
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (11): 1151-1169.DOI: 10.15541/jim20220194
• REVIEW • Previous Articles Next Articles
HUANG Hui1,2( ), CHEN Yu1,2,3(
), CHEN Yu1,2,3( )
)
Received:2022-04-04
Revised:2022-05-02
Published:2022-11-20
Online:2022-06-16
Contact:
CHEN Yu, professor. E-mail: chenyuedu@shu.edu.cnAbout author:HUANG Hui (1994-), female, PhD candidate. E-mail: huanghuiscu@sina.com
Supported by:CLC Number:
HUANG Hui, CHEN Yu. Materdicine and Medmaterial[J]. Journal of Inorganic Materials, 2022, 37(11): 1151-1169.
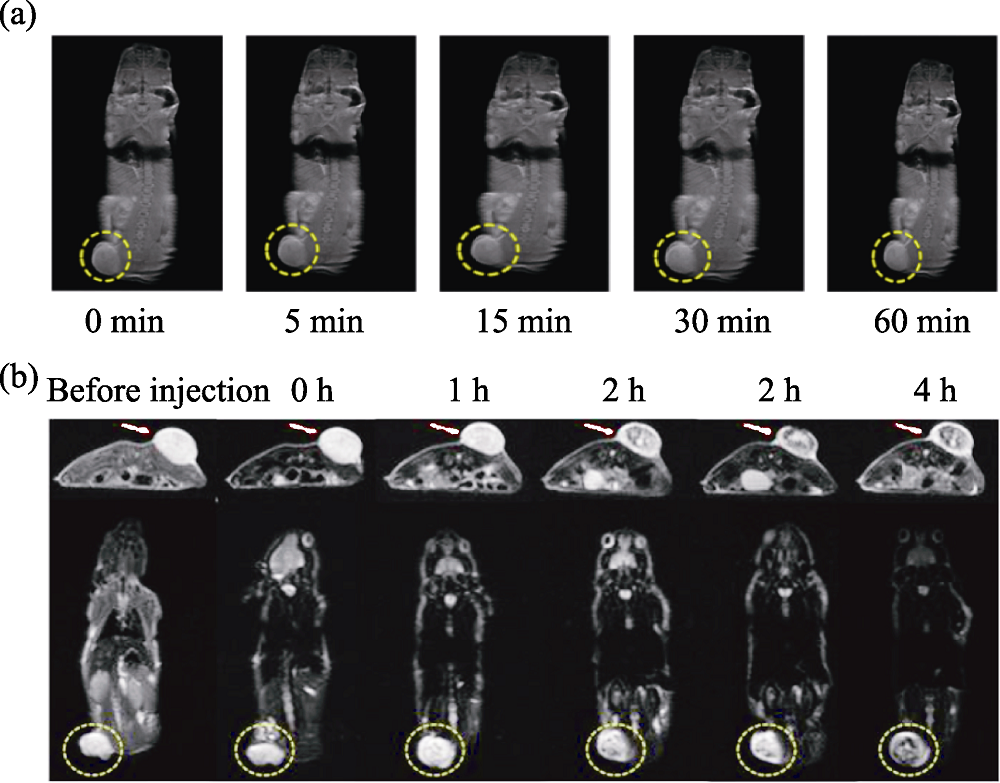
Fig. 1 Medmaterials for magnetic resonance imaging [33,35] (a) T1-weighted magnetic resonance imaging of tumor-bearing mice after intravenous injection of Mn-based nanomaterials; (b) T2-weighted magnetic resonance imaging of tumor-bearing mice after intravenous injection of iron oxide-based nanomaterials
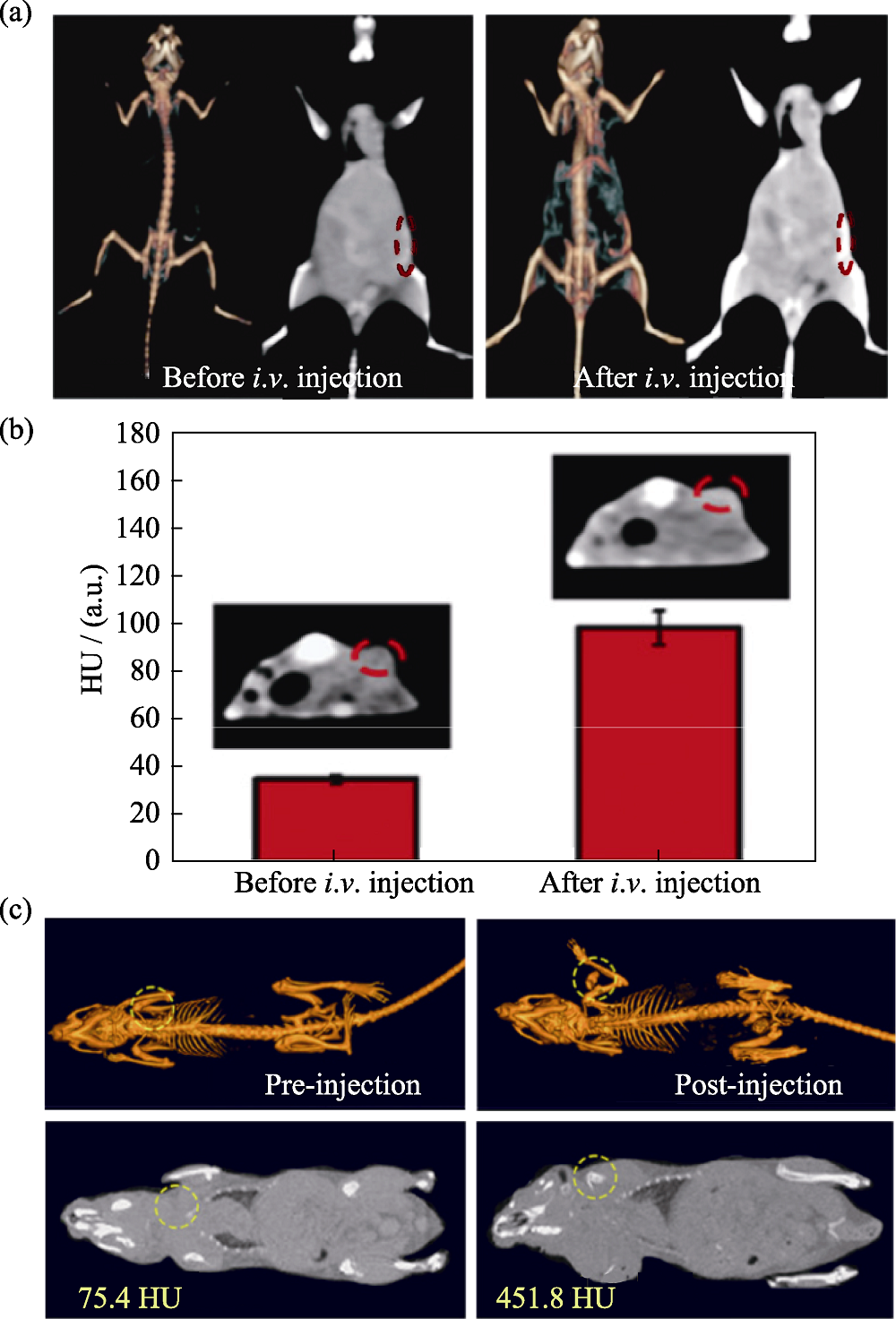
Fig. 2 Medmaterials for computed tomography imaging [43-44] (a) Computed tomography imaging in vivo before and after intravenous administration of W1.33C nanosheets; (b) Signal intensities of