
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (10): 1093-1101.DOI: 10.15541/jim20220027
• RESEARCH ARTICLE • Previous Articles Next Articles
LI Chengjin1,2( ), XUE Yi1,2, ZHOU Xiaoxia2(
), XUE Yi1,2, ZHOU Xiaoxia2( ), CHEN Hangrong2
), CHEN Hangrong2
Received:2022-01-17
Revised:2022-04-18
Published:2022-10-20
Online:2022-04-26
Contact:
ZHOU Xiaoxia, associate professor. E-mail: zhouxiaoxia@mail.sic.ac.cnAbout author:LI Chengjin (1995-), male, Master candidate. E-mail: ChengjinLL@163.com
Supported by:CLC Number:
LI Chengjin, XUE Yi, ZHOU Xiaoxia, CHEN Hangrong. BiZnx/Si Photocathode: Preparation and CO2 Reduction Performance[J]. Journal of Inorganic Materials, 2022, 37(10): 1093-1101.
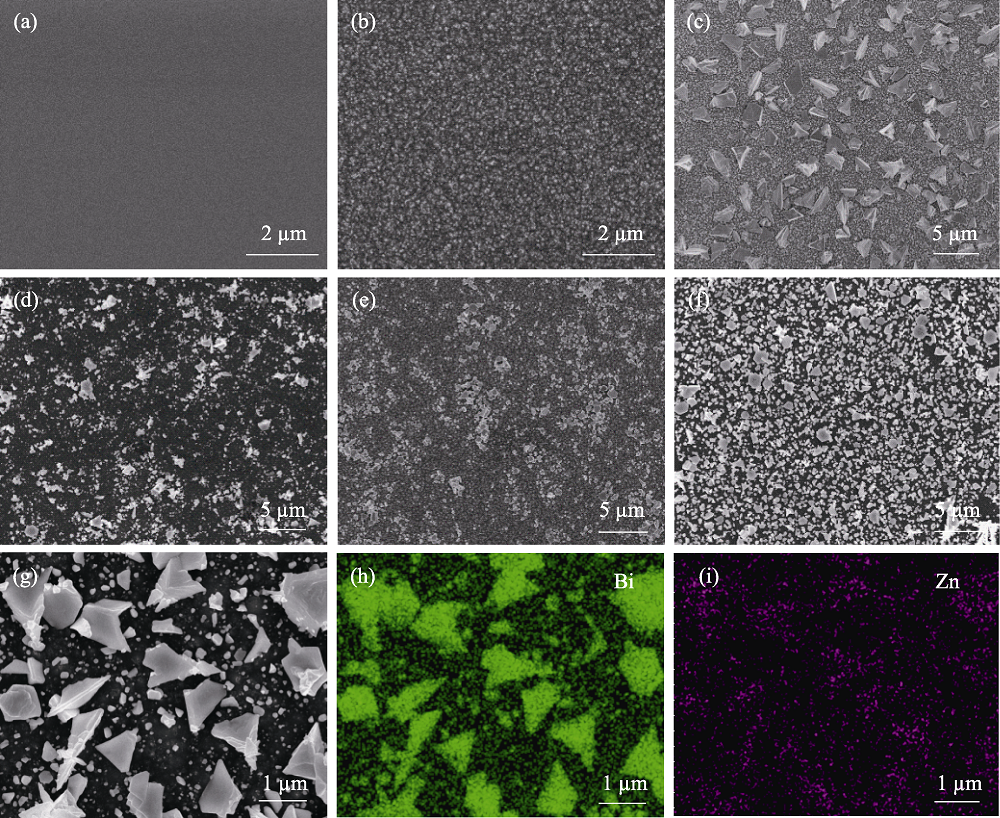
Fig. 3 SEM images of (a) planar-Si, (b) Si treated with NaOH solution, (c) Bi/Si, (d) BiZn1/Si, (e) BiZn2/Si, and (f) BiZn3/Si with (g-i) elemental mappings of BiZn2/Si
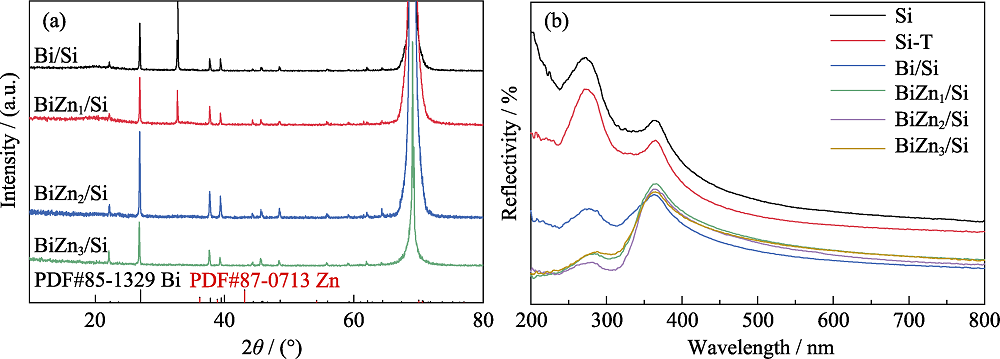
Fig. 4 (a) XRD patterns of Bi/Si, BiZn1/Si, BiZn2/Si, and BiZn3/Si, and (b) UV-Vis reflectivity spectra of Planar-Si, Si-T, Bi/Si, BiZn1/Si, BiZn2/Si and BiZn3/Si Colorful figures are available on website
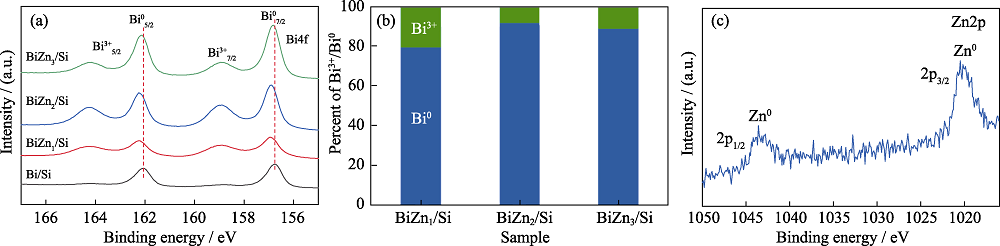
Fig. 5 (a) High resolution Bi4f XPS spectra of Bi/Si, BiZn1/Si, BiZn2/Si and BiZn3/Si, (b) ratios of Bi3+/Bi0 for BiZn1/Si, BiZn2/Si, and BiZn3/Si, and (c) high resolution Zn2p XPS spectrum for BiZn2/Si Colorful figures are available on website
