无机材料学报 ›› 2014, Vol. 29 ›› Issue (10): 1009-1017.DOI: 10.15541/jim20140010
• • 下一篇
李 波, 徐文峰, 廖晓玲
收稿日期:2014-01-06
修回日期:2014-02-27
出版日期:2014-10-20
网络出版日期:2014-09-22
作者简介:李 波(1980–), 男, 博士, 副教授. E-mail: leewave@126.com
基金资助:LI Bo, XU Wen-Feng, LIAO Xiao-Ling
Received:2014-01-06
Revised:2014-02-27
Published:2014-10-20
Online:2014-09-22
About author:LI Bo. E-mail: leewave@126.com
Supported by:摘要:
磷酸钙微球具有良好的渗透性、高的比表面积、低致密度和较好的力学性能,在分离、催化、传感、组织工程和药物释放等方面均有应用。本文综述了近年来磷酸钙陶瓷微球在组织工程和药物释放等骨修复相关领域的研究进展, 介绍了实心、多孔、空心和花瓣状等四种不同结构磷酸钙陶瓷微球制备方法以及在骨修复领域中的应用, 并归纳总结了各类微球具有的优缺点和改进的方向, 为骨修复用磷酸钙微球的设计和制备提供较系统的参考。
中图分类号:
李 波, 徐文峰, 廖晓玲. 磷酸钙微球骨修复材料研究进展[J]. 无机材料学报, 2014, 29(10): 1009-1017.
LI Bo, XU Wen-Feng, LIAO Xiao-Ling. Research Progress in Calcium Phosphate Microspheres for Bone Defect Repair[J]. Journal of Inorganic Materials, 2014, 29(10): 1009-1017.
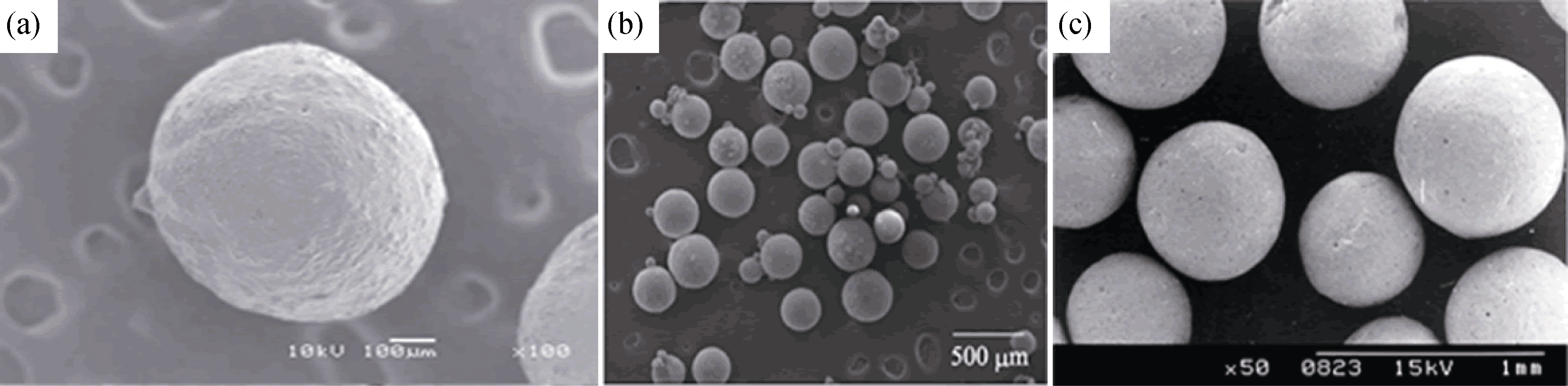
图1 几种典型的CaP陶瓷实心微球[10, 12-13]
Fig. 1 Images of typical calcium phosphate solid microspheres (a) β-TCP microsphere prepared with nitrogen liquid freeze drying method[10]; (b) HA microsphere prepared with CMCS and gel as binder[12]; (c) HA microsphere prepared with emulsion method[13]
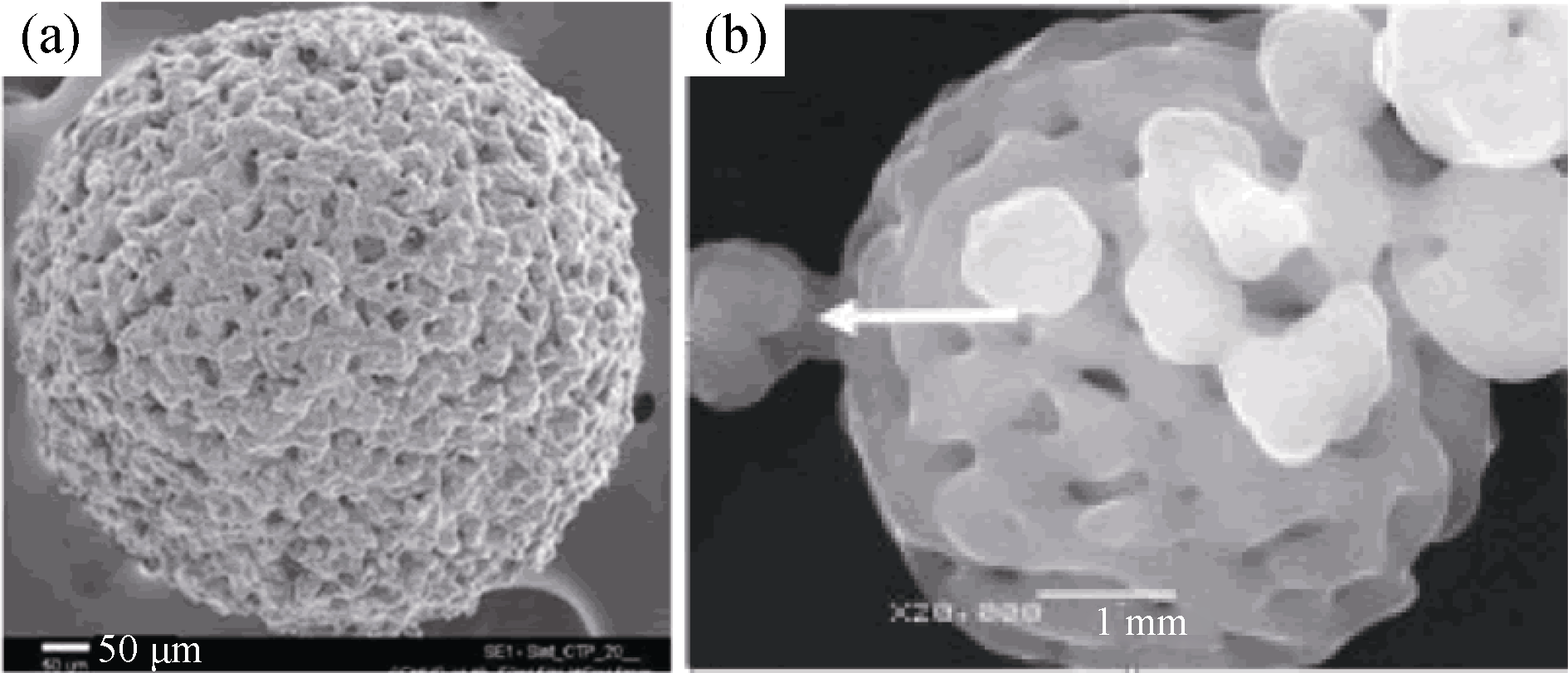
图2 两种典型的多孔CaP微球[18, 23]
Fig. 2 Two typical porous CaP microspheres[18, 23] (a) HA microsphere prepared with alginate salt gel process[18], (b) HA microsphere prepared with spray drying method[23]
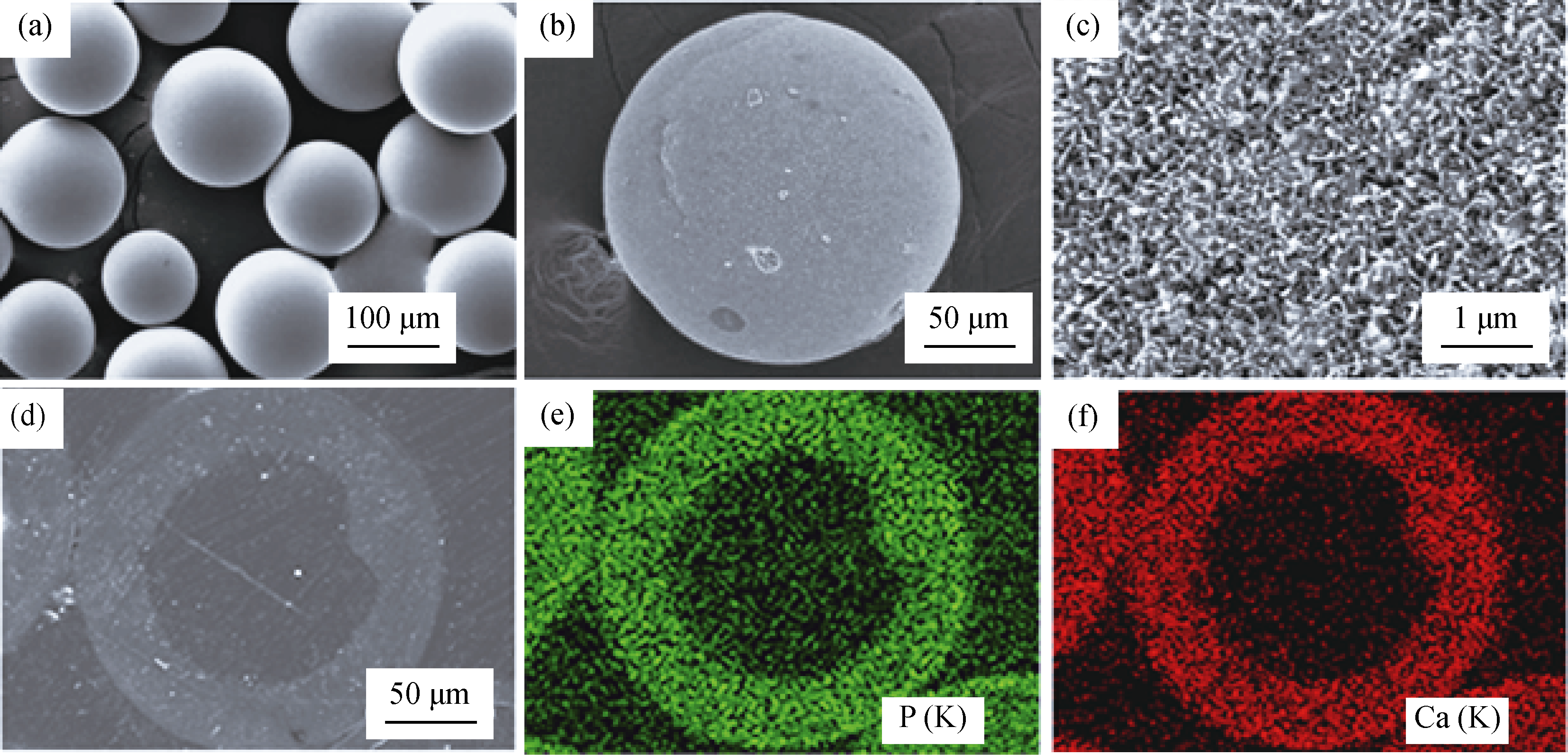
图4 以玻璃微球为硬模板制备的空心CaP陶瓷时, 作为模板用玻璃微球形貌(a), 空心HA微球外表面形貌(b),空心HA表面放大形貌(c), HA空心微球抛光纵切面背散射SEM观察到的形貌(d), X射线能谱显示图片(d)的含P(e)和Ca(f)元素分布 [27]
Fig. 4 SEM images of hollow HA microsphere with glass as hard template. (a) starting glass microspheres as hard template, (b) external surface of hollow HA microsphere, (c) external surface of hollow HA microsphere at high magnification. (d) SEM image in back-scattered mode of a polished cross section of a hollow hydroxyapatite microsphere, (e) and (f) X-ray maps of Ca(K) and P(K) across the planar section shown in (d)[27]
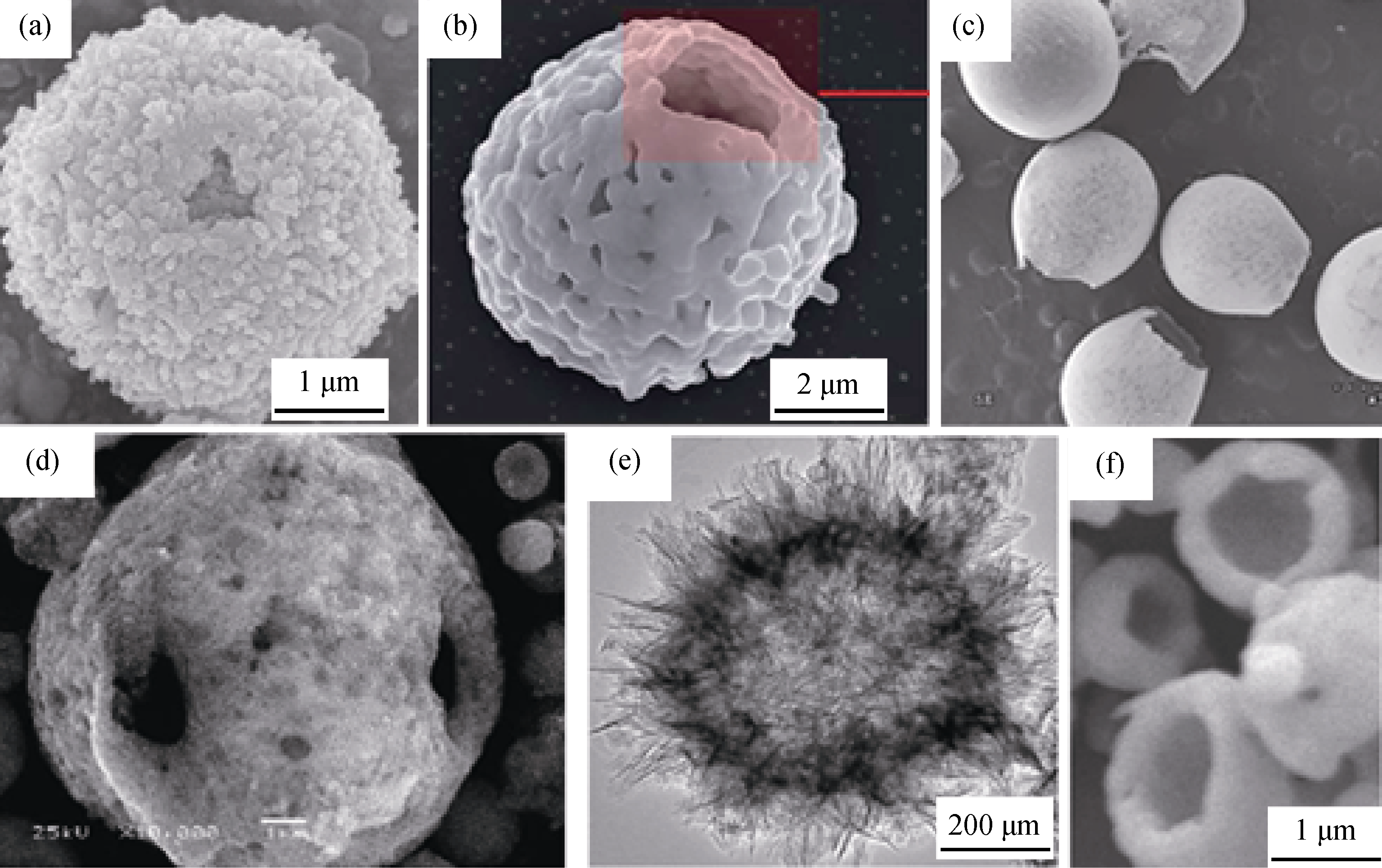
