
Journal of Inorganic Materials ›› 2015, Vol. 30 ›› Issue (7): 694-698.DOI: 10.15541/jim20140672
• Orginal Article • Previous Articles Next Articles
ZHENG Yi-Fan1,2, ZHONG Shu-Bin1, LV De-Yi1, ZHOU Huan2, HUAN Chang-Yong3
Received:2014-12-25
Revised:2015-03-13
Published:2015-07-20
Online:2015-06-25
About author:ZHENG Yi-Fan. E-mail: zhengyifan@zjut.edu.cn
Supported by:CLC Number:
ZHENG Yi-Fan, ZHONG Shu-Bin, LV De-Yi, ZHOU Huan, HUAN Chang-Yong. Effect of Calcination Temperature of Cu-Mn/γ-Al2O3 Catalysts on Performance for Catalytic Oxidation of Toluene[J]. Journal of Inorganic Materials, 2015, 30(7): 694-698.
| Catalysts | T50/℃ | T95/℃ | SSA/(m2•g-1) |
|---|---|---|---|
| Cu-Mn/γ-Al2O3-300 | 263 | 287 | 78 |
| Cu-Mn/γ-Al2O3-400 | 265 | 301 | 73 |
| Cu-Mn/γ-Al2O3-500 | 277 | 324 | 75 |
| Cu-Mn/γ-Al2O3-550 | 276 | 286 | 70 |
| Cu-Mn/γ-Al2O3-600 | 282 | 369 | 57 |
Table 1 T50, T95 and specific surface areas (SSA) for Cu-Mn/γ-Al2O3 catalysts calcined at various temperatures
| Catalysts | T50/℃ | T95/℃ | SSA/(m2•g-1) |
|---|---|---|---|
| Cu-Mn/γ-Al2O3-300 | 263 | 287 | 78 |
| Cu-Mn/γ-Al2O3-400 | 265 | 301 | 73 |
| Cu-Mn/γ-Al2O3-500 | 277 | 324 | 75 |
| Cu-Mn/γ-Al2O3-550 | 276 | 286 | 70 |
| Cu-Mn/γ-Al2O3-600 | 282 | 369 | 57 |
| Calcination temperature/℃ | Crystalline phases | Crystalline phases | |
|---|---|---|---|
| CuO | Cu1.4Mn1.6O4 | ||
| 300 | CuO, Cu0.451Mn0.549O2 , γ-Al2O3 | 18.5 | - |
| 400 | CuO, Cu0.451Mn0.549O2, γ-Al2O3 | 20.0 | - |
| 500 | CuO, Cu0.451Mn0.549O2, γ-Al2O3 | 21.0 | - |
| 550 | CuO, Cu1.4Mn1.6O4 , γ-Al2O3 | 23.0 | 20 |
| 600 | CuO, Cu1.4Mn1.6O4 , γ-Al2O3 | 26.0 | 25 |
Table 2 Crystalline phases and crystalline size of Cu-Mn/γ-Al2O3 catalysts calcined at various temperatures
| Calcination temperature/℃ | Crystalline phases | Crystalline phases | |
|---|---|---|---|
| CuO | Cu1.4Mn1.6O4 | ||
| 300 | CuO, Cu0.451Mn0.549O2 , γ-Al2O3 | 18.5 | - |
| 400 | CuO, Cu0.451Mn0.549O2, γ-Al2O3 | 20.0 | - |
| 500 | CuO, Cu0.451Mn0.549O2, γ-Al2O3 | 21.0 | - |
| 550 | CuO, Cu1.4Mn1.6O4 , γ-Al2O3 | 23.0 | 20 |
| 600 | CuO, Cu1.4Mn1.6O4 , γ-Al2O3 | 26.0 | 25 |
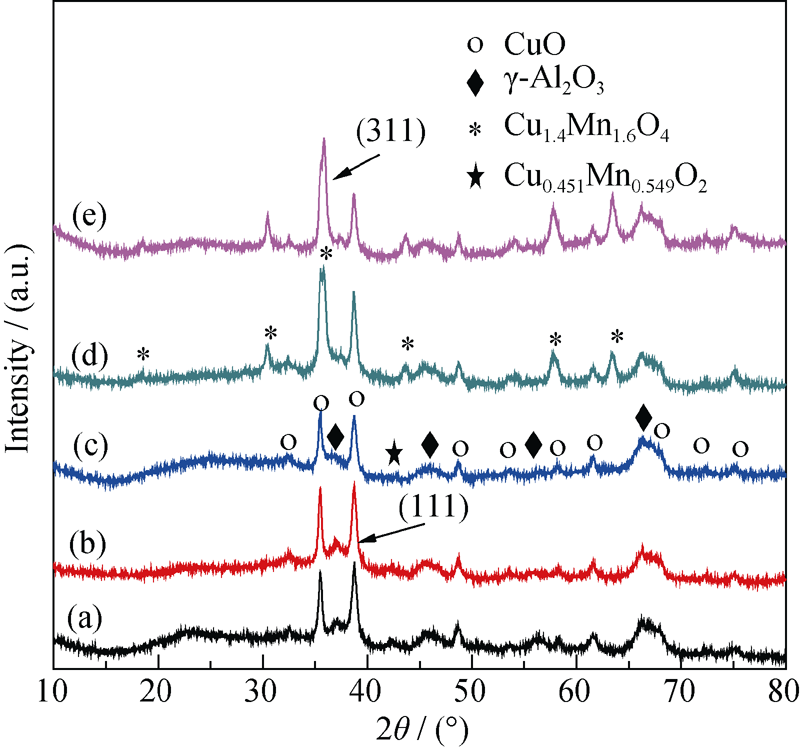
Fig. 3 XRD patterns of Cu-Mn/γ-Al2O3 catalysts calcined at various temperatures (a) Cu-Mn/γ-Al2O3-300; (b) Cu-Mn/γ-Al2O3-400; (c) Cu-Mn/γ-Al2O3-500; (d) Cu-Mn/γ-Al2O3-550; (e) Cu-Mn/γ-Al2O3-600
| Catalysts | Binding energy/eV | Cu +/(Cu ++Cu 2+) | Mn4 +/(Mn3++Mn4 +) | |||
|---|---|---|---|---|---|---|
| Cu + | Cu 2+ | Mn3 + | Mn4+ | |||
| Cu-Mn/γ-Al2O3-300 | - | 933.8 | 641.2 | 642.5 | - | 0.85 |
| Cu-Mn/γ-Al2O3-400 | 931.2 | 933.4 | 641.1 | 642.4 | 0.06 | 0.82 |