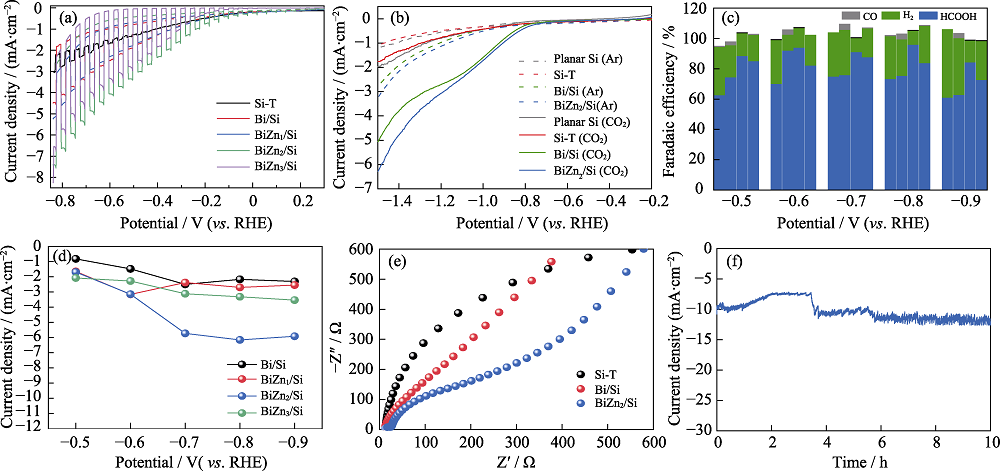
Fig. 6 (a) Transient photocurrent curves of Si-T, Bi/Si, BiZn1/Si, BiZn2/Si, and BiZn3/Si, (b) LSV curves of untreated Si (Planar Si), Si-T, Bi/Si, and BiZn2/Si in Ar or CO2-saturated 0.5 mol·L-1 KHCO3 aqueous solution, (c) FE histograms of CO2 reduction productions and (d) HCOOH current densities for Bi/Si, BiZn1/Si, BiZn2/Si, and BiZn3/Si, (e) electrochemical impedance spectra (EIS) of Si-T, Bi/Si and BiZn2/Si, and (f) stability of BiZn2/Si during 10 h test Colorful figures are available on website
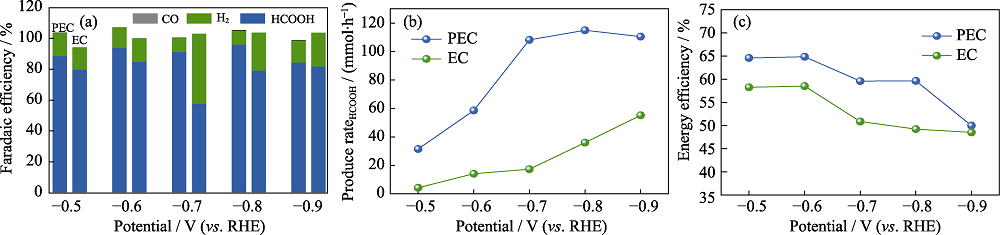
Fig. 7 PEC and EC CO2 reduction performances of BiZn2/Si (a) Faradaic efficiency of CO2 reduction products; (b) HCOOH production rates; (c) EE. Colorful figures are available on website
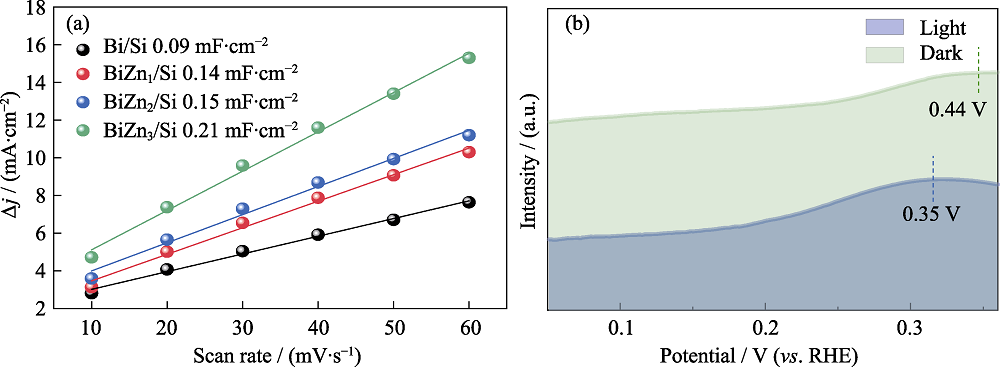
Fig. 8 (a) ECSA lines of Bi/Si, BiZn1/Si, BiZn2/Si, and BiZn3/Si, and (b) oxidation LSV curves of PEC and EC CO2 reduction for BiZn2/Si in 0.1 mol·L-1 KOH aqueous solution
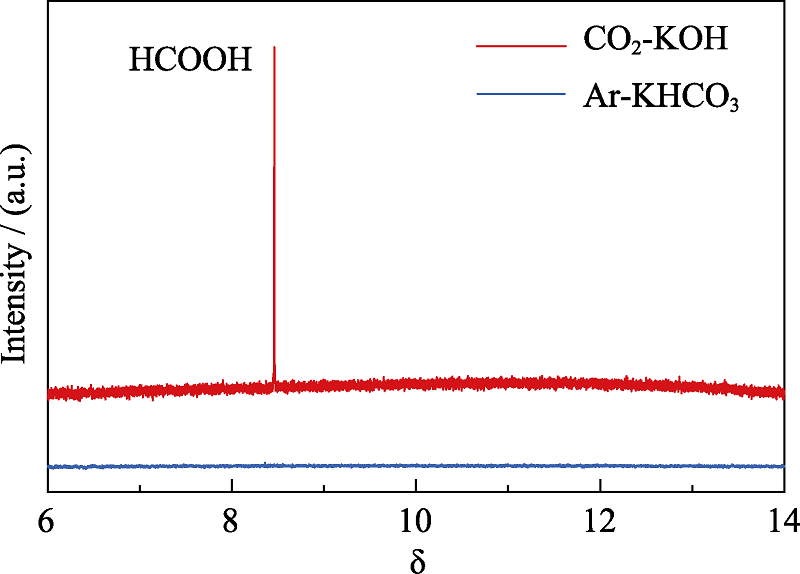
Fig. S6 1H NMR spectrum of the liquid phase products of BiZn2/Si in CO2-saturated 0.5 mol·L-1 KOH and Ar-saturated 0.5 mol·L-1 KHCO3 solutions for photoelechemical reduction of CO2