图5 分别以(a) CaCO3/Fe3O4硬模板法[32]、(b)酵母菌生物模板法[33]、(c) DCM乳液法[34]、(d) 喷雾干燥法[35]、(e) 微波水热法[37]、和(f) 电喷涂法[38]制备的几种典型的空心CaP陶瓷微球
Fig. 5 Six typical hollow calcium phosphate microspheres prepared with (a) CaCO3/Fe3O4 as hard template[32], (b) yeast as bio- template[33], (c) DCM emulsion method[34], (d) spray drying method[35], (e) microwave-hydrothermal method[37], and (f) electrosprayed method[38], respectively
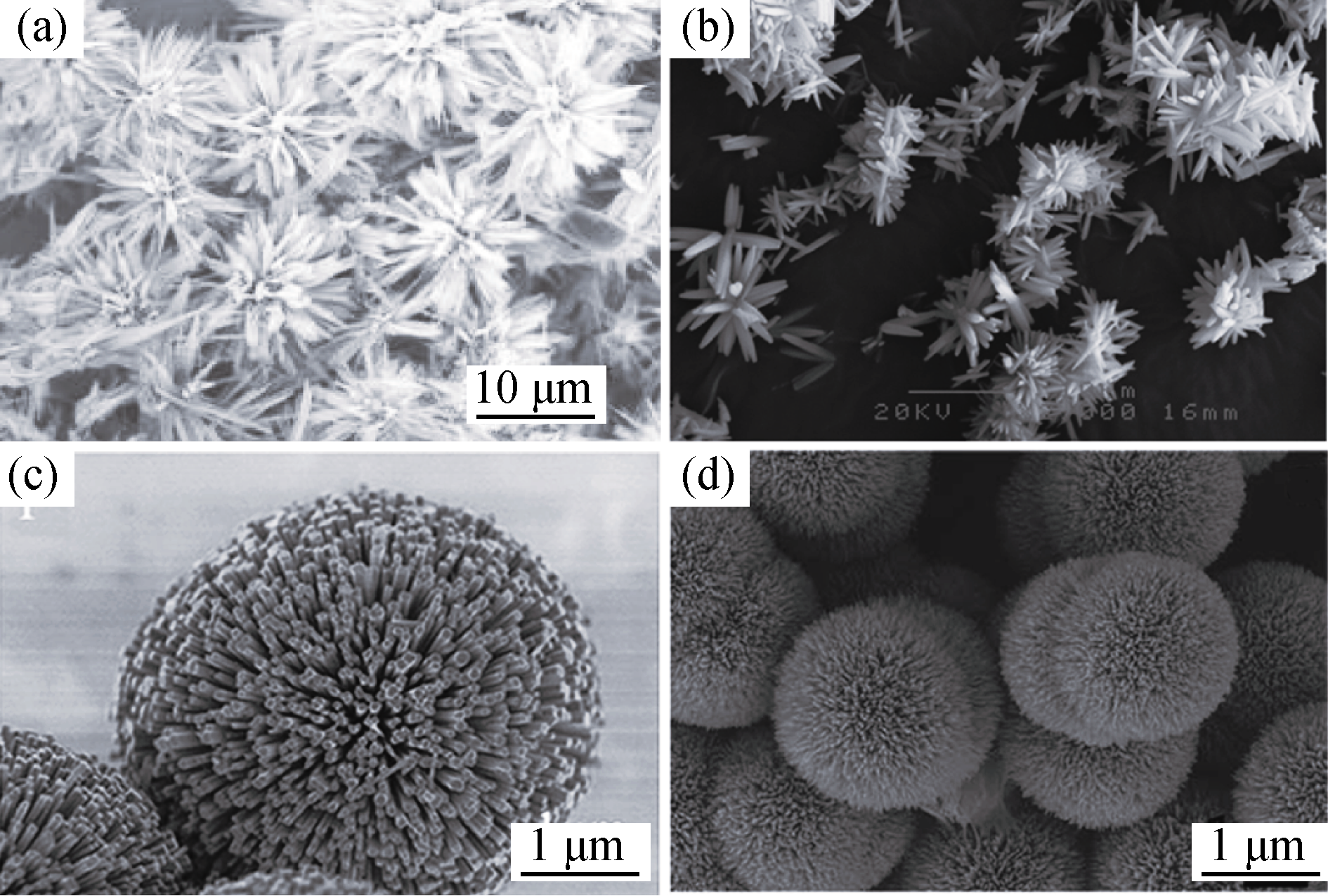
图6 4种典型的花瓣状CaP陶瓷微球SEM照片, 分别是以 (a) 酒石酸氢钾[39]、(b) EDTA[40]、(c) 氟取代结合EDTA [41]和(d) 柠檬酸[42]等为模板制备而成
Fig. 6 Four typical flower-like microspheres prepared with potassium hydrogen tartrate (a)[39], EDTA[40] (b), F- substitution combined with EDTA (c) and citric acid[41] (d) as template, respectively[42]

图8 HA微球与管状多孔HA支架及多孔圆片构建骨修复体(a)和腹膜植入(b)[45], 肌内植入3个月(c)后甲苯胺蓝染色组织切片[46]
Fig. 8 Images of (a) the HA spherules, porous HA tubes, and HA disks fabricated to assemble the novel scaffolds, (b) digital photo shows the implantation of the porous scaffolds at peritoneum pocket[45], (c) photographs of toluidine blue stained spherulite HA-positive assemble scaffold after intramuscular implantation for 3 months[46]. NB represents new bone
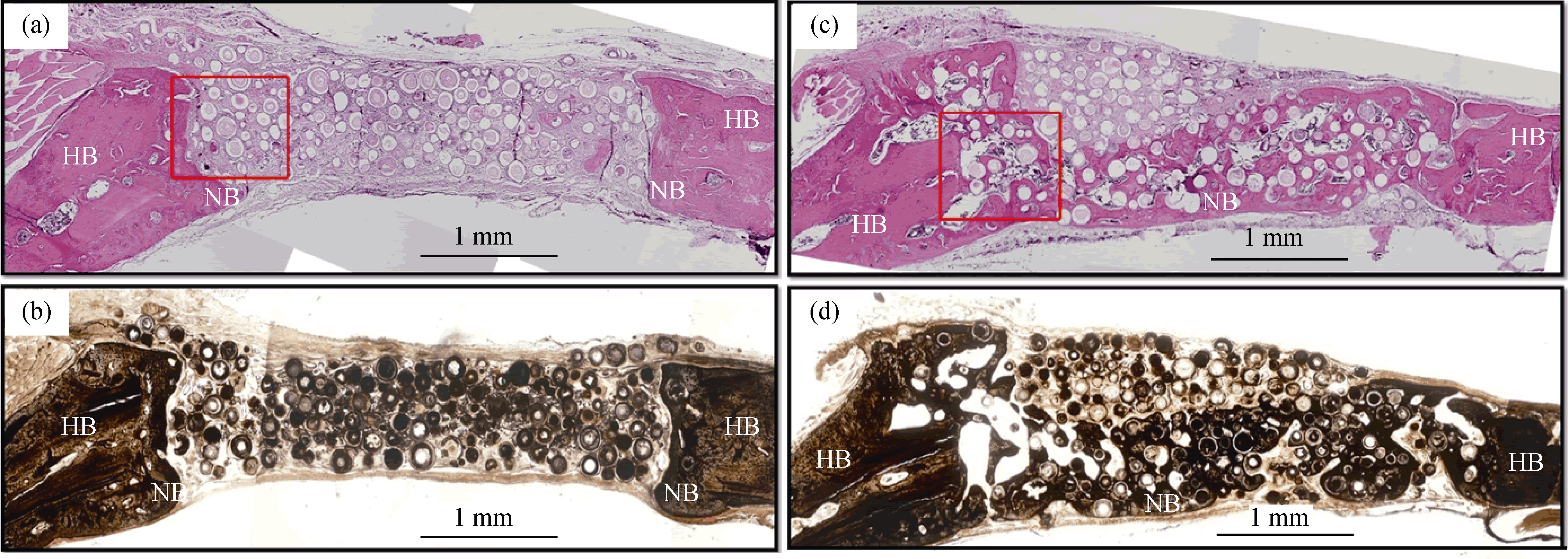
图9 空心CaP陶瓷微球修复大鼠颅骨6w后HE染色(a, c)和von Koss染色(b, d)组织切片, 其中(a, b)为空白对照微球, (c, d)为载1 μg BMP2微球[56]
Fig. 9 (a, c) H&E and (b, d) von Kossa stained sections of rat calvarial defects implanted for 6 weeks with (a, b) as-prepared hollow HA microspheres (without BMP2) and (c, d) hollow HA microspheres loaded with BMP2 (1 μg per defect). HB represents host (old) bone; NB represents new bone[56]
| [1] | WANG H, LEEUWENBURGH S C G, LI Y, et al. The use of micro- and nano-spheres as functional components for bone tissue regeneration. Tissue. Eng. Part B Rev., 2012, 18(1): 24-39. |
| [2] | LIU X, JIN X, MA P X. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat. Mater., 2011, 10(5): 398-406. |
| [3] | FISHER M B, MAUCK R L. Tissue engineering and regenerative medicine: recent innovations and the transition to translation. Tissue Engineering Part B-Reviews, 2013, 19(1): 1-13. |