computed tomography imaging and corresponding coronal plane images (inset) before and after intravenous administration; (c) In vivo computed tomography images of tumor-bearing mice before and after injection of copper/manganese silicate nanosphere-coated lanthanide-doped nanoparticles. HU: Hounsfield unit
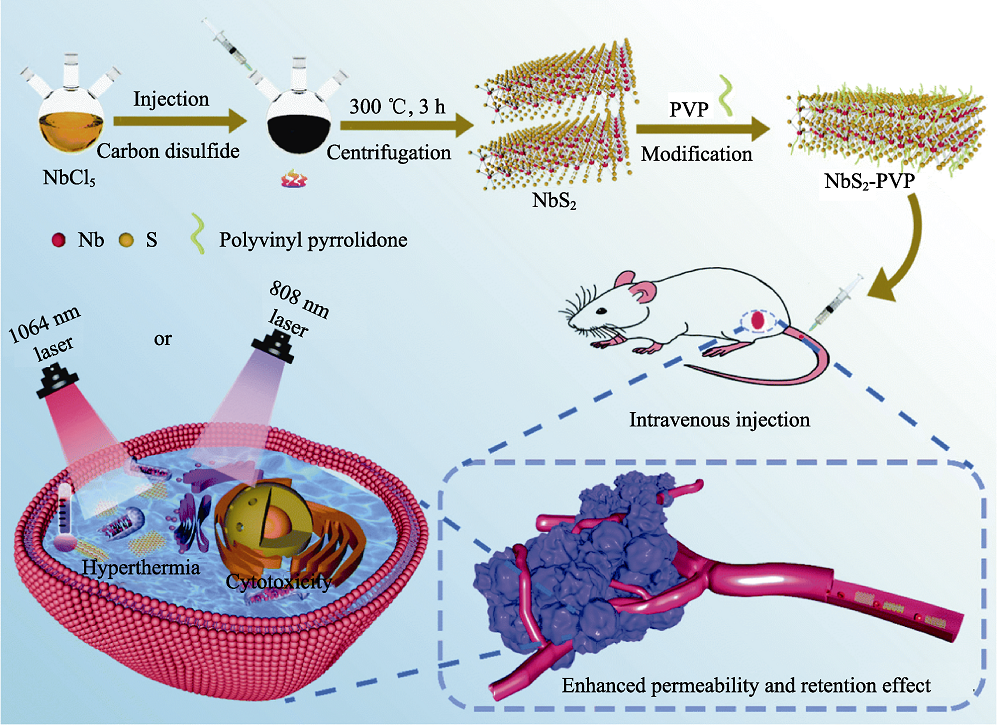
Fig. 3 Medmaterials for photothermal therapy against cancer[55] Scheme illustrating the NbS2 nanosheets-based photothermal tumor therapy in the first near-infrared and second near-infrared biowindows. PVP: Polyvinyl pyrrolidone
