无机材料学报 ›› 2014, Vol. 29 ›› Issue (3): 257-263.DOI: 10.3724/SP.J.1077.2014.13322
聂文波, 肖其昌, 王京亮, 雷钢铁, 肖启振, 李朝晖
收稿日期:2013-06-28
修回日期:2013-08-01
出版日期:2014-03-20
网络出版日期:2014-02-18
作者简介:聂文波(1986-), 男, 硕士研究生. E-mail: 363478547@qq.com
基金资助:NIE Wen-Bo, XIAO Qi-Chang, WANG Jing-Liang, LEI Gang-Tie, XIAO Qi-Zhen, LI Zhao-Hui
Received:2013-06-28
Revised:2013-08-01
Published:2014-03-20
Online:2014-02-18
About author:NIE Wen-Bo. E-mail: 363478547@qq.com
摘要:
采用溶胶-凝胶技术在富锂锰基固溶体Li1.2Mn0.54Co0.13Ni0.13O2表面包覆V2O5, 制备了Li1.2Mn0.54Co0.13Ni0.13O2@V2O5核壳复合材料。通过扫描电镜(SEM)和透射电镜(TEM)观察其形貌, X射线衍射(XRD)分析确定其结构。结果显示, 结晶态的V2O5均匀包覆在类球形Li1.2Mn0.54Co0.13Ni0.13O2颗粒表面, Li1.2Mn0.54Co0.13Ni0.13O2材料的晶体结构在包覆前后保持不变。X射线光电子能谱(XPS)分析结果表明, 该核壳复合材料首次充电时, 锂离子嵌入V2O5包覆层。电化学测试结果表明, 表面包覆15%V2O5的核壳复合材料具有最佳的电化学性能: 0.1C倍率下放电比容量为276 mAh/g, 首次充放电库仑效率达到94%, 50次循环后容量保持率达89%。
中图分类号:
聂文波, 肖其昌, 王京亮, 雷钢铁, 肖启振, 李朝晖. Li1.2Mn0.54Co0.13Ni0.13O2@V2O5核壳复合材料的制备及其电化学性能[J]. 无机材料学报, 2014, 29(3): 257-263.
NIE Wen-Bo, XIAO Qi-Chang, WANG Jing-Liang, LEI Gang-Tie, XIAO Qi-Zhen, LI Zhao-Hui. Preparation of Li1.2Mn0.54Co0.13Ni0.13O2@V2O5 Core-shell Composite and Its Electrochemical Properties[J]. Journal of Inorganic Materials, 2014, 29(3): 257-263.
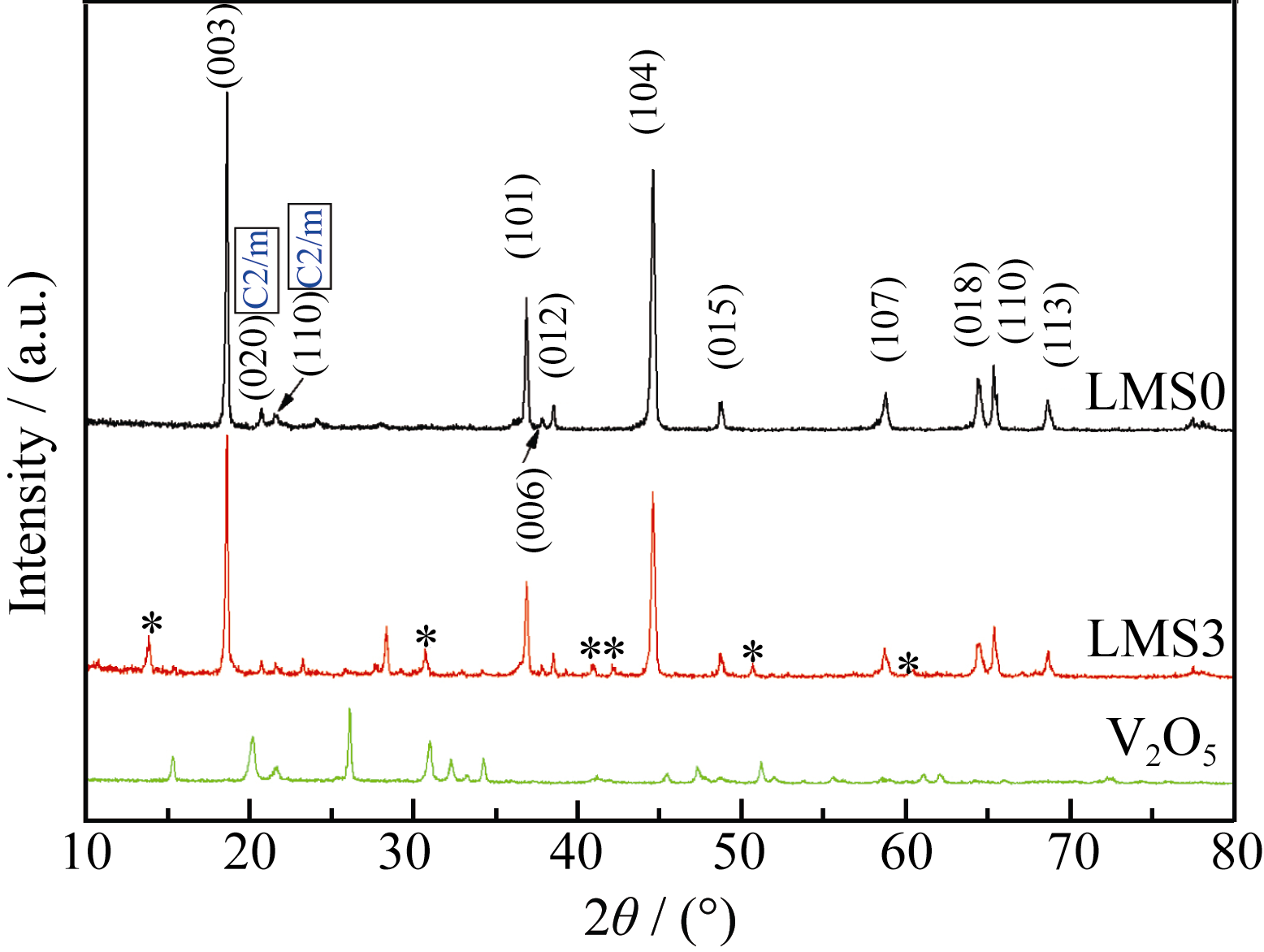
图 1 V2O5、Li1.2Mn0.54Co0.13Ni0.13O2@V2O5核壳复合材料(LMS3)和Li1.2Mn0.54Co0.13Ni0.13O2(LMS0)的XRD图谱
Fig. 1 XRD patterns of V2O5, Li1.2Mn0.54Co0.13Ni0.13O2@V2O5 core-shell composite (LMS3) and Li1.2Mn0.54Co0.13Ni0.13O2 (LMS0)
