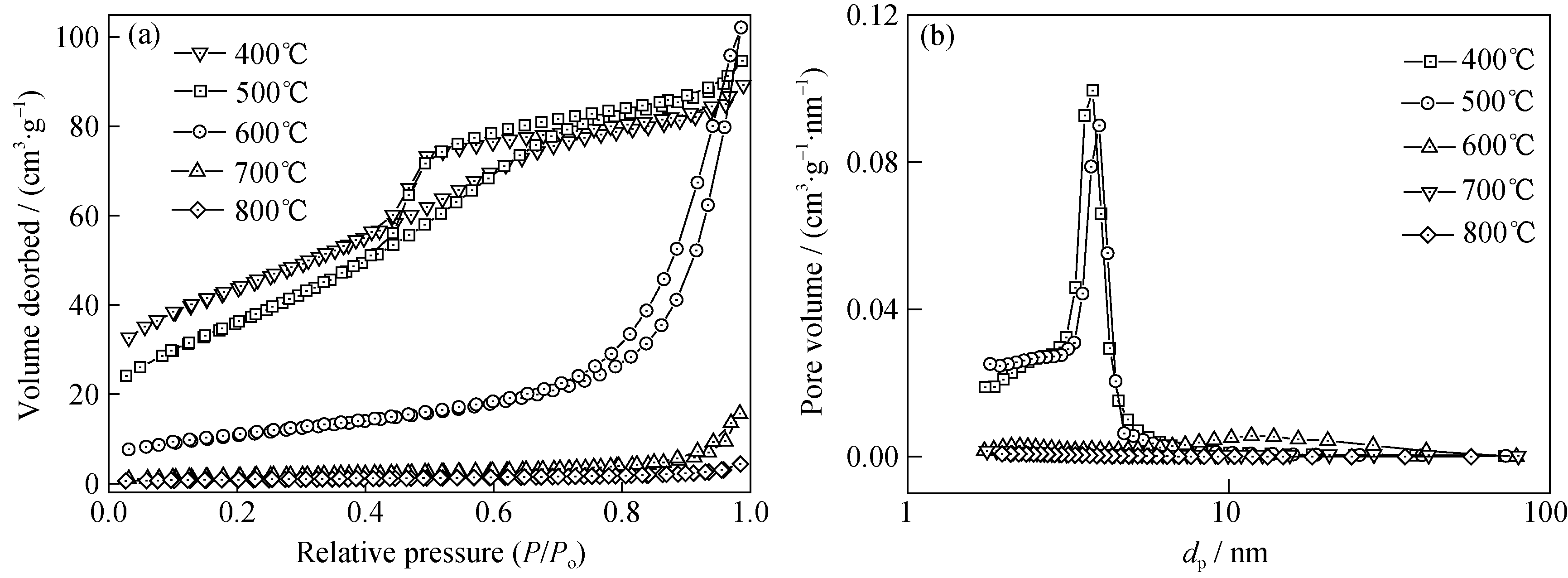
图1 不同焙烧温度制备多孔钼钛氧化物的N2吸附/脱附等温曲线(a)和孔径分布图(b)
Fig. 1 N2 adsorption-desorption isotherms (a) and pore size distributions (b) of porous Mo-TiO2 with different calcination temperatures
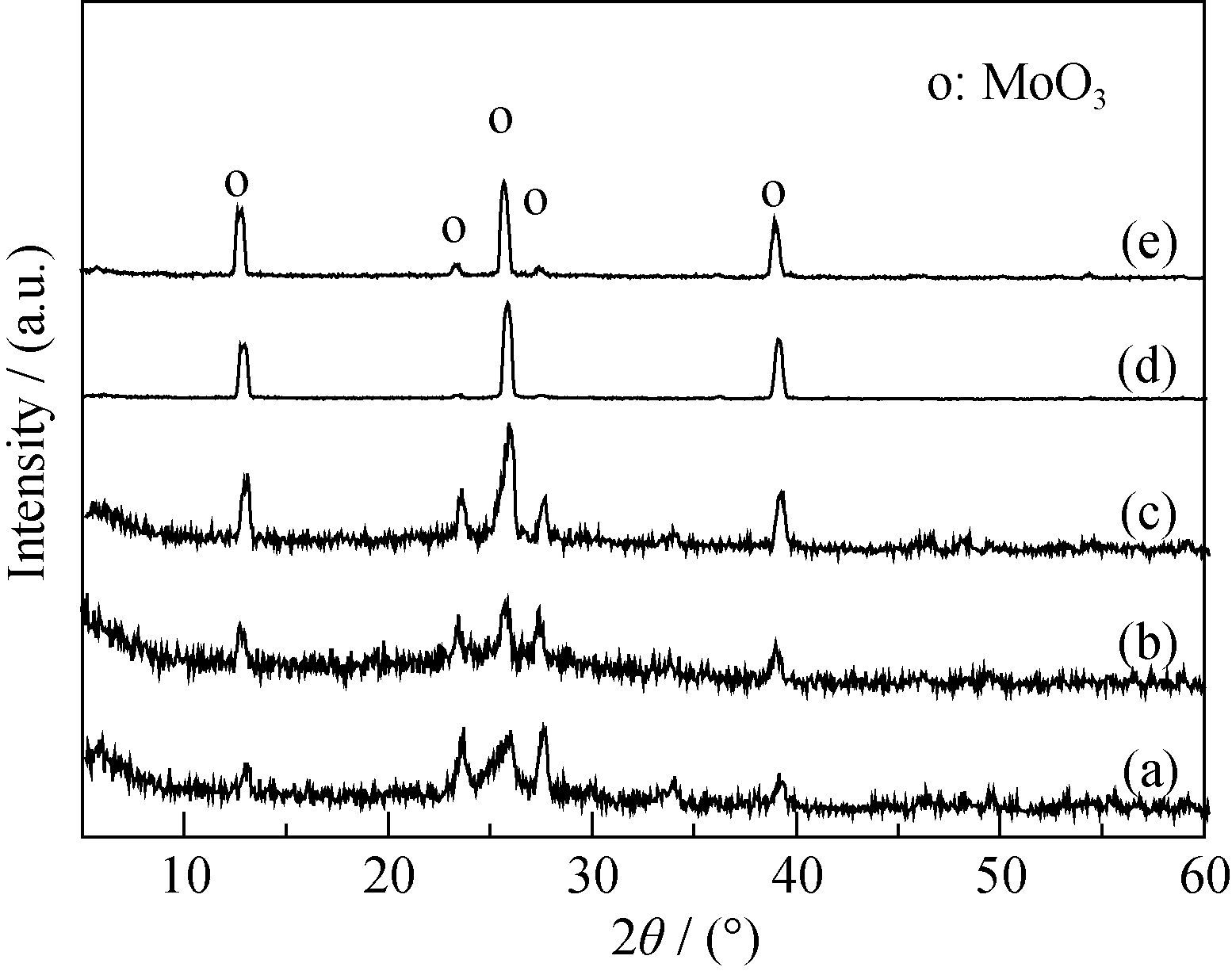
图6 不同焙烧温度制备Mo(I)-TiO2前驱体的XRD图谱
Fig. 6 XRD patterns of precursor Mo(I)-TiO2 calcined at different temperatures(a) 400℃; (b) 500℃; (c) 600℃; (d) 700℃; (e) 800℃
| [1] | RAMIREZ J, LUIS C, GUIDO B.The role of titania support in Mo-based hydrodesulfurization catalysts.Journal of Catalysis, 1999, 184(1): 59-67. |
| [2] | CHEN X B, SAMUEL S M.Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications.Chemical Reviews, 2007, 107(7): 2891-2959. |
| [3] | DAHL M, LIU Y D, YIN Y D.Composite titanium dioxide nanomaterials. Chemical Reviews, 2014, 114(19): 9853-9889. |
| [4] | ZHANG H Z, BANFIELD J F.Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2.The Journal of Physical Chemistry B, 2000, 104(15): 3481-3487. |
| [5] | HANAOR D A H, SORRELL C C. Review of the anatase to rutile phase transformation. Journal of Materials Science, 2011, 46(4): 855-874. |
| [6] | MOHAMMADI M R, FRAY D J, MOHAMMADI A.Sol-Gel nanostructured titanium dioxide: controlling the crystal structure, crystallite size, phase transformation, packing and ordering.Microporous and Mesoporous Materials, 2008, 112(1): 392-402. |
| [7] | ANTONELLI D M, JACKIE Y Y.Synthesis of hexagonally packed mesoporous TiO2 by a modified Sol-Gel method.Angewandte Chemie International Edition in English, 1995, 34(18): 2014-2017. |
| [8] | LEE D W, LEE K H.Novel eco-friendly synthesis of sucrose-templated mesoporous titania with high thermal stability.Microporous and Mesoporous Materials, 2011, 142(1): 98-103. |
| [9] | JUNG J H, HIDEKI K, KJELD J C B, et al. Creation of novel helical ribbon and double-layered nanotube TiO2 structures using an organogel template.Chemistry of Materials, 2002, 14(4): 1445-1447. |