| Sample | BiZn1/Si | BiZn2/Si | BiZn3/Si |
|---|---|---|---|
| (n)Bi/(n)Zn | 142 | 120 | 88 |
Table S1 Bi/Zn molar ratios of different samples in ICP results
| Sample | BiZn1/Si | BiZn2/Si | BiZn3/Si |
|---|---|---|---|
| (n)Bi/(n)Zn | 142 | 120 | 88 |
| Electrode | Electrolyte | Eapp/V | Jtotal/(mA·cm-2) | FEformate | Ref. |
|---|---|---|---|---|---|
| Sn/SnOx | 0.5 mol·L-1 KHCO3 | -0.7 vs. RHE | -2 | ~38% PEC | [ |
| SnO2 | 0.5 mol·L-1 NaOH | -0.6 vs. RHE | -3.5 | 67.6% EC | [ |
| Sn foil | 0.5 mol·L-1 KHCO3 | -2.0 vs. SCE | -28 | 63.5% EC | [ |
| Sn dendrite | 0.1 mol·L-1 KHCO3 | -1.36 vs. RHE | -17.1 | 71.6% EC | [ |
| Sn GDE | 0.5 mol·L-1 KHCO3 | -1. 8 vs. SCE | -22.2 | 78.6% EC | [ |
| 2,2’-bpy-coordinated Cu | 0.5 mol·L-1 KHCO3 | -1.2 vs. RHE | -15 | 57.7% PEC | [ |
| Si/Bi5 | 0.5 mol·L-1 KHCO3 | -1.03 vs. RHE | -24.1 | 72.1% PEC | [ |
| Bi-PMo nanosheets | 0.5 mol·L-1 NaHCO3 | -0.86 vs. RHE | -30 | 93% EC | [ |
| Bi2O3 nanoparticle | 0.5 mol·L-1 NaHCO3 | -1.2 vs. RHE | -22 | 91% EC | [ |
| p-Si/Bi | 0.5 mol·L-1 NaHCO3 | -0.9 vs. RHE | -12 | 90% PEC | [ |
| Bi nanoflakes | 0.1 mol·L-1 KHCO3 | -0.4 vs. RHE | - | 79.5% EC | [ |
| Cu25In75 | 0.5 mol·L-1 NaHCO3 | -0.7 vs. RHE | - | 84.1% EC | [ |
| In1.5Cu0.5 NPs | 0.1 mol·L-1 KHCO3 | -1.2 vs. RHE | -3.59 | 90% EC | [ |
