无机材料学报 ›› 2017, Vol. 32 ›› Issue (3): 225-234.DOI: 10.15541/jim20160192 CSTR: 32189.14.10.15541/jim20160192
• • 下一篇
陈丽能, 晏梦雨, 梅志文, 麦立强
收稿日期:2016-03-28
修回日期:2016-06-16
出版日期:2017-03-20
网络出版日期:2017-02-24
作者简介:陈丽能(1991–), 女, 博士研究生. E-mail: clnsmiles@whut.edu.cn
基金资助:CHEN Li-Neng, YAN Meng-Yu, MEI Zhi-Wen, MAI Li-Qiang
Received:2016-03-28
Revised:2016-06-16
Published:2017-03-20
Online:2017-02-24
About author:CHEN Li-Neng. E-mail: clnsmiles@whut.edu.cn
Supported by:摘要:
锌离子电池是近年来发展起来的一种新型二次水系电池, 具有高能量密度、高功率密度、放电过程高效安全、电池材料无毒廉价、制备工艺简单等优点, 在大型储能等领域具有很高应用价值和发展前景。本文综述了水系锌离子电池的研究进展, 对金属锌作负极的优点和面临的处理问题进行总结, 对已报导的正极材料中锌离子电池的电化学性能和反应机制进行分析, 并通过分析目前多价离子的脱嵌特性对锌离子电池正极材料的发展进行预测。
中图分类号:
陈丽能, 晏梦雨, 梅志文, 麦立强. 水系锌离子电池的研究进展[J]. 无机材料学报, 2017, 32(3): 225-234.
CHEN Li-Neng, YAN Meng-Yu, MEI Zhi-Wen, MAI Li-Qiang. Research Progress and Prospect of Aqueous Zinc Ion Battery[J]. Journal of Inorganic Materials, 2017, 32(3): 225-234.

图3 锌离子电池在0.1 A/g (a)和8 A/g (b)恒电流下的充放电循环曲线[9]
Fig. 3 Charge and discharge curues of zinc ion batteries at current density of 0.1 A/g (a) and 8 A/g (b)[9]

图5 (a)200 mA h/g的电流密度下未改性及加入不同比例活性炭改性的锌负极后锌离子电池的循环性能; (b) 0.1 mV/s扫描速度下未改性和活性炭改性锌负极的锌离子电池的循环伏安曲线[45]
Fig. 5 (a) Cycling performance of rechargeable zinc ion batteries with the unmodified ZnAB and ZnAB+AC (charge/discharge at 200 mA h/g); (b) CV curves of the unmodified ZnAB and ZnAB+12wt%AC at scanning rate of 0.1 mV/s[45]

图6 (a) 不同倍率下锌离子电池的放电曲线; (b) 6C倍率下锌离子电池的循环性能[9]
Fig. 6 (a) Discharge curves of the zinc ion battery at various rates and (b) cycle life performance of the zinc ion battery at a continuous cycling 6C/6C charge/discharge test[9]

图7 (a)锌离子电池第1圈(黑)、第2圈(红)的循环伏安曲线和循环性能曲线; (b)充放电不同阶段时锌负极的XRD图谱: 原电极(1), 半放电(2), 完全放电(3), 完全充电(4), 重新放电(5), 与图(a)中表示对应[68]
Fig. 7 Potential profiles of the zinc/α-MnO2 Zn-ion battery during the first (black line) and the second (red line) cycles, and their cycling performance up to 30 cycles (inset). (b) Ex-situ X-ray diffraction patterns of the electrodes at various charge and discharge stages: original electrode (1), half discharged electrode (2), fully discharged electrode (3), fully recharged electrode (4), and fully re-discharged electrode (5), as indicated in Figure (a)[68]
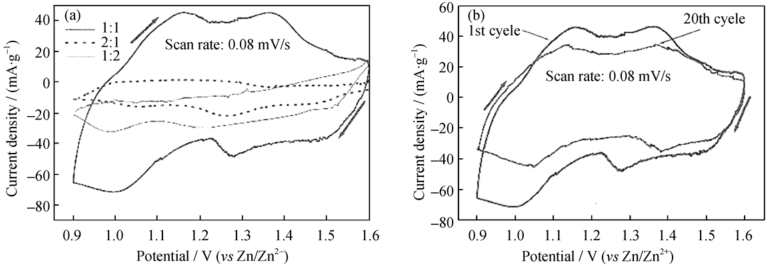
图9 (a)不同比例V2O5/C做电极时电池的循环伏安曲线; (b)V2O5/C做电极时电池循环第1圈和第20圈的循环伏安曲线[44]
Fig. 9 (a) Cycle voltammograms of composite electrodes with different mass ratios of V2O5/C and (b) cycle performance of composite electrodes[44]

图10 (a)电流密度为60 mA/g时电池的循环性能曲线; (b)不同倍率情况下电池的循环性能曲线[73]
Fig. 10 (a) Capacity versus number of cycles of the full cell at the current density of 60 mA/g ; (b) Capacity versus number of cycles of the full cell at different current densities[73]

