无机材料学报 ›› 2015, Vol. 30 ›› Issue (2): 122-128.DOI: 10.15541/jim20140231
马平平1, 2, 刘志坚1, 夏建华1, 卢志超1
收稿日期:2014-05-04
修回日期:2014-06-27
出版日期:2015-02-20
网络出版日期:2015-01-27
基金资助:MA Ping-Ping1, 2, LIU Zhi-Jian1, XIA Jian-Hua1, LU Zhi-Chao1
Received:2014-05-04
Revised:2014-06-27
Published:2015-02-20
Online:2015-01-27
Supported by:摘要:
以LiOH·H2O、FeC2O4·2H2O、NH4VO3和NH4H2PO4为原料, 分别以不同聚合度的聚乙二醇(PEG-200、PEG-600、PEG-1000、PEG-2000、PEG-6000)为碳源, 通过高温固相法合成0.7LiFePO4·0.3Li3V2(PO4)3/C复合正极材料(LFVP/C)。用X射线衍射、拉曼光谱和扫描电镜对材料的结构和形貌进行了表征。充放电测试表明, 在电压范围为2.0~4.3 V 时, PEG-200为碳源的LFVP/C的复合正极材料具有较高的比容量、优良的循环性能和倍率特性。10C条件下其放电容量可以保持120 mAh/g。
中图分类号:
马平平, 刘志坚, 夏建华, 卢志超. 聚乙二醇为碳源合成0.7LiFePO4ּ0.3Li3V2(PO4)3/C 复合正极材料及性能研究[J]. 无机材料学报, 2015, 30(2): 122-128.
MA Ping-Ping, LIU Zhi-Jian, XIA Jian-Hua, LU Zhi-Chao. Electrochemical Performance of 0.7LiFePO4ּ0.3Li3V2(PO4)3/C Cathode Materials Using Polyethylene Glycol as Carbon Source[J]. Journal of Inorganic Materials, 2015, 30(2): 122-128.
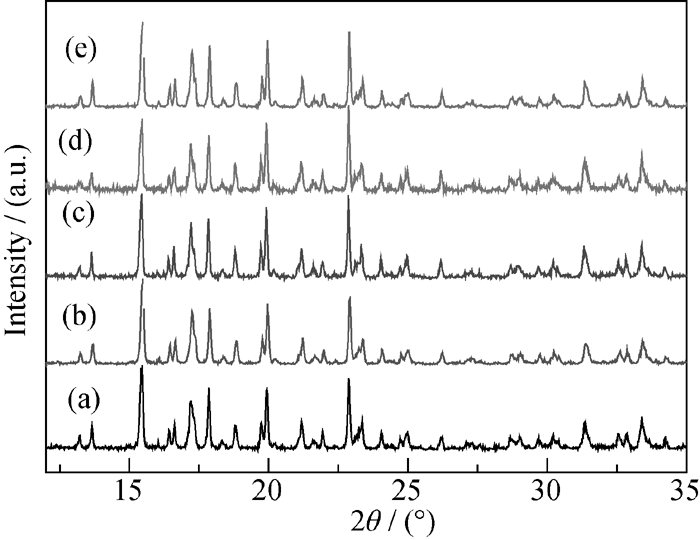
图 2 用不同碳源制备的LFVP/C样品的XRD图谱
Fig. 2 XRD patterns of LFVP/C compounds prepared with different carbon sources (a) PEG-200; (b) PEG-600; (c) PEG-1000; (d) PEG-2000; (e) PEG-6000
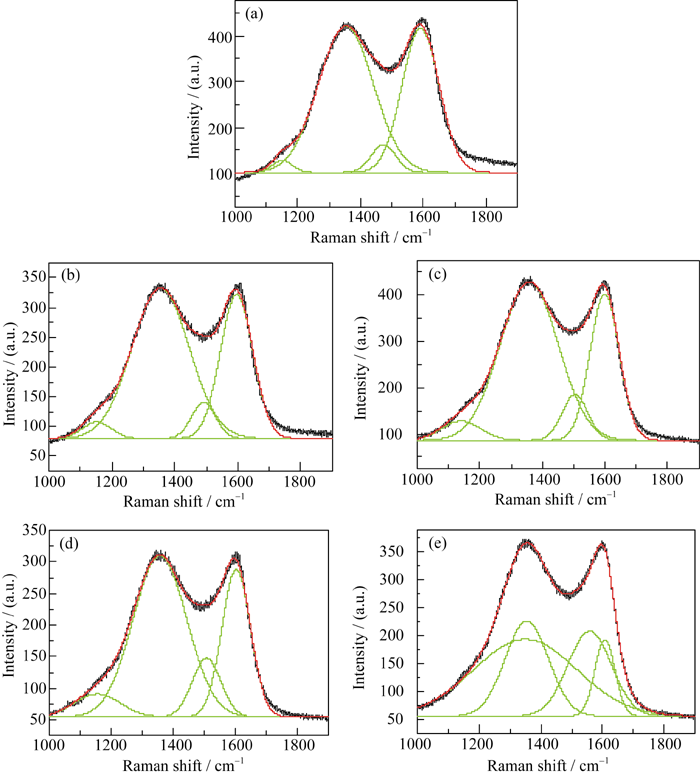
图 3 不同碳源样品的拉曼光谱和拟合结果
Fig. 3 Raman spectra and its fitted result of the products with different carbon sources (a) PEG-200; (b) PEG-600; (c) PEG-1000; (d) PEG-2000; (e) PEG-6000
| Carbon source | Wavenumber/cm-1 | Area/(a.u.) | AD/AG | Asp3/Asp2 | Carbon content | Electrical conductivity/(S?cm-1) | |
|---|---|---|---|---|---|---|---|
| PEG-200 | sp2 | 1352 | 70022 | 1.50 | 0.07 | 1.73% | 1.89×10-3 |
| 1590 | 46753 | ||||||
| sp3 | 1150 | 2035 | |||||
| 1472 | 5886 | ||||||
| PEG-600 | sp2 | 1354 | 57547 | 1.84 | 0.10 | 1.59% | 1.14×10-4 |
| 1596 | 31336 | ||||||
| sp3 | 1151 | 3165 | |||||
| 1491 | 5744 | ||||||
| PEG-1000 | sp2 | 1357 | 78754 | 2.07 | 0.14 | 1.81% | 2.22×10-5 |
| 1599 | 38080 | ||||||
| sp3 | 1139 | 6453 | |||||
| 1504 | 10158 | ||||||
| PEG-2000 | sp2 | 1358 | 56532 | 2.17 | 0.21 | 1.47% | 8.66×10-5 |
| 1604 | 26020 | ||||||
| sp3 | 1156 | 6771 | |||||