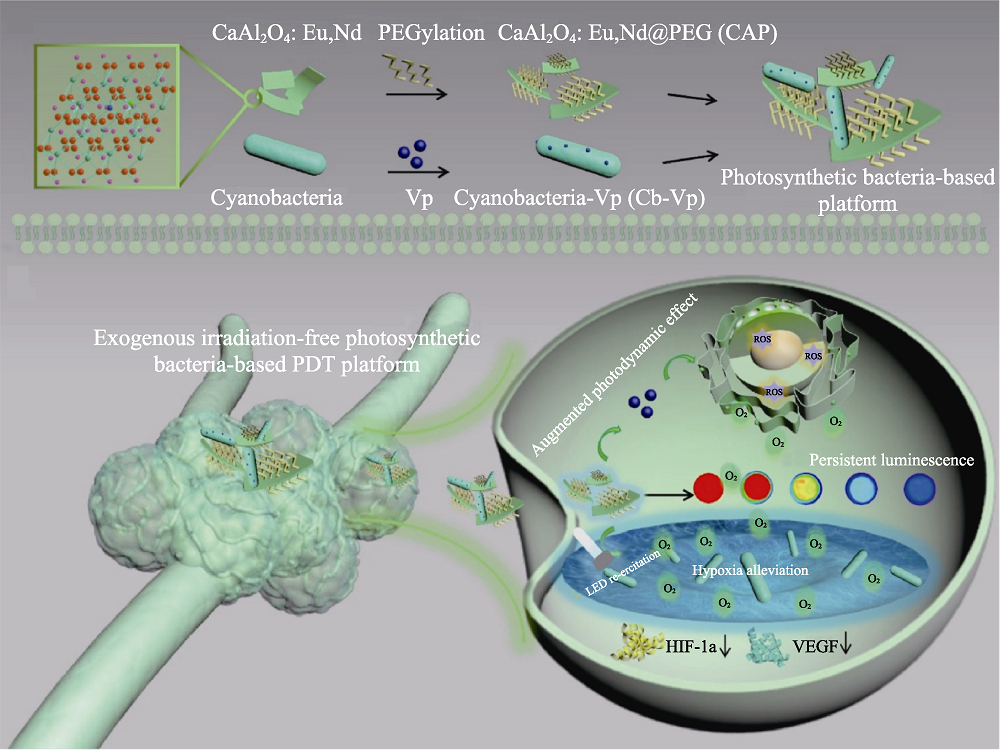
Fig. 4 Medmaterials for photodynamic therapy against cancer[61] Schematic representation of the exogenous irradiation-free photosynthetic bacteria-based system for photodynamic tumor therapy. PDT: Photodynamic therapy; HIF: Hypoxia inducible factor; VEGF: Vascular endothelial growth factor
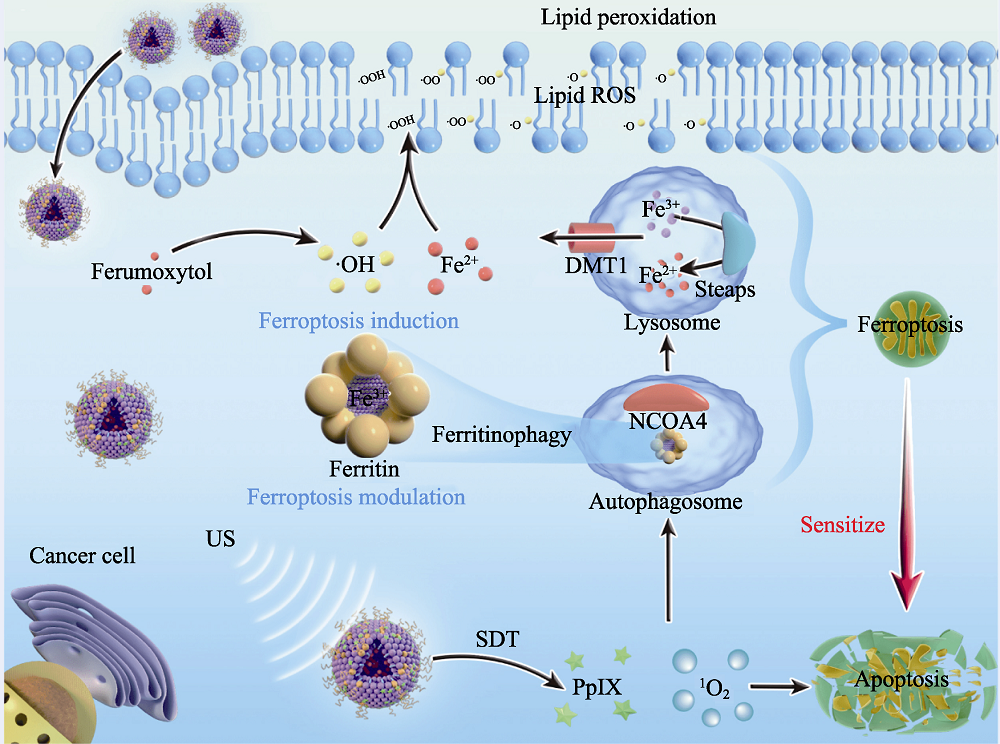
Fig. 5 Medmaterials for tumor sonodynamic therapy [62] Scheme of the underlying therapeutic mechanism of sonodynamic therapy-based ferroptosis-targeting. NCOA4: Nuclear