| Cu-Mn/γ-Al2O3-500 | 931.1 | 933.4 | 641.0 | 642.3 | 0.07 | 0.80 |
| Cu-Mn/γ-Al2O3-550 | 931.4 | 934.1 | 641.0 | 642.5 | 0.12 | 0.86 |
| Cu-Mn/γ-Al2O3-600 | 931.4 | 934.1 | 641.1 | 642.5 | 0.11 | 0.81 |
Table 3 Binding energy of Cu2p and Mn2p and proportion of Cu +and Mn4+ in all Cu and Mn of Cu-Mn/γ-Al2O3 catalysts calcined at various temperatures
| Catalysts | Binding energy/eV | Cu +/(Cu ++Cu 2+) | Mn4 +/(Mn3++Mn4 +) | |||
|---|---|---|---|---|---|---|
| Cu + | Cu 2+ | Mn3 + | Mn4+ | |||
| Cu-Mn/γ-Al2O3-300 | - | 933.8 | 641.2 | 642.5 | - | 0.85 |
| Cu-Mn/γ-Al2O3-400 | 931.2 | 933.4 | 641.1 | 642.4 | 0.06 | 0.82 |
| Cu-Mn/γ-Al2O3-500 | 931.1 | 933.4 | 641.0 | 642.3 | 0.07 | 0.80 |
| Cu-Mn/γ-Al2O3-550 | 931.4 | 934.1 | 641.0 | 642.5 | 0.12 | 0.86 |
| Cu-Mn/γ-Al2O3-600 | 931.4 | 934.1 | 641.1 | 642.5 | 0.11 | 0.81 |
| [1] | LAMB ARTHUR B, BRAY WILLIAM C, FRAZER J C W. The removal of carbon monoxide from air.Journal of Industrial and Engineering Chemistry, 1920, 12(3): 213-221. |
| [2] | AGUERO FABIOLA N, BARBERO BIBIANA P, GAMBARO LUIS, et al.Catalytic combustion of volatile organic compounds in binary mixtures over MnOx/Al2O3 catalyst.Applied Catalysis B: Environmental, 2009, 91(1/2): 108-112. |
| [3] | JONES CHRISTOPHER, COLE KIERAN J, TAYLOR STUART H, et al.Copper manganese oxide catalysts for ambient temperature carbon monoxide oxidation: Effect of calcination on activity.Journal of Molecular Catalysis A: Chemical, 2009, 305(1/2):121-124. |
| [4] | DOGGALI PRADEEP, TERAOKA Y, MUNGSE P, et al.Combustion of volatile organic compounds over Cu-Mn based mixed oxide type catalysts supported on mesoporous Al2O3, TiO2 and ZrO2.Journal of Molecular Catalysis A:Chemical, 2012, 358: 23-30. |
| [5] | ROXANA MORALES MARIA, BARBERO BIBIANA P, LOPEZ TESSY, et al.Evaluation and characterization of Mn-Cu mixed oxide catalysts supported on TiO2 and ZrO2 for ethanol total oxidation.Fuel, 2009, 88(11): 2122-2129. |
| [6] | MORALES MARIA ROXANA, BARBERO BIBIANA P, CADUS LUIS E.Evaluation and characterization of Mn-Cu mixed oxide catalysts for ethanol total oxidation:Influence of copper content.Fuel, 2008, 87(7): 1177-1186. |
| [7] | CAO HONGYAN, LI XIAOSHUANG, CHEN YAOQIANG, et al.Effect of loading content of copper oxides on performance of Mn-Cu mixed oxide catalysts for catalytic combustion of benzene.Journal of Rare Earths, 2012, 30(9): 871-877. |
| [8] | BEHAR SIHAM, GONZALEZ PHILIPPE, AGULHON PIERRE, et al.New synthesis of nanosized Cu-Mn spinels as efficient oxidation catalysts.Catalysis Today, 2012, 189(1): 35-41. |