图11 (a)使用HD-Zn和普通Zn片在5C倍率循环2圈后的循环伏安曲线和(b)5C倍率下HD-Zn和普通Zn片做负极时电池循环过程中的容量保持率[74]
Fig. 11 (a) Effect of HD Zn compared to Zn sheet as an anode on charge and discharge profiles of cycle 2 at 5C rate and (b) fractional capacity vs. cycle number compared for sheet Zn and HD Zn at 5C rate[74]

图12 (a) 在2 mV/s扫描速度下以0.5 mol/L的Na2SO4(1), 0.5 mol/L的K2SO4(2)和1 mol/L的ZnSO4(3)为电解液时锌离子电池的循环伏安曲线, (b) 不同倍率下锌离子电池的循环性能曲线[75]
Fig. 12 (a) CVs of ZnHCF in 0.5 mol/L Na2SO4 (1), 0.5 mol/ L K2SO4 (2), and 1 mol/L ZnSO4 (3) at scan rate of 2 mV/s ; (b) Cycle life tests at a rate of 1 C (square) and 5C (circle), 1C = 60 mA/g[75]
| Species | Diameter/nm | D/(×10-4, m2·s) | ΔE/eV | Storage capacity /(mA h·g-1) |
|---|---|---|---|---|
| Li+ | 0.069 | 13.8 | -5.006 | 63 |
| Na+ | 0.102 | 11.6 | -4.493 | 68 |
| K+ | 0.138 | 7.3 | -5.601 | 53 |
| Mg2+ | 0.066 | 22.8 | -7.282 | 97 |
| Ca2+ | 0.099 | 19.6 | -2.092 | 99 |
| Zn2+ | 0.074 | 8.6 | -5.540 | 220 |
| La3+ | 0.106 | 11.1 | -10.019 | 101 |
表1 单价离子和多价离子的电化学信息
Table 1 Detailed information of univalent and multivalent ions
| Species | Diameter/nm | D/(×10-4, m2·s) | ΔE/eV | Storage capacity /(mA h·g-1) |
|---|---|---|---|---|
| Li+ | 0.069 | 13.8 | -5.006 | 63 |
| Na+ | 0.102 | 11.6 | -4.493 | 68 |
| K+ | 0.138 | 7.3 | -5.601 | 53 |
| Mg2+ | 0.066 | 22.8 | -7.282 | 97 |
| Ca2+ | 0.099 | 19.6 | -2.092 | 99 |
| Zn2+ | 0.074 | 8.6 | -5.540 | 220 |
| La3+ | 0.106 | 11.1 | -10.019 | 101 |
| [1] | LUND H.Renewable energy strategies for sustainable development.Energy, 2007, 32(6): 912-919. |
| [2] | DINCER I, ROSEN M A, Exergy: energy, environment and sustainable development, Newnes, 2012. |
| [3] | ESPINOSA D C R, BERNARDES A M, TEN RIO J A S. An overview on the current processes for the recycling of batteries.Journal of Power Sources, 2004, 135(1): 311-319. |
| [4] | ALIAS N, MOHAMAD A A.Advances of aqueous rechargeable lithium-ion battery: a review.Journal of Power Sources, 2015, 274: 237-251. |
| [5] | OHMORI, SHIGEKAZU, YAMAMOTO, et al. Sodium Ion Battery. U.S. Patent Application.2010: 3-23. |
| [6] | SUO L, BORODIN O, GAO T, et al.“Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries.Science, 2015, 350(6263): 938-943. |
| [7] | YAN J, WANG J, LIU H, et al.Rechargeable hybrid aqueous batteries.Journal of Power Sources, 2012, 216: 222-226. |
| [8] | LUO J Y, CUI W J, HE P, et al.Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte.Nat. Chem., 2010, 2(9): 760-765. |
| [9] | XU C J, LI B, DU H, et al.Energetic zinc ion chemistry: the rechargeable zinc ion battery.Angew. Chem. Int. Ed. Engl., 2012, 51(4): 933-935. |
| [10] | [LEE B, LEE H R, KIM H, et al.Elucidating the intercalation mechanism of zinc ions into alpha-MnO2 for rechargeable zinc batteries.Chem. Commun.(Camb), 2015, 51(45): 9265-9268. |