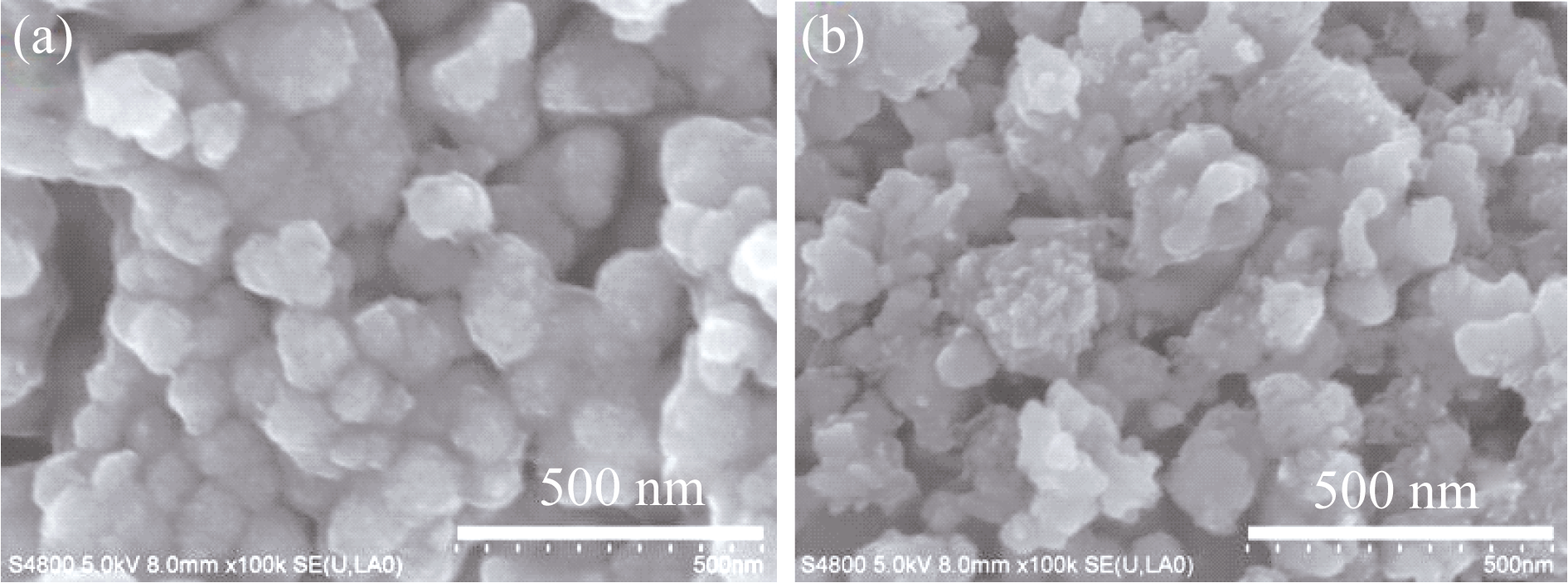
图2 Li1.2Mn0.54Co0.13Ni0.13O2 (a)和Li1.2Mn0.54Co0.13Ni0.13O2@V2O5核壳复合材料(b)的SEM照片
Fig. 2 SEM images of Li1.2Mn0.54Co0.13Ni0.13O2 (a) and Li1.2Mn0.54Co0.13Ni0.13O2@V2O5 core-shell composite
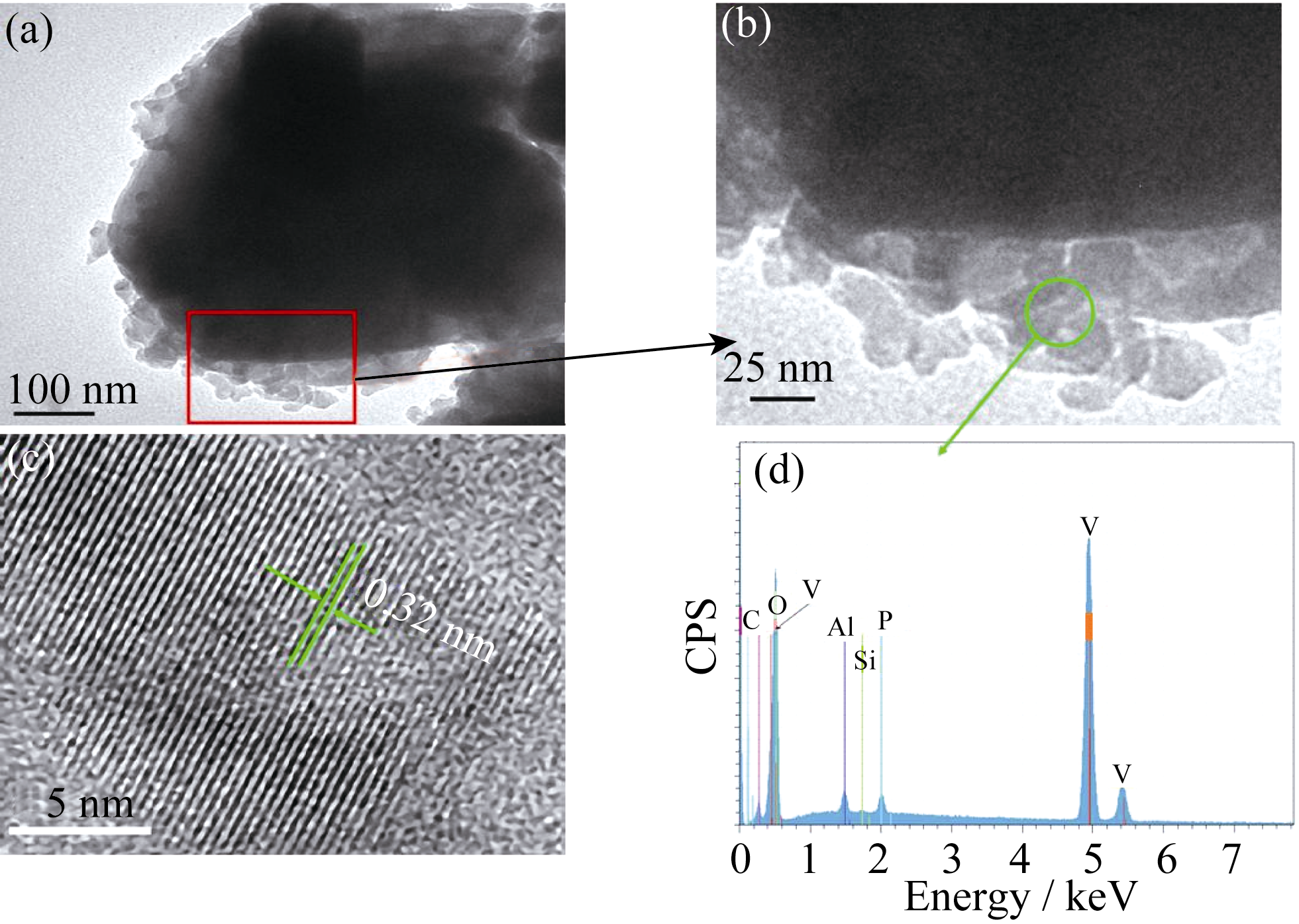
图 3 Li1.2Mn0.54Co0.13Ni0.13O2@V2O5核壳复合材料TEM照片(a, b), 包覆层中V2O5的HRTEM照片(c)及其EDX图谱(d)
Fig. 3 TEM images of Li1.2Mn0.54Co0.13Ni0.13O2@V2O5 core-shell composite (a, b), HRTEM image of the V2O5 in the shell (c) and its EDX spectrum (d)
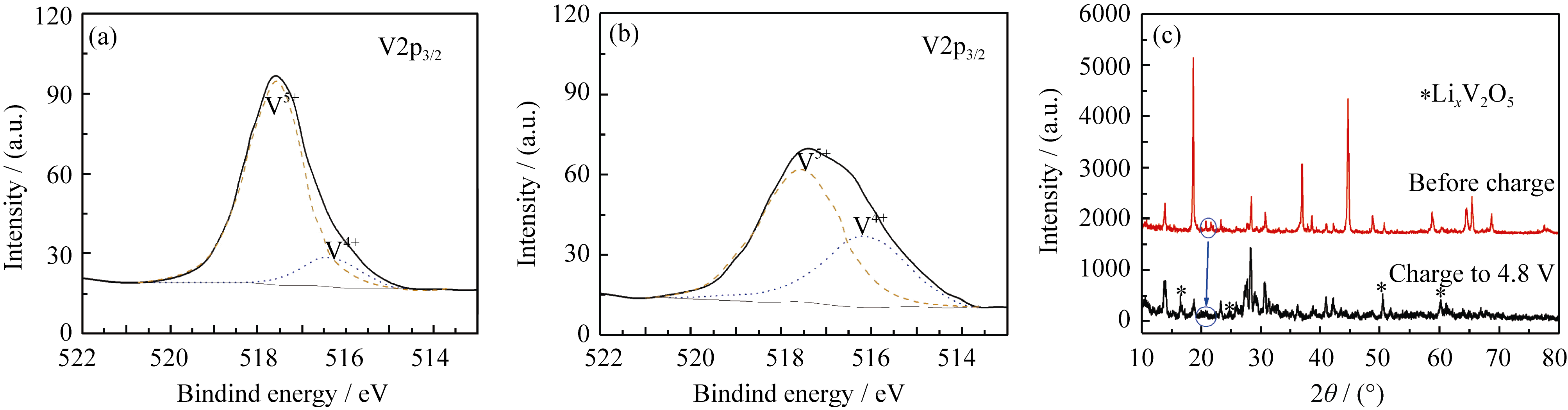
图4 Li1.2Mn0.54Co0.13Ni0.13O2@V2O5核壳复合材料(LMS3)未充电(a)和首次充电至4.8 V后(b)的V2p2/3 XPS图谱, 及其首次充电至4.8 V后XRD图谱(c)
Fig. 4 XPS spectra of V2p2/3 in the Li1.2Mn0.54Co0.13Ni0.13O2@V2O5 core-shell composite (LMS3) before (a) and after (b) charged to 4.8 V in the first cycle, and their XRD patterns
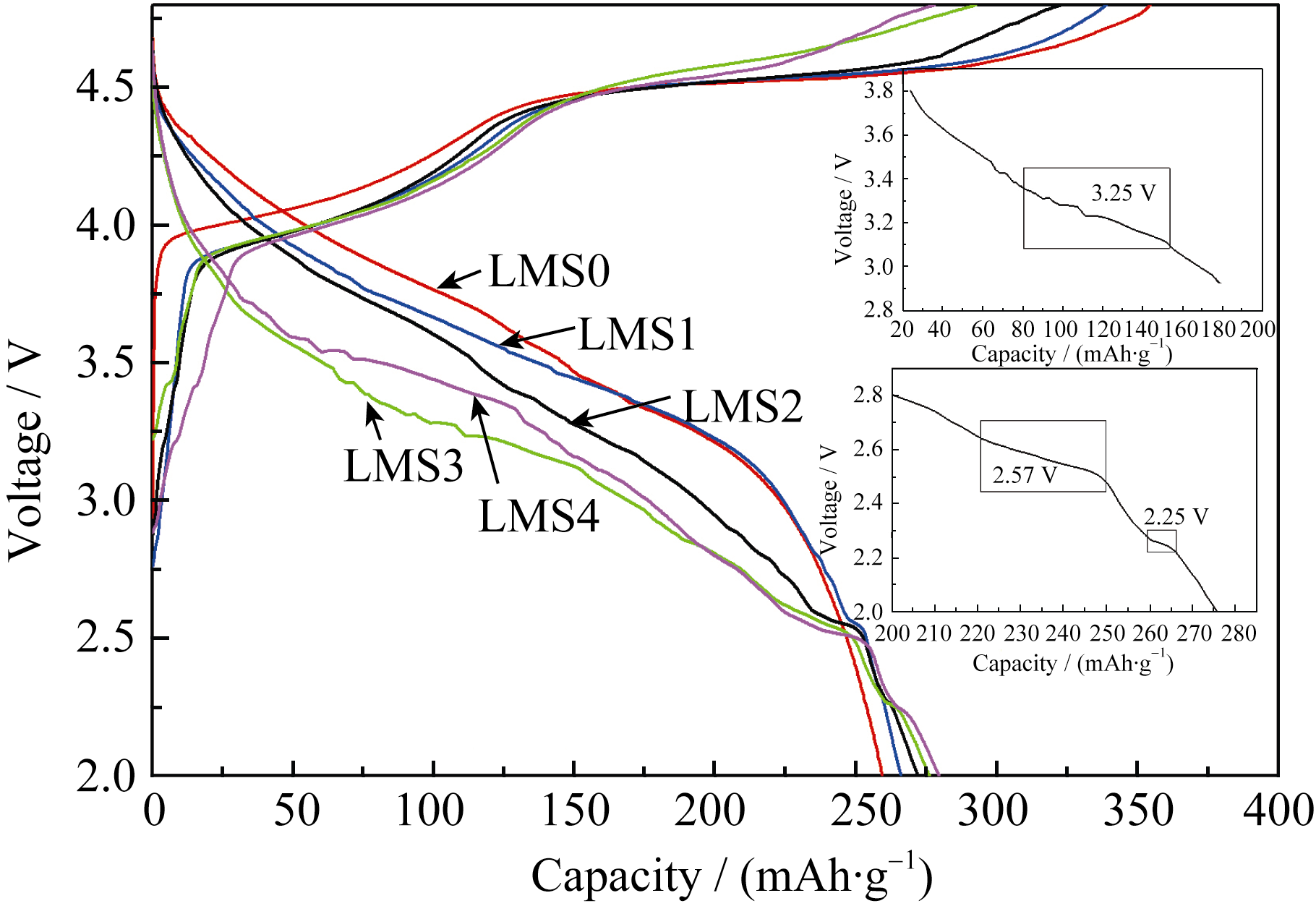
图6 Li1.2Mn0.54Co0.13Ni0.13O2@V2O5核壳复合材料的首次充放电曲线
Fig. 6 First charge/discharge profiles of the Li1.2Mn0.54Co0.13Ni0.13O2 @V2O5 core-shell composites Inset is local discharge curves of sample LMS3
| Mass fraction of the V2O5 in the composite/% | Initial charge capacity/(mAh·g-1) | Initial discharge capacity/(mAh·g-1) | Irreversible capacity/(mAh·g-1) | Coulombic efficiency/% |
|---|---|---|---|---|
| 0 | 354 | 259 | 95 | 73 |
| 5 | 339 | 266 | 73 | 78 |
| 10 | 323 | 272 | 51 | 84 |
| 15 | 292 | 276 | 16 | 94 |