| [9] | LI W B, ZHUANG M, WANG J X.Catalytic combustion of toluene on Cu-Mn/MCM-41 catalysts:Influence of calcination temperature and operating conditions on the catalytic activity.Catalysis Today, 2008, 137(2/3/4): 340-344. |
| [10] | VU VAN HINH, BELKOUCH JARNAL, OULD-DRIS AIESSA, et al.Catalytic oxidation of volatile organic compounds on manganese and copper oxides supported on titania.American Institute of Chemical Engineers Journal, 2008, 54(6): 1585-1591. |
| [11] | CHEN HONG, WANG JIHUI, LI HE, et al. Low temperature combustion of ethylene in a carbon dioxide stream over a cordierite monolith-supported Cu-Mn Hopcalite catalyst. Applied Catalysis A:General, 2012, 427-428: 73-78. |
| [12] | VEPREK S, COCKE D L, KEHL S, et al.Mechanism of the deactivation of hopcalite catalysts studied by XPS, ISS and other techniques.Journal of Catalysis, 1986, 100: 250-263. |
| [13] | OKU MASAOKI, HIROKAWA KICHINOSUKE.X-ray photoelectron spectroscopy of Co3O4, Fe3O4, Mn3O4 and related compounds.Journal of Electron Spectroscopy and Related Phenomena, 1976, 8: 475-481. |
| [14] | MIRZAEI A A, SHATERIAN H R, KAYKHAII M.The X-ray photoelectron spectroscopy of surface composition of aged mixed copper manganese oxide catalysts.Applied Surface Science, 2005, 239(2): 246-254. |
| [15] | TANG QINGHU, GONG XIAONAN, ZHAO PEIZHENG, et al.Copper-manganese oxide catalysts supported on alumina:Physicochemical features and catalytic performances in the aerobic oxidation of benzyl alcohol.Applied Catalysis A:General, 2010, 389(1/2): 101-107. |
| [16] | CHEN HONG, TONG XINLI, LI YONGDAN.Mesoporous Cu-Mn Hopcalite catalyst and its performance in low temperature ethylene combustion in a carbon dioxide stream.Applied Catalysis A: General, 2009, 370(1/2): 59-65. |
| [17] | MORALES M R, BARBERO B P, CADUS L E.Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts.Applied Catalysis B: Environmental, 2006, 67(3/4): 229-236. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | SUN Chen, ZHAO Kunfeng, YI Zhiguo. Research Progress in Catalytic Total Oxidation of Methane [J]. Journal of Inorganic Materials, 2023, 38(11): 1245-1256. |
| [3] | HUANG Xieyi,WANG Peng,YIN Guoheng,ZHANG Shaoning,ZHAO Wei,WANG Dong,BI Qingyuan,HUANG Fuqiang. Removal of Volatile Organic Compounds Driven by Platinum Supported on Amorphous Phosphated Titanium Oxide [J]. Journal of Inorganic Materials, 2020, 35(4): 482-490. |
| [4] | LI Si-Han, ZHANG Chao, WU Chen-Liang, ZHANG He-Feng, YAN Xin-Huan. Pd/CeO2/γ-Al2O3 Catalyst with Low Loading for Catalytic Oxidation of VOCs [J]. Journal of Inorganic Materials, 2019, 34(8): 827-833. |
