
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (3): 269-273.DOI: 10.15541/jim20150467
• RESEARCH PAPER • Previous Articles Next Articles
ZHANG Ya-Ping1, ZHANG An-Yu1, YU Lian-Qing1( ), DONG Kai-Tuo1, LI Yan2, HAO Lan-Zhong1
), DONG Kai-Tuo1, LI Yan2, HAO Lan-Zhong1
Received:2015-09-28
Revised:2015-12-08
Published:2016-03-20
Online:2016-02-24
Supported by:CLC Number:
ZHANG Ya-Ping, ZHANG An-Yu, YU Lian-Qing, DONG Kai-Tuo, LI Yan, HAO Lan-Zhong. Photoelectrochemical Properties of AgX(Cl, Br)-TiO2 Heterojunction Nanocomposites[J]. Journal of Inorganic Materials, 2016, 31(3): 269-273.
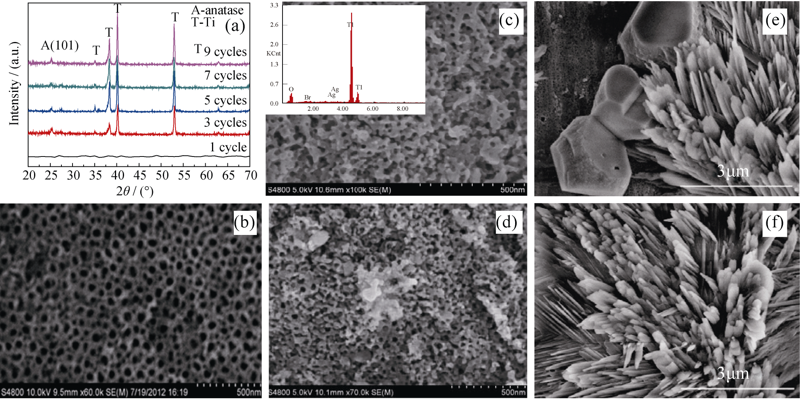
Fig. 1 AgX deposited on TiO2 nanotubes(a) XRD patterns (a) and SEM images of (b) pure TiO2, (c) AgBr after 1 cycle (d) AgBr after 3 cycles, (e) AgCl after 1 cycle and (f) AgCl after 5 cycles
| AgX | Cycles | Photovoltage /V | Photocurrent /(mA·cm-2) | Photoconversion rate/% |
|---|---|---|---|---|
| Blank | 0 | -0.440 | 0.260 | 0.95 |
| AgCl | 1 3 5 7 9 | -0.215 -0.296 -0.287 -0.302 -0.274 | 0.180 0.251 0.231 0.169 0.148 | 0.51 0.58 0.60 0.58 0.48 |
| AgBr | 1 3 5 7 9 | -0.186 -0.586 -0.280 -0.222 -0.400 | 0.258 0.238 0.215 0.268 0.151 | 2.67 0.46 0.58 0.62 0.29 |
Table1 Photoelectric properties of AgX-TiO2 composite
| AgX | Cycles | Photovoltage /V | Photocurrent /(mA·cm-2) | Photoconversion rate/% |
|---|---|---|---|---|
| Blank | 0 | -0.440 | 0.260 | 0.95 |
| AgCl | 1 3 5 7 9 | -0.215 -0.296 -0.287 -0.302 -0.274 | 0.180 0.251 0.231 0.169 0.148 | 0.51 0.58 0.60 0.58 0.48 |
| AgBr | 1 3 5 7 9 | -0.186 -0.586 -0.280 -0.222 -0.400 | 0.258 0.238 0.215 0.268 0.151 | 2.67 0.46 0.58 0.62 0.29 |
| [1] | LIU Z X, YU Y C, LIU J J, et al. AgBr/TiO2 heterojunction type composite catalyst preparation and photocatalytic activity of visible light. Chemical Research, 2010, 21(3): 1008-1011. |
| [2] | FUJISHIMA A, HONDA K.Electrochemical photolysis of water at a semiconductor electrode.Nature, 1972, 238(7): 37-38. |
| [3] | BOER K W.Survey of Semiconductor Physics. New York: Van Nostrand Reinhold, 1990: 249-253. |
| [4] | KIMMEL G A, BAER M, PETRIK N G, et al. Polarization and azimuth-resolved infrared spectroscopy of water on TiO2(110): anisotropy and the hydrogen-bonding network, Phys. Chem. Lett., 2012, 3(6): 778-784. |
| [5] | ZHANG J, ZHANG Y P, YU L Q, et al. Preparation of Ag-modified nano-titanium and their photocatalytic activity. Rare Metals, 2011, 30(1): 267-270. |
| [6] | YU L Q, WANG Q Q, LIU C G, et al. Magnetically stabilized bed for benzene selective hydrogenation. Chemical Engineering & Technology, 2014, 37(3): 1-7. |
| [7] | DONG K T, YU L Q, ZHANG Y P, et al. Green synthesis of sulfur/ graphene nanocomposite and photocatalytic performance. Science of Advanced Materials, 2014, 6(8): 1828-1835. |
| [8] | HU C, HU X X, WANG L S, et al. Visible-light-induced photocatalytic degradation of azodyes in aqueous AgI/TiO2 dispersion. Environ. Sci. Technol, 2006, 40(24): 7903-7907. |
| [9] | LAN Y Q, HU C, HU X X, et al. Efficient destruction of pathogenic bacteria with AgBr/TiO2 under visible light irradiation. Applied Catalysis B: Environmental, 2007, 73(3/4): 354-360. |
| [10] | MOHAMMAD R E, SARA R, SAEED H, MOHAMMAD R G.Apatite-coated Ag/AgBr/TiO2 visible light photocatalyst for destruction of bacteria. J.Am. Chem. Soc., 2007, 129(31): 9552-9553. |