| 20 | 278 | 279 | -1 | 100 |
表1 Li1.2Mn0.54Co0.13Ni0.13O2@V2O5核壳复合材料的首次充放电参数过高温处理具有较高的结晶度, 因此, 所得的核壳复合材料具有优越的循环性能。
Table 1 First discharge/charge parameters of the Li1.2Mn0.54Co0.13Ni0.13O2@V2O5 core-shell composites in the first cycle
| Mass fraction of the V2O5 in the composite/% | Initial charge capacity/(mAh·g-1) | Initial discharge capacity/(mAh·g-1) | Irreversible capacity/(mAh·g-1) | Coulombic efficiency/% |
|---|---|---|---|---|
| 0 | 354 | 259 | 95 | 73 |
| 5 | 339 | 266 | 73 | 78 |
| 10 | 323 | 272 | 51 | 84 |
| 15 | 292 | 276 | 16 | 94 |
| 20 | 278 | 279 | -1 | 100 |
| Sample | Rs / Ω | Rct / Ω | ||
|---|---|---|---|---|
| Before cycle | After 20 cycles | Before cycle | After 20 cycles | |
| LMS0 | 3.94 | 4.02 | 21.6 | 133.0 |
| LMS3 | 4.06 | 4.08 | 40.7 | 55.7 |
表2 等效电路的阻抗参数
Table 2 Impedance parameters of the equipment circuit
| Sample | Rs / Ω | Rct / Ω | ||
|---|---|---|---|---|
| Before cycle | After 20 cycles | Before cycle | After 20 cycles | |
| LMS0 | 3.94 | 4.02 | 21.6 | 133.0 |
| LMS3 | 4.06 | 4.08 | 40.7 | 55.7 |
| [1] | ZHANG JIAN-BO, LIANG FAN, GAO XUE-PING, et al. Advance of lithium ion batteries: The 16th international meeting on lithium batteries. Scientia Sinica Chimica, 2012, 42(8): 1252-1262. |
| [2] | ZHANG QIAN, LIU WEI-WEI, FANG GUO-QING, et al. Structural and electrochemical performances of Li1+2xMn0.3+xNi0.3-3xCr0.4O2 synthesized by spray-dry method. Journal of Inorganic Materials, 2013, 28(06): 616-622. |