| [11] | MCBREEN J.Nickel/zinc batteries.Journal of Power Sources, 1994, 51(1/2): 37-44. |
| [12] | LIU Y, YANG Z, XIE X, et al.Layered double oxides nano-flakes derived from layered double hydroxides: preparation, properties and application in zinc/nickel secondary batteries.Electrochimica Acta, 2015, 185: 190-197. |
| [13] | SMITH D F, GUCINSKI J A.Synthetic silver oxide and mercury-free zinc electrodes for silver-zinc reserve batteries.Journal of Power Sources, 1999, 80(1): 66-71. |
| [14] | MOJTAHEDI M, GOODARZI M, SHARIFI B, et al.Effect of electrolysis condition of zinc powder production on zinc-silver oxide battery operation.Energy Conversion And Management, 2011, 52(4): 1876-1880. |
| [15] | LIANG H W, WU Z Y, CHEN L F, et al.Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: an efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy, 2015, 11: 366-376. |
| [16] | LI Y, GONG M, LIANG Y, et al.Advanced zinc-air batteries based on high-performance hybrid electrocatalysts.Nature communications, 2013, 4: 1805. |
| [17] | R MAINAR A, LEONET O, BENGOECHEA M, et al. Alkaline aqueous electrolytes for secondary zinc-air batteries: an overview.International Journal of Energy Research, 2016. |
| [18] | KANG F Y, CHENG J X,LI B, et al. Rechargeable Zinc Ion Battery. U.S., Patent 8663844. 2014.03.04. |
| [19] | FORMANEK J, JANDOVA J, CAPEK J.Iron removal from zinc liquors originating from hydrometallurgical processing of spent Zn/MnO2 batteries.Hydrometallurgy, 2013, 138: 100-105. |
| [20] | 杨敬东. 二次锌离子电池的制备及其性能研究. 江苏: 南京工业大学硕士学位论文, 2013. |
| [21] | MCLARNON F R, CAIRNS E J.The secondary alkaline zinc electrode.Journal of The Electrochemical Society, 1991, 138(2): 645-656. |
| [22] | LEE J, JU J B, CHO W I, et al.Todorokite-type MnO2 as a zinc-ion intercalating material.Electrochimica Acta, 2013, 112: 138-143. |
| [23] | XU D, LI B, WEI C, et al.Preparation and characterization of MnO2/acid-treated CNT nanocomposites for energy storage with zinc ions.Electrochimica Acta, 2014, 133: 254-261. |
| [24] | ALFARUQI M H, GIM J, SONG J, et al. Electrochemical reaction mechanism in a high capacity zinc-ion battery system.The Electrochemical Society, 2015(3): 285. |
| [25] | TAFUR J P, ABAD J, ROM N E, et al.Charge storage mechanism of MnO2 cathodes in Zn/MnO2 batteries using ionic liquid-based gel polymer electrolytes.Electrochemistry Communications, 2015, 60: 190-194. |
| [26] | FENG F, GENG M, NORTHWOOD D.Electrochemical behaviour of intermetallic-based metal hydrides used in Ni/metal hydride (MH) batteries: a review.International Journal of Hydrogen Energy, 2001, 26(7): 725-734. |