| [4] | HONG Y, FAN H, LI B, et al. Fabrication, biological effects, and medical applications of calcium phosphate nanoceramics. Mat. Sci. Eng. R., 2010, 70(3-6): 225-242. |
| [5] | SADAT-SHOJAI M, KHORASANI M T, DINPANAH- KHOSHDARGI E, et al. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater., 2013, 9(8): 7591-7621. |
| [6] | CHRISTENSON E M, ANSETH K S, VAN DEN BEUCKEN L, et al. Nanobiomaterial applications in orthopedics. J. Orthopaed. Res., 2007, 25(1): 11-22. |
| [7] | FAN J-B, HUANG C, JIANG L, et al. Nanoporous microspheres: from controllable synthesis to healthcare applications. J. Mater. Chem. B, 2013, 1(17): 2222-2235. |
| [8] | PARK J H, PEREZ R A, JIN G Z, et al. Microcarriers designed for cell culture and tissue engineering of bone. Tissue Engineering Part B-Reviews, 2013, 19(2): 172-190. |
| [9] | WANG A J, LU Y P, SUN R X. Recent progress on the fabrication of hollow microspheres. Mat. Sci. Eng. A, 2007, 460: 1-6. |
| [10] | MATSUNO T, HASHIMOTO Y, ADACHI S, et al. Preparation of injectable 3D-formed beta-tricalcium phosphate bead/alginate composite for bone tissue engineering. Dent. Mater. J., 2008, 27(6): 827-834. |
| [11] | HASHIMOTO Y, ADACHI S, MATSUNO T, et al. Effect of an injectable 3D scaffold for osteoblast differentiation depends on bead size. Bio-Med. Mater. Eng., 2009, 19(6): 391-400. |
| [12] | LUO H T, ZHI W, LU X, et al. Research on preparation and biological properties of dense hydroxyapatite spheresJ. Inorg. Mater., 2013, 28(1): 40-44. |
| [13] | SUNNY M C, RAMESH P, VARMA H K. Microstructured microspheres of hydroxyapatite bioceramic. J. Mater. Sci-Mater. Med., 2002, 13(7): 623-632. |
| [14] | KAMITAKAHARA M, IMAI R, IOKU K. Preparation and evaluation of spherical Ca-deficient hydroxyapatite granules with controlled surface microstructure as drug carriers. Mater. Sci. Eng. C, 2013, 33(4): 2446-2450. |
| [15] | PEREZ R A, ALTANKOV G, JORGE-HERRERO E, et al. Micro- and nanostructured hydroxyapatitecollagen microcarriers for bone tissue-engineering applications. J. Tissue. Eng. Regen. Med., 2013, 7(5): 353-361. |