| This work | 0.5 mol·L-1 KHCO3 | -0.8 vs. RHE | -6.45 | 96.1% PEC | This work |
Table S2 Faraday efficiency and current density comparison of different photo/electrocatalysts
| Electrode | Electrolyte | Eapp/V | Jtotal/(mA·cm-2) | FEformate | Ref. |
|---|---|---|---|---|---|
| Sn/SnOx | 0.5 mol·L-1 KHCO3 | -0.7 vs. RHE | -2 | ~38% PEC | [ |
| SnO2 | 0.5 mol·L-1 NaOH | -0.6 vs. RHE | -3.5 | 67.6% EC | [ |
| Sn foil | 0.5 mol·L-1 KHCO3 | -2.0 vs. SCE | -28 | 63.5% EC | [ |
| Sn dendrite | 0.1 mol·L-1 KHCO3 | -1.36 vs. RHE | -17.1 | 71.6% EC | [ |
| Sn GDE | 0.5 mol·L-1 KHCO3 | -1. 8 vs. SCE | -22.2 | 78.6% EC | [ |
| 2,2’-bpy-coordinated Cu | 0.5 mol·L-1 KHCO3 | -1.2 vs. RHE | -15 | 57.7% PEC | [ |
| Si/Bi5 | 0.5 mol·L-1 KHCO3 | -1.03 vs. RHE | -24.1 | 72.1% PEC | [ |
| Bi-PMo nanosheets | 0.5 mol·L-1 NaHCO3 | -0.86 vs. RHE | -30 | 93% EC | [ |
| Bi2O3 nanoparticle | 0.5 mol·L-1 NaHCO3 | -1.2 vs. RHE | -22 | 91% EC | [ |
| p-Si/Bi | 0.5 mol·L-1 NaHCO3 | -0.9 vs. RHE | -12 | 90% PEC | [ |
| Bi nanoflakes | 0.1 mol·L-1 KHCO3 | -0.4 vs. RHE | - | 79.5% EC | [ |
| Cu25In75 | 0.5 mol·L-1 NaHCO3 | -0.7 vs. RHE | - | 84.1% EC | [ |
| In1.5Cu0.5 NPs | 0.1 mol·L-1 KHCO3 | -1.2 vs. RHE | -3.59 | 90% EC | [ |
| This work | 0.5 mol·L-1 KHCO3 | -0.8 vs. RHE | -6.45 | 96.1% PEC | This work |
| [1] |