| [3] | WANG ZHAO, WU FENG, SU YUE-FENG, et al. Preparation and characterization of xLi2MnO3·(1-x)Li[Ni1/3Mn1/3Co1/3]O2 cathode materials for lithium-ion batteries. Acta Physico-Chimica Sinica, 2012, 28(4): 823-830. |
| [4] | THACKERAY M M, KANG S H, JOHNSON C S, et al. Li2MnO3-stabilized LiMO2 (M=Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem., 2007, 17(30): 3112-3125. |
| [5] | WU CHENG-REN, ZHAO CHANG-CHUN, WANG ZHAO- XIANG, et al. Li-rich layer-structured cathode materials for Li-ion batteries. Progress in Chemistry, 2011, 23(10): 2038-2044. |
| [6] | DU KE, HU GUO-RONG. Review of manganese-based solid solution xLi[Li1/3Mn2/3]O2·(1-x)LiMO2. Chinese Science Bulletin, 2012, 57(10): 794-804. |
| [7] | ZHAO Y J, ZHAO C S, FENG H L, et al. Enhanced electrochemical performance of Li[Li0.2Ni0.2Mn0.6]O2 modified by manganese oxide coating for lithium-ion batteries. Electrochem. Solid-State Lett., 2011, 14(1): A1-A5. |
| [8] | SHI S J, TU J P, TANG Y Y, et al. Enhanced cycling stability of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by surface modification of MgO with melting impregnation method. Electrochim. Acta, 2013, 88: 671-679. |
| [9] | ROSINA K J, JIANG M, ZENG D L, et al. Structure of aluminum fluoride coated Li[Li1/9Ni1/3Mn5/9]O2 cathodes for secondary lithium-ion batteries. J. Mater. Chem., 2012, 22(38): 20602-20610. |