| [16] | KOMLEV V S, BARINOV S M, KOPLIK E V. A method to fabricate porous spherical hydroxyapatite granules intended for time- controlled drug release. Biomaterials, 2002, 23(16): 3449-3454. |
| [17] | BERNHARDT A, DITTRICH R, LODE A, et al. Nanocrystalline spherical hydroxyapatite granules for bone repair: in vitro evaluation with osteoblast-like cells and osteoclasts. J. Mater. Sci-Mater. Med., 2013, 24(7): 1755-1766. |
| [18] | RIBEIRO C C, BARRIAS C C, BARBOSA M A. Preparation and characterisation of calcium-phosphate porous microspheres with a uniform size for biomedical applications. J. Mater. Sci-Mater. Med., 2006, 17(5): 455-463. |
| [19] | DO-VAN T, LEE B T. Formation and characterization of porous spherical biphasic calcium phosphate (BCP) granules using PCL. Ceram. Int., 2011, 37(6): 2043-2049. |
| [20] | YANG J H, KIM K H, YOU C K, et al. Synthesis of spherical hydroxyapatite granules with interconnected pore channels using camphene emulsion. J. Biomed. Mater. Res. B Appl. Biomater., 2011, 99B(1): 150-157. |
| [21] | HONG M H, SON J S, KIM K M, et al. Drug-loaded porous spherical hydroxyapatite granules for bone regeneration. J. Mater. Sci-Mater. Med., 2011, 22(2): 349-355. |
| [22] | WANG A, LU Y, ZHU R, et al. Effect of process parameters on the performance of spray dried hydroxyapatite microspheres. Powder Technol., 2009, 191(1/2): 1-6. |
| [23] | WANG A J, LU Y P, ZHU R F, et al. Effect of sintering on porosity, phase, and surface morphology of spray dried hydroxyapatite microspheres. J. Biomed. Mater. Res., 2008, 87A(2): 557-562. |
| [24] | LOU X W, ARCHER L A, YANG Z. Hollow micro-/nanostruc-tures: synthesis and applications. Adv. Mater., 2008, 20(21): 3987-4019. |
| [25] | WANG Y, YAO A, HUANG W, et al. In situ fabrication of hollow hydroxyapatite microspheres by phosphate solution immersion. J. Cryst. Growth, 2011, 327(1): 245-250. |