receptor coactivator 4; US: Ultrasound; SDT: Sonodynamic therapy; PpIX: Protoporphyrin IX; DMT1: Divalent metal transporter 1; STEAPS: Six-transmembrane epithelial antigen of the prostate
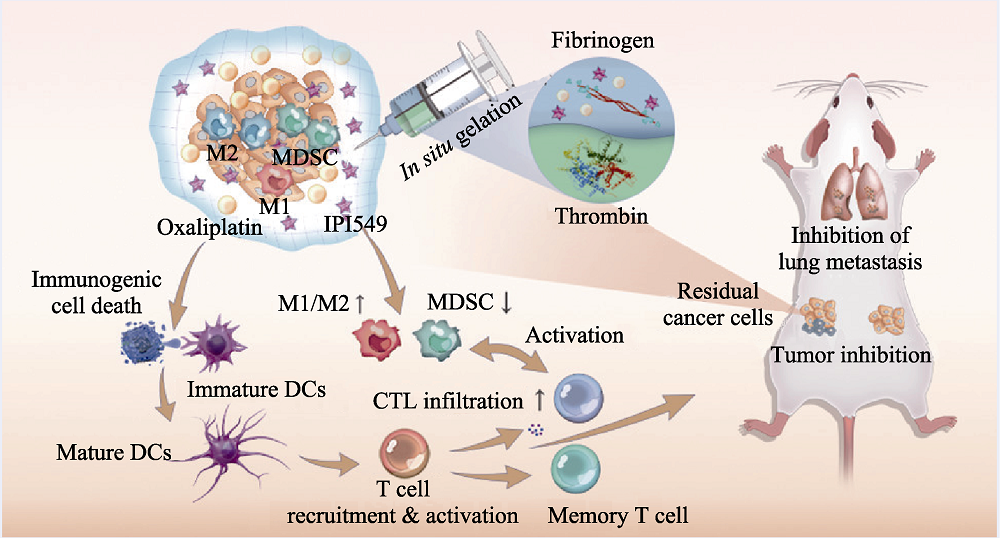
Fig. 6 Medmaterials for tumor immunotherapy[63] Scheme demonstrating the mechanism of chemoimmunotherapeutic approach for inhibiting tumor growth and metastasis M1: M1-like macrophages; M2: M2-like macrophages; MDSC: Myeloid-derived suppressor cells; IPI549: Selective PI3Kγ inhibitor verified in multiple tumor models; DC: Dendritic cells; CTL: Cytotoxic T lymphocytes