| [10] | WU C R, FANG X P, GUO X W, et al. Surface modification of Li1.2Mn0.54Co0.13Ni0.13O2 with conducting polypyrrole. J. Power Sources, 2013, 231: 44-49. |
| [11] | WU F, LI N, SU Y F, et al. Can surface modification be more effective to enhance the electrochemical performance of lithium rich materials?. J. Mater. Chem., 2012, 22(4): 1489-1497. |
| [12] | WANG Z Y, LIU E Z, HE C N, et al. Effect of amorphous FePO4 coating on structure and electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 as cathode material for Li-ion batteries. J. Power Sources, 2013, 236: 25-32. |
| [13] | MARTHA S K, NANDA J, KIM Y, et al. Solid electrolyte coated high voltage layered-layered lithium-rich composite cathode: Li1.2Mn0.525Ni0.175Co0.1O2. J. Mater. Chem. A, 2013, 1(18): 5587-5595. |
| [14] | LIU Y J, LIU S B, WANG Y P, et al. Effect of MnO2 modification on electrochemical performance of LiNi0.2Li0.2Mn0.6O2 layered solid solution cathode. J. Power Sources, 2013, 222: 455-460. |
| [15] | ZHANG H Z, QIAO Q Q, LI G R, et al. Surface nitridation of Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as cathode material for lithium-ion battery. J. Mater. Chem., 2012, 22(26): 13104-13109. |
| [16] | ZHENG J, DENG S N, SHI Z C, et al. The effects of persulfate treatment on the electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material. J. Power Sources, 2013, 221: 108-113. |