ZHU D, LIU J, QIAO S. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Advanced Materials, 2016, 28(18): 3423-3452.
DOI URL |
| [2] |
COSTENTIN C, ROBERT M, SAVEANT J. Catalysis of the electrochemical reduction of carbon dioxide. Chemical Society Reviews, 2013, 42(6): 2423-2436.
DOI PMID |
| [3] |
QIAO J, LIU Y, HONG F, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chemical Society Reviews, 2014, 43(2): 631-675.
DOI PMID |
| [4] | LIANG Z, SHEN R, NG Y, et al. A review on 2D MoS2 cocatalysts in photocatalytic H2 production. Journal of Materials Science & Technology, 2020, 56: 89-121. |
| [5] |
LI X, WEN J, LOW J, et al. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Science China Materials, 2014, 57: 70-100.
DOI URL |
| [6] |
SHEN R, REN D, DING Y, et al. Nanostructured CdS for efficient photocatalytic H2 evolution: a review. Science China Materials, 2020, 63(11): 2153-2188.
DOI URL |
| [7] |
LI X, YU J, JARONIEC M, et al. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chemical Reviews, 2019, 119(6): 3962-4179.
DOI URL |
| [8] |
JOUNY M, LUC W, JIAO F. General techno-economic analysis of CO2 electrolysis systems. Industrial & Engineering Chemistry Research, 2018, 57(6): 2165-2177.
DOI URL |
| [9] |
SPONHOLZ P, MELLMANN D, JUNGE H, et al. Towards a practical setup for hydrogen production from formic acid. ChemSusChem, 2013, 6(7): 1172-1176.