| [5] | GUO Rui-hua, MO Yi-Jie, AN Sheng-Li, ZHANG Jie-Yu, ZHOU Guo-Zhi. Cerium Oxide Hollow Sphere: Controllable Synthesis and Its Effect on Electrocatalytic Performance of Pt-based Catalysts [J]. Journal of Inorganic Materials, 2018, 33(7): 779-786. |
| [6] | YAN Shi-Sheng, PENG Hong-Yan, ZHAO Zhi-Bin, PAN Meng-Mei, YANG Da-Li, A Jin-Hua, YE Guo-Lin, WANG Chong-Tai, GUO Xin-Wei. Nitrogen-doped Diamond Electrode Property and Anodic Catalytic Degradation of Nitrobenzene [J]. Journal of Inorganic Materials, 2018, 33(5): 565-569. |
| [7] | ZHAI Chun-Yang, SUN Ming-Juan, DU Yu-Kou, ZHU Ming-Shan. Noble Metal/Semiconductor Photoactivated Electrodes for Direct Methanol Fuel Cel [J]. Journal of Inorganic Materials, 2017, 32(9): 897-903. |
| [8] | LI Min, LUO Yuan, XU Wei-Jia, LIU Jia-Xiang. DMFC Anode Catalyst Fe3O4@Pt Particles: Synthesis and Catalytic Performanc [J]. Journal of Inorganic Materials, 2017, 32(9): 916-922. |
| [9] | YU Zheng-Fa, WANG Xu-Zhen, HOU Ya-Nan, ZHAO Zong-Bin, Rui Li, QIU Jie-Shan. Facile Preparation of Nitrogen-doped Porous Carbons via Salt Melt Synthesis with Efficient Catalytic Desulfurization Performance [J]. Journal of Inorganic Materials, 2017, 32(7): 770-776. |
| [10] | ZHANG Yi, ZHANG Xiao-Feng, He Xiao-Lei, HUANG Huo-Di, LE Li-Juan, LIN Shen. Preparation and Electrocatalytic Property of Pt/{GN/CuPW11}n Composite Films toward Methanol Oxidation [J]. Journal of Inorganic Materials, 2017, 32(10): 1075-1082. |
| [11] | JIN Zhao, CHEN Qi-Han, ZHENG Meng-Jia, ZHAO Peng, LI Qian, CUI Xiao-Qiang. Fabrication of Au Nanoparticles / Bilayer TiO2 Nanotube Periodical Structure and Electrocatalytic Oxidation of Ethanol [J]. Journal of Inorganic Materials, 2016, 31(3): 241-247. |
| [12] | MO Wen-Long, MA Feng-Yun, LIU Yue-E, LIU Jing-Mei, ZHONG Mei, Aisha· Nulahong. Influence of Calcination Temperature on Performance of NiO/γ-Al2O3 Catalyst for CO2-CH4 Reforming to Produce Syngas [J]. Journal of Inorganic Materials, 2016, 31(3): 234-240. |
| [13] | LIU Yang-Long, ZHENG Yu-Ying, SHANG Peng-Bo. Preparation, Characterization and Photocatalytic Property of Eu-doped TiO2 Hollow Microspheres [J]. Journal of Inorganic Materials, 2015, 30(7): 699-705. |
| [14] | HUA Cheng-Jiang, WANG Ming-Hui, LUAN Guo-You, LIU Yan, WU Hua. Rapid in situ Crystallization and Catalytic Performance of Cu3(BTC)2-based Film on Copper Mesh [J]. Journal of Inorganic Materials, 2015, 30(5): 529-534. |
| [15] | HUANG Xiao-Ling, LIN Zhou, LIAN Xing-Yi, ZHANG Xiao-Feng, LIN Shen. Preparation and Electrocatalytic Properties of Pd/PMo12-GN Composite towards Formic Acid Oxidation [J]. Journal of Inorganic Materials, 2014, 29(7): 722-728. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||