| [17] | HUANG XIA, YANG FEI, HU GUO-RONG, et al. Modification of Li[Li0.2Ni0.2Mn0.6]O2 as cathode material for rechargeable lithium batteries by acid-leaching. Chinese Journal of Inorganic Chemistry, 2012, 28(5): 983-988. |
| [18] | SONG B H, LAI M O, LU L. Influence of Ru substitution on Li-rich 0.55Li2MnO3·0.45LiNi1/3Co1/3Mn1/3O2 cathode for Li-ion batteries. Electrochim. Acta, 2012, 80: 187-195. |
| [19] | GAO J, KIM J, MANTHIRAM A. High capacity Li[Li0.2Mn0.54Ni0.13Co0.13]O2-V2O5 composite cathodes with low irreversible capacity loss for lithium ion batteries. Electrochem. Commun., 2009, 11(1): 84-86. |
| [20] | LEE E S, MANTHIRAM A. High capacity Li[Li0.2Mn0.54Ni0.13Co0.13] O2-VO2(B) composite cathodes with controlled irreversible capacity loss for lithium-ion batteries. J. the Electrochem. Soc., 2011, 158(1): A47-A50. |
| [21] | GAO J, KIM J, MANTHIRAM A. Eliminating the irreversible capacity loss of high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode by blending with other lithium insertion hosts. J. Power Sources, 2009, 191(2): 644-647. |
| [22] | WANG J L, XIAO Q C, YU J J, et al. Layered lithium-rich Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials prepared by different methods and its electrochemical properties. Scientific Journal of Frontier Chemical Development, 2012, 2(4): 81-88. |