| [11] | ZANG Y J, FARNOOD R.Photocatalytic activity of AgBr /TiO2 in water under simulated sunlight irradiation.Applied Catalysis B: Environmental, 2008, 79(4): 334-340. |
| [12] | WANG F, LIU Y, DONG W, et al. Tuning TiO2 photoelectrochemical properties by nanoring/nanotube combined structure. J. Phys. Chem. C, 2011, 115(30): 14635-14640. |
| [13] | TANG J W, DURRANT J R, KLUG D R.Mechanism of photocatalytic water splitting in TiO2 reaction of water with photoholes, importance of charge carrier dynamics, and evidence for four-hole chemistry. J. Am. Chem. Soc., 2008, 130(42): 13885-13891. |
| [14] | YU L Q, ZHANG Y P, ZHI Q Q, et al. Enhanced photoelectrochemical and sensing performance of novel TiO2 arrays to H2O2, Sensors and Actuator B: Chemical, 2015, 211: 111-115. |
| [15] | YU L Q, ZHI Q Q, ZHANG Y P, et al. Photocatalytic properties of TiO2 porous network film. Journal of Nanoscience and Nanotechnology, 2015, 15: 6576-6581. |
| [16] | YU L Q, DONG K T, ZHANG Y P, et al. Tuned n/n or n/p heterojunctions for reduced graphene oxide and titania nanosheets and their electrochemical properties. Materials Chemistry and Physics, 2014, 48(15): 803-809. |
| [17] | ZHANG Q Q, TANG B.Colloidal silver chloride preparation and photocatalytic antibacterial properties of antibacterial film.Journal of Nanjing university of Technology, 2004, 28(5): 547-551. |
| [18] | LI G P, LI J, LUO Y J, et al. AgBr nanoclusters: PAMAM dendrimer template was prepared and its photocatalytic performance. Journal of Inorganic Chemistry, 2007, 23(2): 253-257. |
| [19] | NIU J F, YAO B H, WEI J, et al. Preparation of Ag/AgCl supported TiO2 plasma photocatalysts and photocatalytic degradation of chloramphenicol Ag/AgCl. Journal of Xi'an University of Technology, 2011, 27(4): 77-81. |
| [20] | ZANG Y J, RAMIN F.Photocatalytic activity of AgBr/ TiO2 in water under simulated sunlight irradiation.Appl. Catal. B., 2008, 79(4): 334-340. |
| [21] | GAO L, ZHNG S, ZHANG Q H. Nano Titanium Oxide Photocatalytic Materials and Applications. Chemical Industry Press, 2003: 31-35. |
| [22] | ZHANG F, LI Q L, YANG J J.Study of TiO2 photocatalyst in the visible light. Chinese Journal of Catalysis, 1999, 20(3): 329-332. |
| [23] | WANG W J, HUANG B B, ZHANG X Y, et al. Highly efficient visible-light plasmonic photo catalys Ag&AgBr. Chem. Eur. J., 2009, 15(8): 1821-1824. |
| [24] | YU L Q, ZHANG Z P, ZHOU X Y, et al. Photoelectrochemical properties of Ag-TiO2 nanotube arrays. Journal of China University of Petroleum, 2015, 39(3): 183-187. |
| [25] | KHAN S U M, Al-Shahry M, Ingler Jr W B. Efficient photochemical water splitting by a chemically modified n-TiO2.Science, 2002, 297(5590): 2243-2245. |
| [26] | YU L Q, LIU R S, ZHANG Y P, et al. Photoelectrochemical property of Fe-N modified titania nanotube array films. Journal of Optoelectronics and Advanced Materials, 2014, 16(5/6): 519-523. |
| [27] | JOHN S E, MOHAPATRA S K, MISRA M.Double-wall anodic titania nanotube arrays for water photooxidation.Langmuir, 2009, 14(25): 8240-8247. |
| [1] | YANG Yan, ZHANG Faqiang, MA Mingsheng, WANG Yongzhe, OUYANG Qi, LIU Zhifu. Low Temperature Sintering of ZnAl2O4 Ceramics with CuO-TiO2-Nb2O5 Composite Oxide Sintering Aid [J]. Journal of Inorganic Materials, 2025, 40(6): 711-718. |