| [27] | FETCENKO M, OVSHINSKY S, REICHMAN B, et al.Recent advances in NiMH battery technology.Journal of Power Sources, 2007, 165(2): 544-551. |
| [28] | LI M, LIU J, HAN W.Recycling and management of waste lead-acid batteries: a mini-review.Waste Management & Research, 2016: 0734242X16633773. |
| [29] | Y YAMAGUCHI, Lead Acid Batteries. Encyclopedia of Applied Electrochemistry. Springer New York, 2014: 1161-1165. |
| [30] | MAI L, WEI Q, TIAN X, et al.Electrochemical nanowire devices for energy storage.Nanotechnology, 2014, 13(1): 10-15. |
| [31] | AN Q, WEI Q, ZHANG P, et al.Three-dimensional interconnected vanadium pentoxide nanonetwork cathode for high-rate long-life lithium batteries.Small, 2015, 11(22): 2654-2660. |
| [32] | ZHAO Y, FENG J, LIU X, et al.Self-adaptive strain-relaxation optimization for high-energy lithium storage material through crumpling of graphene.Nat. Commun., 2014, 5: 4565. |
| [33] | ZHANG L, LIU Y, CHEN C, et al.Research on electrode materials for sodium-ion batteries.Chinese Journal of Inorganic Chemistry, 2015, 31(9): 1739-1750. |
| [34] | JUNG J W, LEE C L, YU S, et al.Electrospun nanofibers as a platform for advanced secondary batteries: a comprehensive review.J. Mater. Chem. A, 2016, 4(3): 703-750. |
| [35] | LUO W, SHEN F, BOMMIER C, et al.Na-ion battery anodes: materials and electrochemistry.Acc Chem Res, 2016, 49(2): 231-240. |
| [36] | BERTIN F C H, ESPINOSA D C R, TEN RIO J A S. 10.1 Nickel-Cadmium (NiCd) Batteries. Electronic Waste: Recycling Techniques, 2015: 129. |
| [37] | GALUSHKIN N, YAZVINSKAYA N, GALUSHKIN D.Ni-Cd batteries as hydrogen storage units of high-capacity.ECS Electrochemistry Letters, 2013, 2(1): A1-A2. |
| [38] | MAI L, TIAN X, XU X, et al.Nanowire electrodes for electrochemical energy storage devices.Chemical Reviews, 2014, 114(23): 11828-11862. |
| [39] | TAFUR J P, ABAD J, ROM N E, et al.Charge storage mechanism of MnO2 cathodes in Zn/MnO2 batteries using ionic liquid-based gel polymer electrolytes.Electrochemistry Communications, 2015, 60: 190-194. |
| [40] | 章小鸽, 仲海峰, 程东妹, 等. 锌的腐蚀与电化学, 冶金工业出版社, 2008: 3-6 441-443. |
| [41] | LASKA C A, AUINGER M, BIEDERMANN P U, et al.Effect of hydrogen carbonate and chloride on zinc corrosion investigated by a scanning flow cell system.Electrochimica Acta, 2015, 159: 198-209. |
| [42] | 李洪飞. 锌离子电池锌负极材料的制备及性能研究. 北京: 清华大学硕士学位论文, 2012. |
| [43] | ZHU X, DOAN T N L, YU Y, et al. Enhancing rate performance of LiMn2O4 cathode in rechargeable hybrid aqueous battery by hierarchical carbon nanotube/acetylene black conductive pathways.Ionics, 2015, 22(1): 71-76. |
| [44] | TAO B W, LIU J, LI S, et al.Electrochemical properties of V2O5/C composite in aqueous solution used for zinc secondary battery.Acta Physico-Chimica Sinica, 2005, 21(3): 338-342. |