| 1508 | 10759 | ||||||
| PEG-6000 | sp2 | 1354 | 30012 | 2.68 | 2.01 | 1.68% | 1.17×10-5 |
| 1607 | 11208 | ||||||
| sp3 | 1349 | 57748 | |||||
| 1558 | 27004 | ||||||
表 1 不同碳源合成LFVP/C样品的 ID/IG ratio、碳含量和电导率结果
Table 1 Results of ID/IG ratio, carbon content and electronic conductivity of LFVP/C compounds prepared with different carbon sources
| Carbon source | Wavenumber/cm-1 | Area/(a.u.) | AD/AG | Asp3/Asp2 | Carbon content | Electrical conductivity/(S?cm-1) | |
|---|---|---|---|---|---|---|---|
| PEG-200 | sp2 | 1352 | 70022 | 1.50 | 0.07 | 1.73% | 1.89×10-3 |
| 1590 | 46753 | ||||||
| sp3 | 1150 | 2035 | |||||
| 1472 | 5886 | ||||||
| PEG-600 | sp2 | 1354 | 57547 | 1.84 | 0.10 | 1.59% | 1.14×10-4 |
| 1596 | 31336 | ||||||
| sp3 | 1151 | 3165 | |||||
| 1491 | 5744 | ||||||
| PEG-1000 | sp2 | 1357 | 78754 | 2.07 | 0.14 | 1.81% | 2.22×10-5 |
| 1599 | 38080 | ||||||
| sp3 | 1139 | 6453 | |||||
| 1504 | 10158 | ||||||
| PEG-2000 | sp2 | 1358 | 56532 | 2.17 | 0.21 | 1.47% | 8.66×10-5 |
| 1604 | 26020 | ||||||
| sp3 | 1156 | 6771 | |||||
| 1508 | 10759 | ||||||
| PEG-6000 | sp2 | 1354 | 30012 | 2.68 | 2.01 | 1.68% | 1.17×10-5 |
| 1607 | 11208 | ||||||
| sp3 | 1349 | 57748 | |||||
| 1558 | 27004 | ||||||
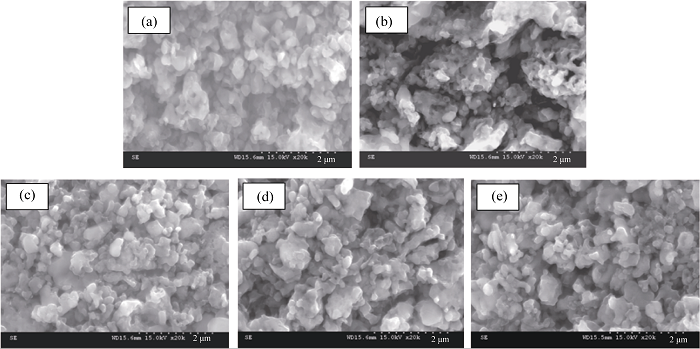
图4 不同碳源合成LFVP/C样品的扫描电镜照片
Fig. 4 SEM images of LFVP/C compounds prepared with different carbon sources (a) PEG-200; (b) PEG-600; (c) PEG-1000; (d) PEG-2000; (e) PEG-6000
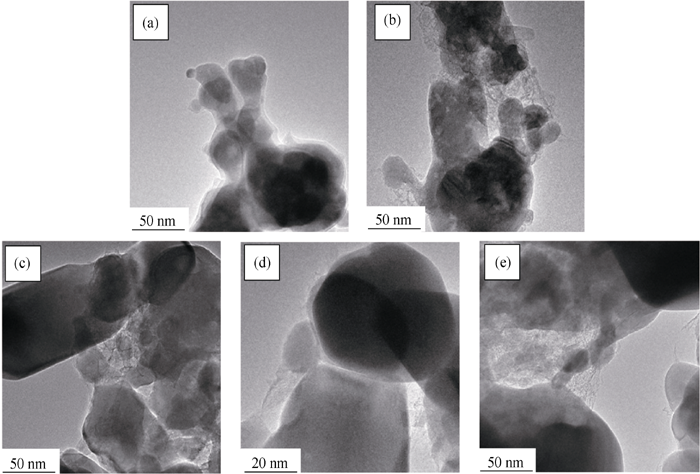
图5 不同碳源合成LFVP/C样品的透射电镜照片
Fig. 5 TEM images of LFVP/C compounds prepared with different carbon sources (a) PEG-200; (b) PEG-600; (c) PEG-1000; (d) PEG-2000; (e) PEG-6000
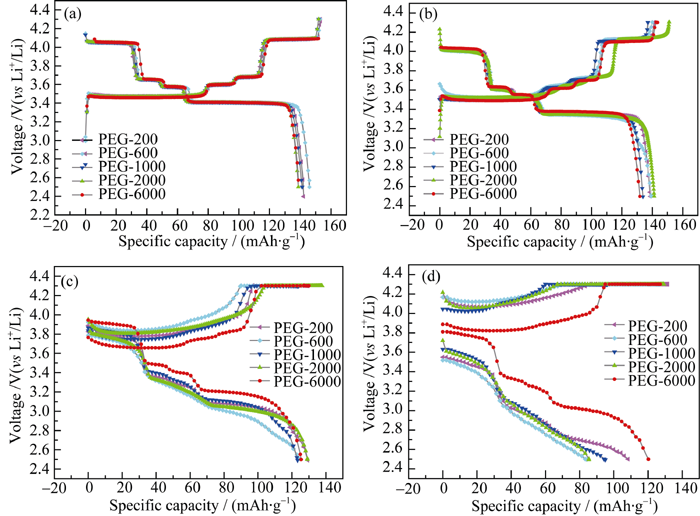
图6 不同碳源合成样品在不同倍率下的充放电曲线
Fig. 6 Charge-discharge curves of LFVP/C compounds prepared with different carbon sources at different rates (a) 2C; (b) 1C; (c) 5C; (d) 10C
| Carbon source | 0.2C | 1C | 5C | 10C |
|---|---|---|---|---|
| PEG-200 | 134 | 134 | 126 | 120 |
| PEG-600 | 142 | 138 | 130 | 112 |
| PEG-1000 | 145 | 139 | 126 | 79 |
| PEG-2000 | 142 | 141 | 132 | 95 |
| PEG-6000 | 140 | 141 | 129 | 72 |