| [10] | KASUGA T, MASAYOSHI H, AKIHIKO H, et al.Formation of titanium oxide nanotube.Langmuir, 1998, 14(12): 3160-3163. |
| [11] | CORTES J M A, ESCOBAR J, ANGELES C, et al. Highly dispersed CoMoS phase on titania nanotubes as efficient HDS catalysts.Catalysis Today, 2008, 130(1): 56-62. |
| [12] | DZWIGAJ S, LOUIS C, BREYSSE M, et al.New generation of titanium dioxide support for hydrodesulfurization.Applied Catalysis B: Environmental, 2003, 41(1): 181-191. |
| [13] | INOUE S, AKIHIRO M, HIDEHIKO K, et al.Preparation of novel titania support by applying the multi-gelation method for ultra- deep HDS of diesel oil.Applied Catalysis A: General, 2004, 269(1): 7-12. |
| [14] | HE M, LU X H, FENG X, et al. A simple approach to mesoporous fibrous titania from potassium dititanate.Chemical Communications, 2004(19): 2202-2203. |
| [15] | OKADA K, NOBUO Y, YOSHIKAZU K, et al.Effect of silica additive on the anatase-to-rutile phase transition.Journal of the American Ceramic Society, 2001, 84(7): 1591-1596. |
| [16] | YANG J, HUANG Y X, Ferreira J M F. Inhibitory effect of alumina additive on the titania phase transformation of a Sol--Gel-derived powder.Journal of Materials Science Letters, 1997, 16(23): 1933-1935. |
| [17] | ZHANG J, Li M J, FENG Z C, et al.UV Raman spectroscopic study on TiO2. I. phase transformation at the surface and in the bulk.The Journal of Physical Chemistry B, 2006, 110(2): 927-935. |
| [18] | YUAN C Z, ZHOU L, HOU L R.Facile fabrication of self-supported three-dimensional porous reduced graphene oxide film for electrochemical capacitors.Materials Letters, 2014, 124: 253-255. |
| [19] | WANG Z, Chen G, DING K.Self-supported catalysts. Chemical Reviews, 2008, 109(2): 322-359. |
| [20] | MAKWANA N M, RAUL Q C, PARKIN I P, et al.A simple and low-cost method for the preparation of self-supported TiO2-WO3 ceramic heterojunction wafers. Journal of Materials Chemistry A, 2014, 2(41): 17602-17608. |