| [26] | WANG Y, MOO Y X, CHEN C, et al. Fast precipitation of uniform CaCO3 nanospheres and their transformation to hollow hydroxyapatite nanospheres. J. Colloid Interf. Sci., 2010, 352(2): 393-400. |
| [27] | FU H, RAHAMAN M N, DAY D E. Effect of process variables on the microstructure of hollow hydroxyapatite microspheres prepared by a glass conversion method. J. Am. Ceram. Soc., 2010, 93(10): 3116-3123. |
| [28] | YAO A, AI F, LIU X, et al. Preparation of hollow hydroxyapatite microspheres by the conversion of borate glass at near room temperature. Mater. Res. Bull., 2010, 45(1): 25-28. |
| [29] | GUO Y, ZHOU Y, JIA D, et al. Fabrication and characterization of hydroxycarbonate apatite with mesoporous structure. Micropor. Mesopor. Mater., 2009, 118(1/2/3): 480-488. |
| [30] | GUO Y-P, LIN T-S, ZHOU Y, et al. Fabrication of monodisperse mesoporous hydroxycarbonate apatite microspheres by emulsion method. Micropor. Mesopor. Mater., 2010, 127(3): 245-249. |
| [31] | GUO Y J, WANG Y Y, CHEN T, et al. Hollow carbonated hydroxyapatite microspheres with mesoporous structure: Hydrothermal fabrication and drug delivery property. Mater. Sci. Eng. C, 2013, 33(6): 3166-3172. |
| [32] | LIN K, CHEN L, LIU P, et al. Hollow magnetic hydroxyapatite microspheres with hierarchically mesoporous microstructure for pH- responsive drug delivery. CrystEngComm., 2013, 15(15): 2999-3008. |
| [33] | HUANG M, WANG Y. Synthesis of calcium phosphate microcapsules using yeast-based biotemplate. J. Mater. Chem., 2012, 22(2): 626-630. |
| [34] | LEE H H, HONG S J, KIM C H, et al. Preparation of hydroxyapatite spheres with an internal cavity as a scaffold for hard tissue regeneration. J. Mater. Sci-Mater. Med., 2008, 19(9): 3029-3034. |
| [35] | SUN R, LU Y, CHEN K. Preparation and characterization of hollow hydroxyapatite microspheres by spray drying method. Mater. Sci. Eng. C, 2009, 29(4): 1088-1092. |
| [36] | JIAO Y, LU Y P, XIAO G Y, et al. Preparation and characterization of hollow hydroxyapatite microspheres by the centrifugal spray drying method. Powder Technol., 2012, 217: 581-584. |