DOI PMID |
| [10] |
AGARWAL A, ZHAI Y, HILL D, et al. The electrochemical reduction of carbon dioxide to formate/formic acid: engineering and economic feasibility. ChemSusChem, 2011, 4(9): 1301-1310.
DOI PMID |
| [11] |
HORI Y, WAKEBE H, TSUKAMOTO T, et al. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochimica Acta, 1994, 39(11): 1833-1839.
DOI URL |
| [12] |
KIM S, DONG W, GIM S, et al. Shape-controlled bismuth nanoflakes as highly selective catalysts for electrochemical carbon dioxide reduction to formate. Nano Energy, 2017, 39: 44-52.
DOI URL |
| [13] |
HAN N, WANG Y, YANG H, et al. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate. Nature Communications, 2018, 9(1): 1320.
DOI URL |
| [14] |
HE S, NI F, WANG L, et al. The p-orbital delocalization of main-group metals to boost CO2 electroreduction. Angewandte Chemie International Edition, 2018, 57(49): 16114-16119.
DOI URL |
| [15] |
MIAO C, YUAN G. Morphology-controlled Bi2O3 nanoparticles as catalysts for selective electrochemical reduction of CO2 to formate. ChemElectroChem, 2018, 5(23): 3741-3747.
DOI URL |
| [16] |
YANG H, HAN N, DENG J, et al. Selective CO2 reduction on 2D mesoporous Bi nanosheets. Advanced Energy Materials, 2018, 8(35): 1801536.
DOI URL |
| [17] |
GUO S, ZHANG Y, ZHANG X, et al. Phosphomolybdic acid-assisted growth of ultrathin bismuth nanosheets for enhanced electrocatalytic reduction of CO2 to formate. ChemSusChem, 2019, 12(5): 1091-1100.
DOI URL |
| [18] |
LU P, GAO D, HE H, et al. Facile synthesis of a bismuth nanostructure with enhanced selectivity for electrochemical conversion of CO2 to formate. Nanoscale, 2019, 11(16): 7805-7812.
DOI URL |
| [19] |
BHUPENDRA K, MARK L, JESSE F, et al. Photochemical and photoelectrochemical reduction of CO2. The Annual Review of Physical Chemistry, 2012, 63(1): 541-569.
DOI URL |
| [20] |
SUN K, SHEN S, LIANG Y, et al. Enabling silicon for solar-fuel production. Chemical Reviews, 2014, 114(17): 8662-8719.
DOI PMID |
| [21] |
WHITE J, BARUCH M, PANDER J, et al. Light-driven heterogeneous reduction of carbon dioxide: photocatalysts and photoelectrodes. Chemical Reviews, 2015, 115(23): 12888-12935.
DOI PMID |
| [22] |
MAEDA K. Metal-complex/semiconductor hybrid photocatalysts and photoelectrodes for CO2 reduction driven by visible light. Advanced Materials, 2019, 31(25): 1808205.
DOI URL |
| [23] |
DING P, HU Y, DENG J, et al. Controlled chemical etching leads to efficient silicon-bismuth interface for photoelectrochemical CO2 reduction to formate. Materials Today Chemistry, 2019, 11: 80-85.
DOI URL |
| [24] |
GONG Q, DING P, XU M, et al. Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction. Nature Communications, 2019, 10(1): 2807.
DOI PMID |
| [25] |
LI Z, FENG Y, LI Y, et al. Fabrication of Bi/Sn bimetallic electrode for high-performance electrochemical reduction of carbon dioxide to formate. Chemical Engineering Journal, 2022, 428: 130901.
DOI URL |
| [26] |
EON H, TIMOSHENKO J, SCHOLTEN F, et al. Operando insight into the correlation between the structure and composition of CuZn nanoparticles and their selectivity for the electrochemical CO2 reduction. Journal of the American Chemical Society, 2019, 141(50): 19879-19887.