| [23] | YU J J, YANG J, NIE W B, et al. A porous vanadium pentoxide nanomaterial as cathode material for rechargeable lithium batteries. Electrochim. Acta, 2013, 89: 292-299. |
| [24] | JOHNSON C S, LI N, LEFIEF C, et al. Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3·(1-x) LiMn0.333Ni0.333Co0.333O2 (0≤x≤0.7). Chem. Mater., 2008, 20(19): 6095-6106. |
| [25] | ALAMARGUY D, CASTLE J E, LIBERATORE M, et al. Distribution of intercalated lithium in V2O5 thin films determined by SIMS depth profiling. Surf. Interface Anal., 2006, 38: 847-850. |
| [26] | EGUCHI M, OZAWA K, SAKKA Y. Preparation and lithium insertion property of layered LixV2O5·nH2O. J. Power Sources, 2003, 119-121: 201-204. |
| [27] | SEMENENKO D A, ITKIS D M, POMERANTSEVA E A, et al. LixV2O5 nanobelts for high capacity lithium-ion battery cathodes. Electrochem. Commun., 2010, 12(9): 1154-1157. |
| [28] | ROZIER P, SAVARIAULT J M, GALY J. A new interpretation of the LixV2O5 electrochemical behaviour for 1<x<3. Solid State Ionics, 1997, 98(3/4): 133-144. |
| [29] | XIONG X H, WANG Z X, GUO H J, et al. Enhanced electrochemical properties of lithium-reactive V2O5 coated on the LiNi0.8Co0.1Mn0.1O2 cathode material for lithium ion batteries at 60℃. J. Mater. Chem. A, 2013, 1: 1284-1288. |
| [30] | Liu J, Wang Q Y, Reeja-Jayan B, et al. Carbon-coated high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes. Electrochem. Commun., 2010, 12(6): 750-753. |