| [45] | LI H, XU C, HAN C, et al.Enhancement on cycle performance of Zn anodes by activated carbon modification for neutral rechargeable zinc ion batteries.Journal of The Electrochemical Society, 2015, 162(8): A1439-A1444. |
| [46] | 屠振密, 李宁, 胡会利, 等.电沉积纳米晶材料技术. 国防工业出版社, 2008: 24-36. |
| [47] | LANG J, FU Q.Development of zinc electrodes for secondary akaline batteries.Marine Electric & Electronic Engineering, 2010, 30(7): 47-50. |
| [48] | 崔存仓. 锌-空气电池空气电极的制备及研究. 黑龙江: 哈尔滨工业大学硕士学位论文, 2013. |
| [49] | NEBURCHILOV V, WANG H, MARTIN J J, et al.A review on air cathodes for zinc-air fuel cells.Journal of Power Sources, 2010, 195(5): 1271-1291. |
| [50] | YANG H, CAO Y, AI X, et al.Improved discharge capacity and suppressed surface passivation of zinc anode in dilute alkaline solution using surfactant additives.Journal of Power Sources, 2004, 128(1): 97-101. |
| [51] | XU M, IVEY D, QU W, et al.Study of the mechanism for electrodeposition of dendrite-free zinc in an alkaline electrolyte modified with 1-ethyl-3-methylimidazolium dicyanamide.Journal of Power Sources, 2015, 274: 1249-1253. |
| [52] | 蒋巍. 空气电池锌电极的制备与研究. 河北: 河北工业大学硕士学位论文, 2005. |
| [53] | DEMIRKRAN N.Copper cementation with zinc recovered from spent zinc-carbon batteries and dissolution of cement copper in hydrochloric acid solutions.Industrial & Engineering Chemistry Research, 2013, 52(24): 8157-8166. |
| [54] | NINDHIA T G T, SURATA I W, SWASTIKA I D G P, et al. Reuse of carbon paste from used zinc-carbon battery for biogas desulfurizer with clay as a binder.International Journal of Environmental Science and Development, 2016, 7(3): 203-206. |
| [55] | XIA Y, ZHU D, SI S, et al.Nickel foam-supported polyaniline cathode prepared with electrophoresis for improvement of rechargeable Zn battery performance.Journal of Power Sources, 2015, 283: 125-131. |
| [56] | ZHOU J, XUE K, PAN G.AC impedance study of the aqueous Zn/V2O5 secondary battery.Acta Physico-Chimica Sinica, 2000, 16(5): 454-458. |
| [57] | GUISAO J P T, ROMERO A J F. Interaction between Zn2+ cations and n-methyl-2-pyrrolidone in ionic liquid-based gel polymer electrolytes for Zn batteries.Electrochimica Acta, 2015, 176: 1447-1453. |
| [58] | WANG K, GAO S, DU Z, et al.MnO2-Carbon nanotube composite for high-areal-density supercapacitors with high rate performance.Journal of Power Sources, 2016, 305: 30-36. |
| [59] | 魏春光. 二氧化锰的隧道调控和电化学离子存储性能研究. 北京: 清华大学博士学位论文, 2013. |
| [60] | INGALE N D, GALLAWAY J W, NYCE M, et al.Rechargeability and economic aspects of alkaline zinc-manganese dioxide cells for electrical storage and load leveling.Journal of Power Sources, 2015, 276: 7-18. |