DOI URL |
| [27] |
KUMAR A, BUI V, LEE J, et al. Moving beyond bimetallic-alloy to single-atom dimer atomic-interface for all-pH hydrogen evolution. Nature Communications, 2021, 12(1): 6766.
DOI PMID |
| [28] |
WU Z, CARACCIOLO D, MASWADEH Y, et al. Alloying-realloying enabled high durability for Pt-Pd-3D-transition metal nanoparticle fuel cell catalysts. Nature Communications, 2021, 12(1): 859.
DOI URL |
| [29] |
LEE W, KO Y, KIM J, et al. High crystallinity design of Ir-based catalysts drives catalytic reversibility for water electrolysis and fuel cells. Nature Communications, 2021, 12(1): 4271.
DOI PMID |
| [30] |
WANG Y, LI Y, LIU J, et al. BiPO4-derived 2D nanosheets for efficient electrocatalytic reduction of CO2 to liquid fuel. Angewandte Chemie International Edition, 2021, 60(14): 7681-7685.
DOI URL |
| [31] |
LUO W, ZHANG J, LI M, et al. Boosting CO production in electrocatalytic CO2 reduction on highly porous Zn catalysts. ACS Catalysis, 2019, 9(5): 3783-3791.
DOI URL |
| [32] |
ZHANG X, SU X, ZHENG Y, et al. Strongly coupled cobalt diselenide monolayers for selective electrocatalytic oxygen reduction to H2O2 under acidic conditions. Angewandte Chemie International Edition, 2019, 60: 26922-26931.
DOI URL |
| [1] | JIN Yuxiang, SONG Erhong, ZHU Yongfu. First-principles Investigation of Single 3d Transition Metals Doping Graphene Vacancies for CO2 Electroreduction [J]. Journal of Inorganic Materials, 2024, 39(7): 845-852. |
| [2] | LI Yuejun, CAO Tieping, SUN Dawei. Bi4O5Br2/CeO2 Composite with S-scheme Heterojunction: Construction and CO2 Reduction Performance [J]. Journal of Inorganic Materials, 2023, 38(8): 963-970. |
| [3] | JIA Xin, LI Jinyu, DING Shihao, SHEN Qianqian, JIA Husheng, XUE Jinbo. Synergy Effect of Pd Nanoparticles and Oxygen Vacancies for Enhancing TiO2 Photocatalytic CO2 Reduction [J]. Journal of Inorganic Materials, 2023, 38(11): 1301-1308. |
| [4] | GAO Wa, XIONG Yujie, WU Congping, ZHOU Yong, ZOU Zhigang. Recent Progress on Photocatalytic CO2 Reduction with Ultrathin Nanostructures [J]. Journal of Inorganic Materials, 2022, 37(1): 3-14. |
| [5] | TIAN Jianjian, MA Xia, WANG Min, YAO Heliang, HUA Zile, ZHANG Lingxia. Sn Quantum Dots for Electrocatalytic Reduction of CO2 to HCOOH [J]. Journal of Inorganic Materials, 2021, 36(12): 1337-1342. |
| [6] | ZHANG Qingming, ZHU Min, ZHOU Xiaoxia. CuO/ZnO Composite Electrocatalyst: Preparation and Reduction of CO2 to Syngas [J]. Journal of Inorganic Materials, 2021, 36(11): 1145-1153. |
| [7] | LIU Yaxin, WANG Min, SHEN Meng, WANG Qiang, ZHANG Lingxia. Bi-doped Ceria with Increased Oxygen Vacancy for Enhanced CO2 Photoreduction Performance [J]. Journal of Inorganic Materials, 2021, 36(1): 88-94. |
| [8] | SUN Hai-Jian,LIU Hui-Ling. Preparation of Cerium-doped TiO2/Ti Photoelectrodes and Photoelectrocatalytic Performance under Visible Light [J]. Journal of Inorganic Materials, 2007, 22(6): 1065-1069. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||