| [2] | MA Binbin, ZHONG Wanling, HAN Jian, CHEN Liangyu, SUN Jingjing, LEI Caixia. ZIF-8/TiO2 Composite Mesocrystals: Preparation and Photocatalytic Activity [J]. Journal of Inorganic Materials, 2024, 39(8): 937-944. |
| [3] | CHEN Haiyan, TANG Zhipeng, YIN Liangjun, ZHANG Linbo, XU Xin. Low-frequency Microwave Absorption of CIPs@Mn0.8Zn0.2Fe2O4-CNTs Composites [J]. Journal of Inorganic Materials, 2024, 39(1): 71-80. |
| [4] | TIAN Yubin, TIAN Chaofan, LI Sen, ZHAO Yongxin, XING Tao, LI Zhi, CHEN Xiaoru, XIANG Shuairong, DAI Pengcheng. Biomass-derived High-conductivity Carbon Cloth: Preparation and Application as Gas Diffusion Layers in Fuel Cells [J]. Journal of Inorganic Materials, 2023, 38(11): 1316-1322. |
| [5] | WANG Mengtao, SUO Jun, FANG Dong, YI Jianhong, LIU Yichun, Olim RUZIMURADOV. Visible-light Catalytic Performance of ITO/TiO2 Nanotube Array Composite [J]. Journal of Inorganic Materials, 2023, 38(11): 1292-1300. |
| [6] | JIA Xin, LI Jinyu, DING Shihao, SHEN Qianqian, JIA Husheng, XUE Jinbo. Synergy Effect of Pd Nanoparticles and Oxygen Vacancies for Enhancing TiO2 Photocatalytic CO2 Reduction [J]. Journal of Inorganic Materials, 2023, 38(11): 1301-1308. |
| [7] | AN Lin, WU Hao, HAN Xin, LI Yaogang, WANG Hongzhi, ZHANG Qinghong. Non-precious Metals Co5.47N/Nitrogen-doped rGO Co-catalyst Enhanced Photocatalytic Hydrogen Evolution Performance of TiO2 [J]. Journal of Inorganic Materials, 2022, 37(5): 534-540. |
| [8] | WU Qiuqin, YAO Fenfa, JIN Chuanhong, ZHENG Yifan. One-dimensional Sub-stoichiometric W3O8 Nanowires Filled Carbon Nanotubes [J]. Journal of Inorganic Materials, 2022, 37(4): 413-419. |
| [9] | LÜ Qingyang, ZHANG Yuting, GU Xuehong. Fabrication of Hollow Fiber Supported TiO2 Ultrafiltration Membranes via Ultrasound-assisted Sol-Gel Method [J]. Journal of Inorganic Materials, 2022, 37(10): 1051-1057. |
| [10] | LIU Fangfang, CHUAN Xiuyun, YANG Yang, LI Aijun. Influence of N/S Co-doping on Electrochemical Property of Brucite Template Carbon Nanotubes [J]. Journal of Inorganic Materials, 2021, 36(7): 711-717. |
| [11] | XIAO Xiang, GUO Shaoke, DING Cheng, ZHANG Zhijie, HUANG Hairui, XU Jiayue. CsPbBr3@TiO2 Core-shell Structure Nanocomposite as Water Stable and Efficient Visible-light-driven Photocatalyst [J]. Journal of Inorganic Materials, 2021, 36(5): 507-512. |
| [12] | XI Wen, LI Haibo. Preparation of TiO2/Ti3C2Tx Composite for Hybrid Capacitive Deionization [J]. Journal of Inorganic Materials, 2021, 36(3): 283-291. |
| [13] | LIU Cai, LIU Fang, HUANG Fang, WANG Xiaojuan. Preparation and Photocatalytic Properties of Alga-based CDs-Cu-TiO2 Composite Material [J]. Journal of Inorganic Materials, 2021, 36(11): 1154-1162. |
| [14] | Li Cuixia, SUN Huizhen, JIN Haize, SHI Xiao, LI Wensheng, KONG Wenhui. Construction and Photocatalytic Performance of 3D Hierarchical Pore rGO/TiO2 Composites [J]. Journal of Inorganic Materials, 2021, 36(10): 1039-1046. |
| [15] | WANG Ping,LI Xinyu,SHI Zhanling,LI Haitao. Synergistic Effect of Ag and Ag2O on Photocatalytic H2-evolution Performance of TiO2 [J]. Journal of Inorganic Materials, 2020, 35(7): 781-788. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||