| [21] | SUN J B, LI B, CAI K P, et al.TiO2 Photolysis device fabricated by direct ink write assembly.Journal of Inorganic Materials, 2011, 26(3): 300-304. |
| [22] | SPURR R A, HOWARD M.Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer.Analytical Chemistry, 1957, 29(5): 760-762. |
| [23] | SHENG Q R, YUAN S, ZHANG J L, et al.Synthesis of mesoporous titania with high photocatalytic activity by nanocrystalline particle assembly.Microporous and Mesoporous Materials, 2006, 87(3): 177-184. |
| [24] | LI L C, WANG Y F, SHI K Z, et al.Preparation and characterization of mesoporous MoO3/TiO2 composite with high surface area by self-Supporting and ammonia method.Catalysis Letters, 2012, 142: 480-485. |
| [25] | ZHANG J, XU Q, FENG Z C, et al.Importance of the relationship between surface phases and photocatalytic activity of TiO2.Angewandte Chemie International Edition in English, 2008, 47(9): 1766-1769. |
| [26] | LI W, BAI Y, LIU C, et al.Highly thermal stable and highly crystalline anatase TiO2 for photocatalysis.Environmental Science & Technology, 2009, 43(14): 5423-5428. |
| [27] | SONG R Q, XU A W, DENG B, et al.Novel multilamellar mesostructured molybdenum oxide nanofibers and nanobelts: synthesis and characterization.The Journal of Physical Chemistry B, 2005, 109(48): 22758-22766. |
| [28] | BADICA P.Preparation through the vapor transport and growth mechanism of the first-order hierarchical structures of MoO3 belts on sillimanite fibers.Crystal growth & design, 2007, 7(4): 794-801. |
| [29] | ONO T, KAMISUKI H, HISASHI H, et al.A comparison of oxidation activities and structures of Mo oxides highly dispersed on group IV oxide supports.Journal of Catalysis, 1989, 116(1): 303-307. |
| [30] | THORNE A, KRUTH A, TUNSTALL D, et al.Formation, structure, and stability of titanate nanotubes and their proton conductivity.The Journal of Physical Chemistry B, 2005, 109(12): 5439-5444. |