表 2 不同碳源LFVP/C样品放电容量(mAh/g)对比
Table 2 Comparison of discharge capability (mAh/g) of LFVP/C compounds prepared with different carbon sources
| Carbon source | 0.2C | 1C | 5C | 10C |
|---|---|---|---|---|
| PEG-200 | 134 | 134 | 126 | 120 |
| PEG-600 | 142 | 138 | 130 | 112 |
| PEG-1000 | 145 | 139 | 126 | 79 |
| PEG-2000 | 142 | 141 | 132 | 95 |
| PEG-6000 | 140 | 141 | 129 | 72 |
| [1] | RUI X, DING N, LIU J, et al.Analysis of the chemical diffusion coefficient of lithium ions in Li3V2(PO4)3 cathode material.Electrochim. Acta, 2010, 55: 2384-2390. |
| [2] | ZHOU F, COCOCCIONI M, KANG K, et al.The Li intercalation potential of LiMPO4 and LiMSiO4 olivines with M = Fe, Mn, Co, Ni.Electrochem Commun., 2004, 6: 1144-1148. |
| [3] | LEE J, TEJA A S.Synthesis of LiFePO4 micro and nanoparticles in supercritical water.Materials Letters, 2006, 60: 2105-2109. |
| [4] | ZHENG J C, LI X H, WANG Z X, et al.LiFePO4 with enhanced performance synthesized by a novel synthetic route.J. Power Sources, 2008, 184: 574-577. |
| [5] | YUAN L X, WANG Z H, ZHANG W X, et al.Development and challenges of LiFePO4 cathode material for lithium-ion batteries.Energy & Environmental Science, 2011, 4: 269-284. |
| [6] | CHUNG S Y, BLOKING J T, CHIANG Y M.Electronically conductive phospho-olivines as lithium storage electrodes.Nature Materials, 2002, 1: 123-128. |
| [7] | RAVET N, CHOUINARD Y, MAGNAN J F, et al. Electroactivity of natural and synthetic triphylite. J. Power Sources, 2001, 97-98: 503-507. |
| [8] | OH S W, MYUNG S T, OH S M, et al.Double carbon coating of LiFePO4 as high rate electrode for rechargeable lithium batteries .Adv. Mater. , 2010, 22: 4842-4845. |
| [9] | ZHENG J, LI X, WANG Z, et al.Novel synthesis of LiFePO4-Li3V2(PO4)3 composite cathode material by aqueous precipitation and lithiation.J. Power Sources, 2010, 195(9): 2935-2938. |
| [10] | GUO Y, HUANG Y, JIA D, et al.Preparation and electrochemical properties of high-capacity LiFePO4-Li3V2(PO4)3/C composite for lithium-ion batteries.J. Power Sources, 2014, 246: 912-917. |