| [37] | WANG K W, ZHU Y J, CHEN F, et al. Microwave-assisted synthesis of hydroxyapatite hollow microspheres in aqueous solution. Mater. Lett., 2011, 65(15/16): 2361-2363. |
| [38] | ELTOHAMY M, SHIN U S, WON J E, et al. Electrosprayed tricalcium phosphate spherical microcups and antibiotic drug delivery. Mater. Lett., 2011, 65(13): 2043-2046. |
| [39] | MA M G. Hierarchically nanostructured hydroxyapatite: hydrothermal synthesis, morphology control, growth mechanism, and biological activity. Int. J. Nanomedicine, 2012, 7: 1781-1791. |
| [40] | KANG N H, KIM S J, SONG S H, et al. Hydroxyapatite synthesis using EDTA. J. Cran. Surg., 2013, 24(3): 1042-1045. |
| [41] | WANG Y, WU C, LIN K, et al. Facile fabrication of nanorod- assembled fluorine-substituted hydroxyapatite (FHA) microspheres. Chem. Asian. J., 2013, 8(5): 990-996. |
| [42] | YANG H, HAO L, DU C, et al. A systematic examination of the morphology of hydroxyapatite in the presence of citrate. RSC Adv., 2013, 3(45): 23184-23189. |
| [43] | YANG H, HAO L, ZHAO N, et al. Hierarchical porous hydroxyapatite microsphere as drug delivery carrier. CrystEngComm., 2013, 15(29): 5760-5763. |
| [44] | MA Y, HAO L, DU S, et al. Synthesis of hydroxyapatite microspheres by hydrothermal method under the control of sodium citrate. J. Inorg. Mater., 2014, 29(3): 284-288. |
| [45] | PENG Q, JIANG F, HUANG P, et al. A novel porous bioceramics scaffold by accumulating hydroxyapatite spherules for large bone tissue engineering in vivo. I. Preparation and characterization of scaffold. J. Biomed. Mater. Res., 2010, 93A(3): 920-929. |
| [46] | WANG H, ZHI W, LU X, et al. Comparative studies on ectopic bone formation in porous hydroxyapatite scaffolds with complementary pore structures. Acta Biomater., 2013, 9(9): 8413-8421. |
| [47] | LARANJEIRA M S, FERNANDES M H, MONTEIRO F J. Innovative macroporous granules of nanostructured-hydroxyapatite agglomerates: bioactivity and osteoblast-like cell behaviour. J. Biomed. Mater. Res., 2010, 95A(3): 891-900. |