| [31] | SHAHEEN W M, SELIM M M.Thermal decompositions of pure and mixed manganese carbonate and ammonium molybdate tetrahydrate.Journal of Thermal Analysis and Calorimetry, 2000, 59(3): 961-970. |
| [32] | ZENG T, BAI Y, LI H, et al.Preparation of polyhedral copper oxide nanoparticles by molten-salt method and their catalytic performance.Journal of Inorganic Materials, 2015, 30(4): 439-442. |
| [33] | WU X, LI H F, ZHOU J L, et al.Fabrication and properties of highly pure BiFeO3 using a method of solid state reaction-molten salt synthesis with non-equilibrium Process. Journal of Inorganic Materials, 2014, 29(11): 1151-1155. |
| [1] | 周帆, 毕辉, 黄富强. 用稻壳制备亚甲基蓝高吸附容量的超高比表面积活性炭[J]. 无机材料学报, 2021, 36(8): 893-903. |
| [2] | 翟婉如,王佳惠,王茂槐,杜雪梅,魏淑贤. 金属有机框架材料中CO2/N2吸附与分离的理论研究[J]. 无机材料学报, 2020, 35(6): 697-702. |
| [3] | 杨景锋, 王齐华, 王廷梅. 氧化铝气凝胶的合成与性能[J]. 无机材料学报, 2018, 33(3): 259-265. |
| [4] | 白雪君, 刘 婵, 侯 敏, 王 彪, 曹 辉, 付俊杰. 锂离子电池硅/碳纳米管/石墨烯自支撑负极材料研究[J]. 无机材料学报, 2017, 32(7): 705-712. |
| [5] | 王浩, 李琳, 王春雷, 王倩, 梁长海, 王同华. 聚酰亚胺基高比表面活性炭及其电化学性能研究[J]. 无机材料学报, 2017, 32(11): 1181-1187. |
| [6] | 许 健, 汤哲鹏, 彭雨晴, 顾传青, Koyo Norinaga, 李爱军. 比表面积与入口气体分压对热解炭微观结构影响的数值模拟研究[J]. 无机材料学报, 2016, 31(12): 1327-1334. |
| [7] | 赵丹丹, 于彦龙, 高东子, 曹亚安. 金红石二氧化钛纳米片的性质及其光催化活性[J]. 无机材料学报, 2016, 31(1): 1-6. |
| [8] | 盛赵旻, 王 樱, 常程康, 刘晓荣, 章冬云, 刘 艳. 硫模板法制备多孔石墨纳米笼及其性能表征[J]. 无机材料学报, 2013, 28(12): 1376-1380. |
| [9] | 高兆辉, 张 浩, 曹高萍, 韩敏芳, 杨裕生. 模板法制备介孔VN纳米电极材料及其电容性能[J]. 无机材料学报, 2012, 27(12): 1261-1265. |
| [10] | 丁保宏, 王海彦, 朱雪梅, 纪晓飞, 臧树良,. SiO2-TiO2-ZrO2 复合氧化物的制备及其在加氢脱硫中的应用[J]. 无机材料学报, 2011, 26(4): 381-386. |
| [11] | 杨 涛, 祝迎春, 钱霍飞, 袁建辉, 许钫钫. 微米空心碳球串珠结构的制备与形成机理[J]. 无机材料学报, 2011, 26(2): 139-144. |
| [12] | 申梓刚,路朋献,胡 行. 利用激光刻蚀技术提高Ba0.5Sr0.5Co0.8Fe0.2O3-δ膜的透氧能力[J]. 无机材料学报, 2010, 15(2): 221-224. |
| [13] | 谢 健,张宏晔,欧阳喜辉,嵇天浩,肖志勇,孙家跃. 由层状钛酸盐纳米管制备SrTiO3纳米粒子及其表征[J]. 无机材料学报, 2008, 23(2): 262-266. |
| [14] | 朱建喜,何宏平,杨丹,郭九皋,谢先德. 柱撑蒙脱石及其热处理产物孔性研究[J]. 无机材料学报, 2004, 19(2): 324-328. |
| [15] | 张青红,高濂,孙静. 煅烧温度对二氧化钛纳米晶性能的影响[J]. 无机材料学报, 2001, 16(5): 833-838. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||