| [11] | HONG J, WANG C S, CHEN X, et al.Vanadium modified LiFePO4 cathode for Li-ion batteries.Electrochem. Solid-State Lett., 2009, 12: A33-A38. |
| [12] | XIANG J Y, TU J P, ZHANG L, et al.Improved electrochemical performances of 9LiFePO4ּLi3V2(PO4)3/C composite prepared by a simple solid-state method.J. Power Sources, 2010, 195: 8331-8335. |
| [13] | ZHANG B, ZHENG J C, YANG Z H.Structural properties of composite cathode material LiFePO4-Li3V2(PO4)3.Ionics, 2011, 17: 859-862. |
| [14] | LI X L, KANG F Y.A novel network composite cathode of LiFePO4/multiwalled carbon nanotubes with high rate capability for lithium ion batteries.Electrochemistry Communications, 2007, 9: 663-666. |
| [15] | SARAVANAN K, REDDY M V, BALAYA P, et al.Storage performance of LiFePO4 nanoplates.J. Mater. Chem., 2009, 19: 605-610. |
| [16] | BARKER J, SAÏDI M Y, SWOYER J L. A carbothermal reduction method for the preparation of electroactive materials for lithium ion applications.J. Electrochem. Soc., 2003, 150: A684-A688. |
| [17] | NIEN Y H, CAREY J R, CHEN J S.Physical and electrochemical properties of LiFePO4/C composite cathode prepared from various polymer-containing precursors.J. Power Sources, 2009, 193: 822-827. |
| [18] | DOBRYSZYCKI J, BIALLOZOR S.On some organic inhibitors of zinc corrosion in alkaline media.Corros. Sci., 2001, 43: 1309-1319. |
| [19] | HUANG C W, LI Y Y.In situ synthesis of platelet graphite nanofibers from thermal decomposition of poly(ethylene glycol).J. Phys. Chem. B, 2006, 110: 23242-23246. |
| [20] | WANG L N, ZHAN X C, ZHANG Z G, et al.A soft chemistry synthesis routine for LiFePO4-C using a novel carbon source.J. Alloys Compd. , 2008, 456: 461-465. |
| [21] | KIM D K, PARK H M, JUNG S J, et al.Effect of synthesis conditions on the properties of LiFePO4 for secondary lithium batteries.J. Power Sources, 2006, 159: 237-240. |
| [22] | WANG L N, ZHANG Z G, ZHANG K L.A simple, cheap soft synthesis routine for LiFePO4 using iron(III) raw material.J. Power Sources, 2007, 167: 200-205. |