| [61] | CHEN Y, XU C, SHI S.Manganese dioxides nanosheets/graphene as the zinc-ion intercalating materials for high capacity zinc ion battery. Contemporary Chemical Industry, 2015, 44(6): 1197-1199. |
| [62] | YIN Y, LIU C, FAN S.A new type of secondary hybrid battery showing excellent performances.Nano Energy, 2015, 12: 486-493. |
| [63] | ALFARUQI M H, MATHEW V, GIM J, et al.Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-Ion battery system.Chemistry of Materials, 2015, 27(10): 3609-3620. |
| [64] | STANI A, TAUCHER-MAUTNER W, KORDESCH K, et al.Development of flat plate rechargeable alkaline manganese dioxide-zinc cells.Journal of Power Sources, 2006, 153(2): 405-412. |
| [65] | CHENG F Y, CHEN J, GOU X L, et al.High-power alkaline zn-mno2 batteries using γ-MnO2 nanowires/nanotubes and electrolytic Zinc powder.Advanced Materials, 2005, 17(22): 2753-2756. |
| [66] | XU C, CHEN Y, SHI S, et al.Secondary batteries with multivalent ions for energy storage.Sci. Rep., 2015, 5: 14120. |
| [67] | XU C, CHIANG S W, MA J, et al.Investigation on zinc ion storage in alpha manganese dioxide for zinc ion battery by electrochemical impedance spectrum.Journal of the Electrochemical Society, 2013, 160(1): A93-A97. |
| [68] | LEE B, YOON C S, LEE H R, et al.Electrochemically-induced reversible transition from the tunneled to layered polymorphs of manganese dioxide.Sci Rep, 2014, 4: 6066. |
| [69] | JIA Z, WANG B, WANG Y. Copper hexacyanoferrate with a well-defined open framework as a positive electrode for aqueous zinc ion batteries. Materials Chemistry And Physics, 2015, 149- 150: 601-606. |
| [70] | ZHANG L, CHEN L, ZHOU X, et al.Morphology-dependent electrochemical performance of zinc hexacyanoferrate cathode for zinc-ion battery.Sci. Rep., 2015, 5: 18263. |
| [71] | PASTA M, WESSELLS C D, LIU N, et al.Full open-framework batteries for stationary energy storage.Nat. Commun., 2014, 5: 3007. |
| [72] | WESSELLS C D, HUGGINS R A, CUI Y.Copper hexacyanoferrate battery electrodes with long cycle life and high power.Nature communications, 2011, 2: 550. |
| [73] | TROCOLI R, LA MANTIA F.An aqueous zinc-ion battery based on copper hexacyanoferrate.ChemSusChem, 2015, 8(3): 481-485. |
| [74] | GUPTA T, KIM A, PHADKE S, et al.Improving the cycle life of a high-rate, high-potential aqueous dual-ion battery using hyper-dendritic zinc and copper hexacyanoferrate.Journal of Power Sources, 2016, 305: 22-29. |
| [75] | ZHANG L, CHEN L, ZHOU X, et al.Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system.Advanced Energy Materials, 2015, 5(2): 1614-6840. |
| [76] | PAN J, WEN Y, CHENG J, et al.Zinc deposition and dissolution in sulfuric acid onto a graphite-resin composite electrode as the negative electrode reactions in acidic zinc-based redox flow batteries.Journal of Applied Electrochemistry, 2013, 43(5): 541-551. |