| [48] | LEE J H, KO I H, JEON S H, et al. Micro-structured hydroxyapatite microspheres for local delivery of alendronate and BMP-2 carriers. Mater. Lett., 2013, 105: 136-139. |
| [49] | SUN R, CHEN K, LU Y. Fabrication and dissolution behavior of hollow hydroxyapatite microspheres intended for controlled drug release. Mater. Res. Bull., 2009, 44(10): 1939-1942. |
| [50] | YANG Y H, LIU C H, LIANG Y H, et al. Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J. Mater. Chem. B, 2013, 1(19): 2447-2450. |
| [51] | JIANG F, WANG D P, WANG H, et al. Analysis of several factors for drug controlled release from apatite microspheres. J. Chin. Ceramic Soc., 2013, 41(10): 1347-1353. |
| [52] | XIA W, GRANDFIELD K, SCHWENKE A, et al. Synthesis and release of trace elements from hollow and porous hydroxyapatite spheres. Nanotechnology, 2011, 22(30): 305610-1-10. |
| [53] | PARK J S, HONG S J, KIM H Y, et al. Evacuated calcium phosphate spherical microcarriers for bone regeneration. Tissue Eng. Part A, 2010, 16(5): 1681-1691. |
| [54] | JIN G Z, KIM J H, PARK J H, et al. Performance of evacuated calcium phosphate microcarriers loaded with mesenchymal stem cells within a rat calvarium defect. J. Mater. Sci-Mater. Med., 2012, 23(7): 1739-1748. |
| [55] | FU H, RAHAMAN M N, BROWN R F, et al. Evaluation of bone regeneration in implants composed of hollow HA microspheres loaded with transforming growth factor beta 1 in a rat calvarial defect model. Acta Biomater., 2013, 9(3): 5718-5727. |
| [56] | XIAO W, FU H, RAHAMAN M N, et al. Hollow hydroxyapatite microspheres: A novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration. Acta Biomater., 2013, 9(9): 8374-8383. |
| [57] | GREEN D W, BOLLAND B J R F, KANCZLER J M, et al. Augmentation of skeletal tissue formation in impaction bone grafting using vaterite microsphere biocomposites. Biomaterials, 2009, 30(10): 1918-1927. |
| [58] | WU K C W, YANG Y H, LIANG Y H, et al. Facile synthesis of hollow mesoporous hydroxyapatite nanoparticles for intracellular bio-imaging. Curr. Nanosci., 2011, 7(6): 926-931. |