| [23] | DOEFF M M, HU Y, MCLARNON F, et al.Effect of surface carbon structure on the electrochemical performance of LiFePO4.Electrochem. Solid-State Lett., 2003, 6: A207-A209. |
| [24] | HU Y, DOEFF M M, KOSTECKI R, et al.Electrochemical performance of Sol-Gel synthesized LiFePO4 in lithium batteries.J. Electrochem. Soc., 2004, 151: A1279-A1285. |
| [25] | KOSTECKI R, SCHNYDER B, ALLIATA D, et al.Surface studies of carbon films from pyrolyzed photoresist.Thin Solid Films, 2001, 396: 36-43. |
| [26] | SALAH A A, MAUGER A, ZAGHIB K, et al.Reduction Fe3+ of impurities in LiFePO4 from pyrolysis of organic precursor used for carbon deposition.J. Electrochem. Soc., 2006, 153: A1692-A1701. |
| [1] | 孔国强, 冷明哲, 周战荣, 夏池, 沈晓芳. Sb掺杂O3型Na0.9Ni0.5Mn0.3Ti0.2O2钠离子电池正极材料[J]. 无机材料学报, 2023, 38(6): 656-662. |
| [2] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [3] | 李涛, 曹鹏飞, 胡力涛, 夏勇, 陈一, 刘跃军, 孙翱魁. NH4+扩层MoS2的制备及其储锌性能研究[J]. 无机材料学报, 2023, 38(1): 79-86. |
| [4] | 朱河圳, 王选朋, 韩康, 杨晨, 万睿哲, 吴黎明, 麦立强. 超高镍LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2正极材料的储锂稳定性的提升机制[J]. 无机材料学报, 2022, 37(9): 1030-1036. |
| [5] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [6] | 宿拿拿, 韩静茹, 郭印毫, 王晨宇, 石文华, 吴亮, 胡执一, 刘婧, 李昱, 苏宝连. 基于ZIF-8的三维网络硅碳复合材料锂离子电池性能研究[J]. 无机材料学报, 2022, 37(9): 1016-1022. |
| [7] | 王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029. |
| [8] | 陈莹, 栾伟玲, 陈浩峰, 朱轩辰. 基于应力场的锂离子电池正极多尺度失效研究[J]. 无机材料学报, 2022, 37(8): 918-924. |
| [9] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [10] | 苏东良, 崔锦, 翟朋博, 郭向欣. 石榴石型Li6.4La3Zr1.4Ta0.6O12对Si/C负极表面固体电解质中间相的调控机制研究[J]. 无机材料学报, 2022, 37(7): 802-808. |
| [11] | 肖美霞, 李苗苗, 宋二红, 宋海洋, 李钊, 毕佳颖. 表面端基卤化Ti3C2 MXene应用于锂离子电池高容量电极材料的研究[J]. 无机材料学报, 2022, 37(6): 660-668. |
| [12] | 王禹桐, 张非凡, 许乃才, 王春霞, 崔立山, 黄国勇. 水系锂离子电池负极材料LiTi2(PO4)3的研究进展[J]. 无机材料学报, 2022, 37(5): 481-492. |
| [13] | 李文博, 黄民松, 李月明, 李驰麟. 双盐镁电池CoS2正极材料的电化学性能研究[J]. 无机材料学报, 2022, 37(2): 173-181. |
| [14] | 李昆儒, 胡省辉, 张正富, 郭玉忠, 黄瑞安. 源于溪木贼的高性能锂离子电池三维多孔生物质硅/碳复合负极材料[J]. 无机材料学报, 2021, 36(9): 929-935. |
| [15] | 王影, 张文龙, 邢彦锋, 曹苏群, 戴新义, 李晶泽. 非晶态磷酸锂包覆钛酸锂电极在0.01~3.00 V电压范围的性能研究[J]. 无机材料学报, 2021, 36(9): 999-1005. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||