| [77] | PADIGI P, THIEBES J, SWAN M, et al.Prussian green: a high rate capacity cathode for potassium ion batteries.Electrochimica Acta, 2015, 166: 32-39. |
| [78] | MULDOON J, BUCUR C B, GREGORY T.Quest for nonaqueous multivalent secondary batteries: magnesium and beyond.Chemical Reviews, 2014, 114(23): 11683-11720. |
| [79] | LIN M C, GONG M, LU B, et al.An ultrafast rechargeable aluminium-ion battery.Nature, 2015, 520(7547): 325-328. |
| [80] | PADIGI P, GONCHER G, EVANS D, et al.Potassium barium hexacyanoferrate -a potential cathode material for rechargeable calcium ion batteries.Journal of Power Sources, 2015, 273: 460-464. |
| [81] | KOMABA S, HASEGAWA T, DAHBI M, et al.Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors.Electrochemistry Communications, 2015, 60: 172-175. |
| [82] | XU C, WEI C, LI B, et al.Charge storage mechanism of manganese dioxide for capacitor application: effect of the mild electrolytes containing alkaline and alkaline-earth metal cations.Journal of Power Sources, 2011, 196(18): 7854-7859. |
| [83] | NAYAK P K, MUNICHANDRAIAH N.Reversible insertion of a trivalent cation onto MnO2 leading to enhanced capacitance.Journal of The Electrochemical Society, 2011, 158(5): A585-A591. |
| [1] | 魏相霞, 张晓飞, 徐凯龙, 陈张伟. 增材制造柔性压电材料的现状与展望[J]. 无机材料学报, 2024, 39(9): 965-978. |
| [2] | 杨鑫, 韩春秋, 曹玥晗, 贺桢, 周莹. 金属氧化物电催化硝酸盐还原合成氨研究进展[J]. 无机材料学报, 2024, 39(9): 979-991. |
| [3] | 刘鹏东, 王桢, 刘永锋, 温广武. 硅泥在锂离子电池中的应用研究进展[J]. 无机材料学报, 2024, 39(9): 992-1004. |
| [4] | 黄洁, 汪刘应, 王滨, 刘顾, 王伟超, 葛超群. 基于微纳结构设计的电磁性能调控研究进展[J]. 无机材料学报, 2024, 39(8): 853-870. |
| [5] | 陈乾, 苏海军, 姜浩, 申仲琳, 余明辉, 张卓. 超高温氧化物陶瓷激光增材制造及组织性能调控研究进展[J]. 无机材料学报, 2024, 39(7): 741-753. |
| [6] | 王伟明, 王为得, 粟毅, 马青松, 姚冬旭, 曾宇平. 以非氧化物为烧结助剂制备高导热氮化硅陶瓷的研究进展[J]. 无机材料学报, 2024, 39(6): 634-646. |
| [7] | 蔡飞燕, 倪德伟, 董绍明. 高熵碳化物超高温陶瓷的研究进展[J]. 无机材料学报, 2024, 39(6): 591-608. |
| [8] | 吴晓晨, 郑瑞晓, 李露, 马浩林, 赵培航, 马朝利. SiCf/SiC陶瓷基复合材料高温环境损伤原位监测研究进展[J]. 无机材料学报, 2024, 39(6): 609-622. |
| [9] | 赵日达, 汤素芳. 多孔碳陶瓷化改进反应熔渗法制备陶瓷基复合材料研究进展[J]. 无机材料学报, 2024, 39(6): 623-633. |
| [10] | 方光武, 谢浩元, 张华军, 高希光, 宋迎东. CMC-EBC损伤耦合机理及一体化设计研究进展[J]. 无机材料学报, 2024, 39(6): 647-661. |
| [11] | 张幸红, 王义铭, 程源, 董顺, 胡平. 超高温陶瓷复合材料研究进展[J]. 无机材料学报, 2024, 39(6): 571-590. |
| [12] | 张慧, 许志鹏, 朱从潭, 郭学益, 杨英. 大面积有机-无机杂化钙钛矿薄膜及其光伏应用研究进展[J]. 无机材料学报, 2024, 39(5): 457-466. |
| [13] | 李宗晓, 胡令祥, 王敬蕊, 诸葛飞. 氧化物神经元器件及其神经网络应用[J]. 无机材料学报, 2024, 39(4): 345-358. |
| [14] | 鲍可, 李西军. 化学气相沉积法制备智能窗用热致变色VO2薄膜的研究进展[J]. 无机材料学报, 2024, 39(3): 233-258. |
| [15] | 胡梦菲, 黄丽萍, 李贺, 张国军, 吴厚政. 锂/钠离子电池硬碳负极材料的研究进展[J]. 无机材料学报, 2024, 39(1): 32-44. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||