| [59] | KIMURA I, KANATANI M, WATANABE K. Adhesion of hollow calcium- deficient hydroxyapatite microspheres onto titanium. Dent. Mater. J., 2009, 28(6): 700-707. |
| [60] | FU Q, HONG Y, LIU X, et al. A hierarchically graded bioactive scaffold bonded to titanium substrates for attachment to bone. Biomaterials, 2011, 32(30): 7333-7346. |
| [61] | FU H, RAHAMAN M N, BROWN R F, et al. Evaluation of BSA protein release from hollow hydroxyapatite microspheres into PEG hydrogel. Mater. Sci. Eng. C, 2013, 33(4): 2245-2250. |
| [62] | FU H, RAHAMAN M N, DAY D E, et al. Hollow hydroxyapatite microspheres as a device for controlled delivery of proteins. J. Mater. Sci-Mater. Med., 2011, 22(3): 579-591. |
| [1] | 丁玲, 蒋瑞, 唐子龙, 杨运琼. MXene材料的纳米工程及其作为超级电容器电极材料的研究进展[J]. 无机材料学报, 2023, 38(6): 619-633. |
| [2] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [3] | 陈强, 白书欣, 叶益聪. 热管理用高导热碳化硅陶瓷基复合材料研究进展[J]. 无机材料学报, 2023, 38(6): 634-646. |
| [4] | 林俊良, 王占杰. 铁电超晶格的研究进展[J]. 无机材料学报, 2023, 38(6): 606-618. |
| [5] | 牛嘉雪, 孙思, 柳鹏飞, 张晓东, 穆晓宇. 铜基纳米酶的特性及其生物医学应用[J]. 无机材料学报, 2023, 38(5): 489-502. |
| [6] | 苑景坤, 熊书锋, 陈张伟. 聚合物前驱体转化陶瓷增材制造技术研究趋势与挑战[J]. 无机材料学报, 2023, 38(5): 477-488. |
| [7] | 杜剑宇, 葛琛. 光电人工突触研究进展[J]. 无机材料学报, 2023, 38(4): 378-386. |
| [8] | 杨洋, 崔航源, 祝影, 万昌锦, 万青. 柔性神经形态晶体管研究进展[J]. 无机材料学报, 2023, 38(4): 367-377. |
| [9] | 游钧淇, 李策, 杨栋梁, 孙林锋. 氧化物双介质层忆阻器的设计及应用[J]. 无机材料学报, 2023, 38(4): 387-398. |
| [10] | 林思琪, 李艾燃, 付晨光, 李荣斌, 金敏. Zintl相Mg3X2(X=Sb, Bi)基晶体生长及热电性能研究进展[J]. 无机材料学报, 2023, 38(3): 270-279. |
| [11] | 陈昆峰, 胡乾宇, 刘锋, 薛冬峰. 多尺度晶体材料的原位表征技术与计算模拟研究进展[J]. 无机材料学报, 2023, 38(3): 256-269. |
| [12] | 张超逸, 唐慧丽, 李宪珂, 王庆国, 罗平, 吴锋, 张晨波, 薛艳艳, 徐军, 韩建峰, 逯占文. 新型GaN与ZnO衬底ScAlMgO4晶体的研究进展[J]. 无机材料学报, 2023, 38(3): 228-242. |
| [13] | 齐占国, 刘磊, 王守志, 王国栋, 俞娇仙, 王忠新, 段秀兰, 徐现刚, 张雷. GaN单晶的HVPE生长与掺杂进展[J]. 无机材料学报, 2023, 38(3): 243-255. |
| [14] | 谢兵, 蔡金峡, 王铜铜, 刘智勇, 姜胜林, 张海波. 高储能密度聚合物基多层复合电介质的研究进展[J]. 无机材料学报, 2023, 38(2): 137-147. |
| [15] | 刘岩, 张珂颖, 李天宇, 周菠, 刘学建, 黄政仁. 陶瓷材料电场辅助连接技术研究现状及发展趋势[J]. 无机材料学报, 2023, 38(2): 113-124. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||