| [1] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [2] | 吴锐, 张敏慧, 金成韵, 林健, 王德平. 光热核壳TiN@硼硅酸盐生物玻璃纳米颗粒的降解和矿化性能[J]. 无机材料学报, 2023, 38(6): 708-716. |
| [3] | 马晓森, 张丽晨, 刘砚超, 汪全华, 郑家军, 李瑞丰. 13X@SiO2合成及其甲苯吸附性能[J]. 无机材料学报, 2023, 38(5): 537-543. |
| [4] | 朱河圳, 王选朋, 韩康, 杨晨, 万睿哲, 吴黎明, 麦立强. 超高镍LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2正极材料的储锂稳定性的提升机制[J]. 无机材料学报, 2022, 37(9): 1030-1036. |
| [5] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [6] | 宿拿拿, 韩静茹, 郭印毫, 王晨宇, 石文华, 吴亮, 胡执一, 刘婧, 李昱, 苏宝连. 基于ZIF-8的三维网络硅碳复合材料锂离子电池性能研究[J]. 无机材料学报, 2022, 37(9): 1016-1022. |
| [7] | 王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029. |
| [8] | 陈莹, 栾伟玲, 陈浩峰, 朱轩辰. 基于应力场的锂离子电池正极多尺度失效研究[J]. 无机材料学报, 2022, 37(8): 918-924. |
| [9] | 迟聪聪, 屈盼盼, 任超男, 许馨, 白飞飞, 张丹洁. SiO2@Ag@SiO2@TiO2核壳结构的制备及其光催化降解性能[J]. 无机材料学报, 2022, 37(7): 750-756. |
| [10] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [11] | 苏东良, 崔锦, 翟朋博, 郭向欣. 石榴石型Li6.4La3Zr1.4Ta0.6O12对Si/C负极表面固体电解质中间相的调控机制研究[J]. 无机材料学报, 2022, 37(7): 802-808. |
| [12] | 肖美霞, 李苗苗, 宋二红, 宋海洋, 李钊, 毕佳颖. 表面端基卤化Ti3C2 MXene应用于锂离子电池高容量电极材料的研究[J]. 无机材料学报, 2022, 37(6): 660-668. |
| [13] | 王禹桐, 张非凡, 许乃才, 王春霞, 崔立山, 黄国勇. 水系锂离子电池负极材料LiTi2(PO4)3的研究进展[J]. 无机材料学报, 2022, 37(5): 481-492. |
| [14] | 陈小梅, 陈颖, 袁霞. 核壳材料Co3O4@SiO2催化环己基过氧化氢分解[J]. 无机材料学报, 2022, 37(1): 65-71. |
| [15] | 李昆儒, 胡省辉, 张正富, 郭玉忠, 黄瑞安. 源于溪木贼的高性能锂离子电池三维多孔生物质硅/碳复合负极材料[J]. 无机材料学报, 2021, 36(9): 929-935. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||