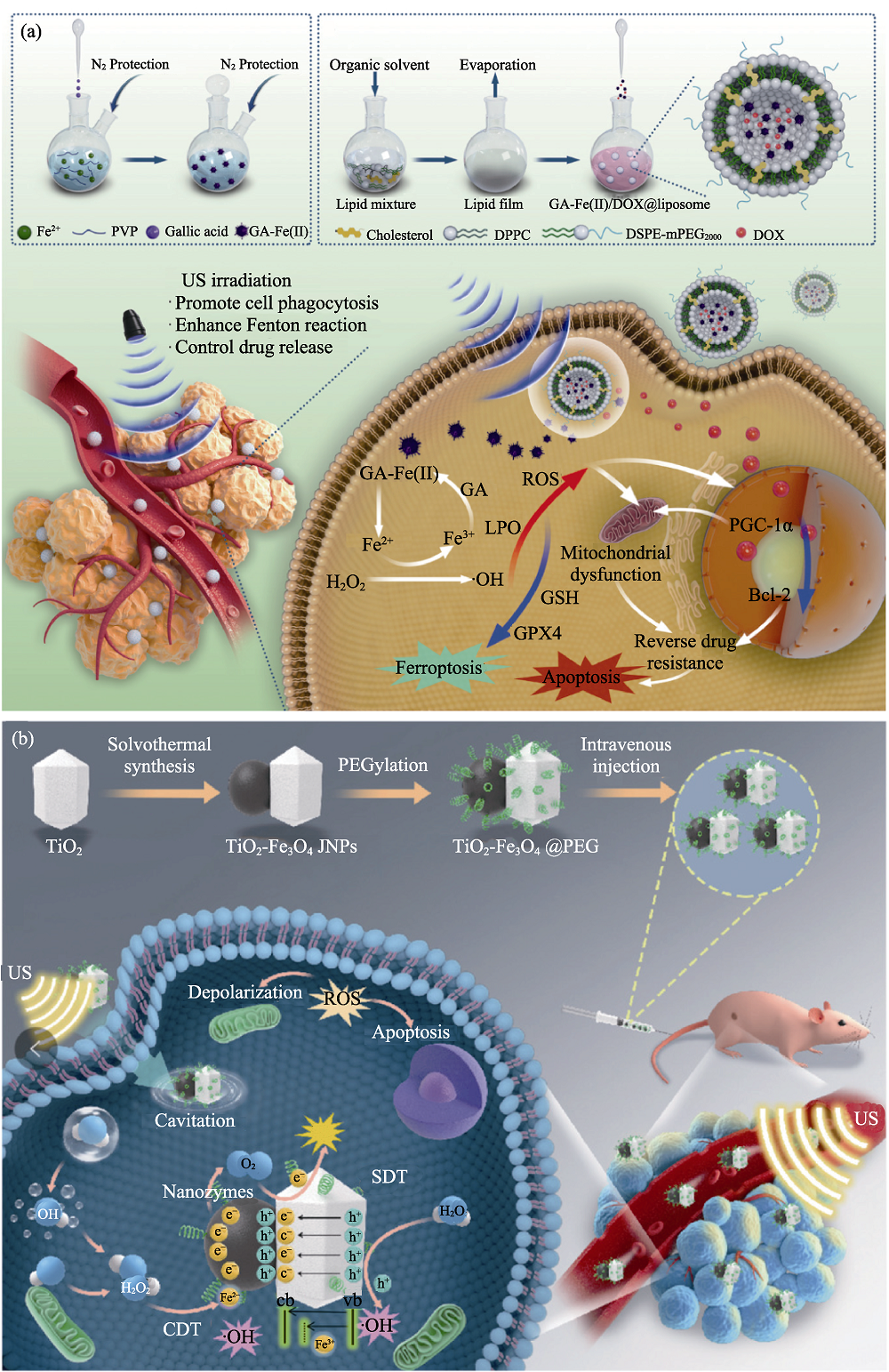
Fig. 7 Medmaterials for tumor synergistic therapy[74-75] (a) Scheme illustrating the function of GA-Fe(II)/DOX@liposome for reversing drug resistance by ultrasound-augmented nanocatalytic ferroptosis; (b) Schematic illustration of the synergistic enhancement of chemodynamic and sonodynamic therapy mediated by TiO2-Fe3O4 Janus nanosonosensitizers. LPO: Lipid peroxidation; GA: Gallic acid; US: Ultrasound; SDT: Sonodynamic therapy; CDT: Chemodynamic therapy
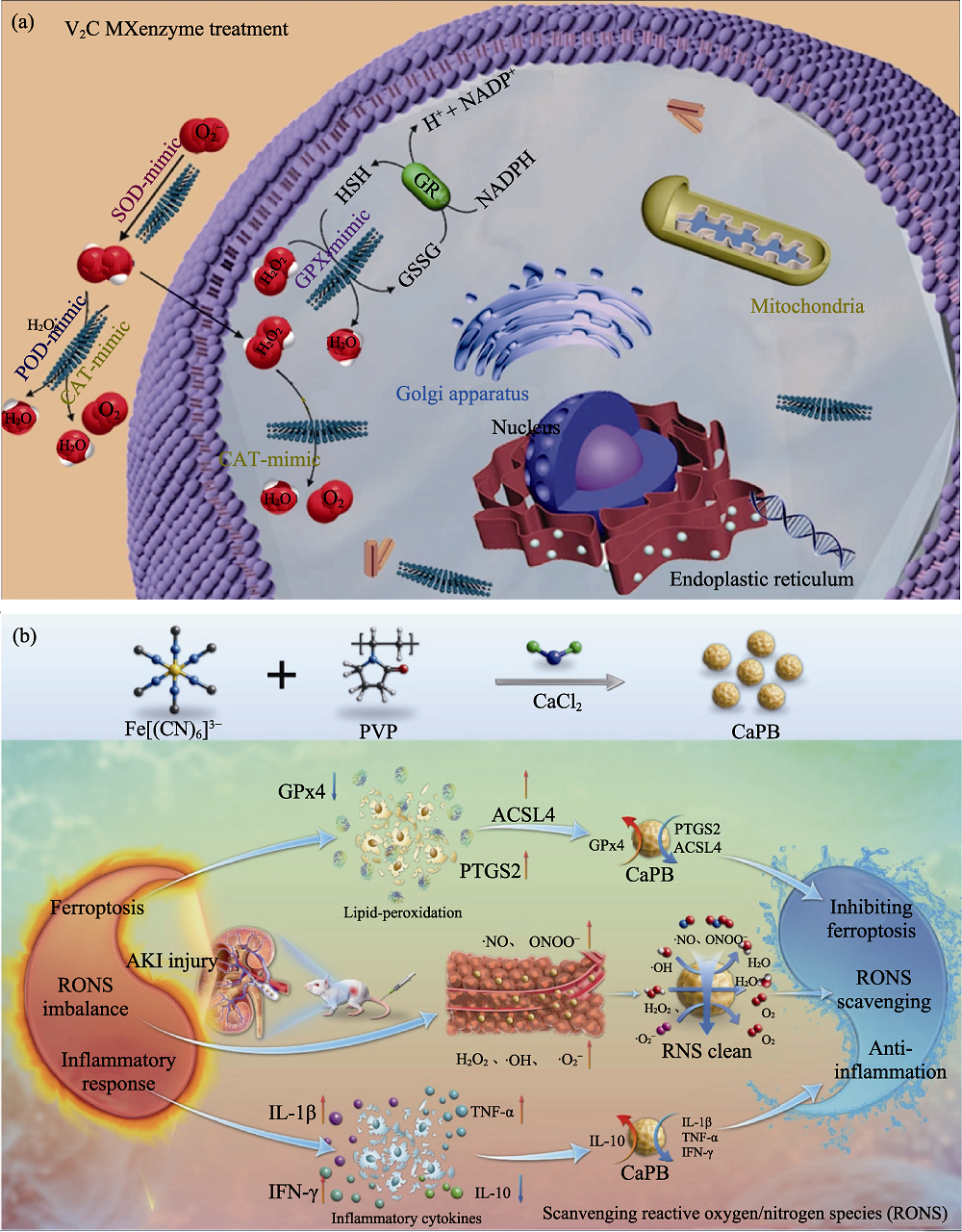
Fig. 8 Medmaterials for theranostics of ROS-scavenging related diseases and acute kidney injury [101-102] (a) Scheme indicating ROS-scavenging activities of V2C MXene with multiple enzyme-like natures; (b) Scheme representing CaPB nanozymes in the acute kidney injury treatment. ROS: Reactive oxygen species; MXene: Transition metal carbides, carbonitrides and nitrides; GPx4: Glutathione peroxidase 4; ACSL4: Acyl-CoA synthetase long chain family member 4; PTGS2: Prostaglandin-endoperoxide synthase 2; RONS: Reactive oxygen and nitrogen species
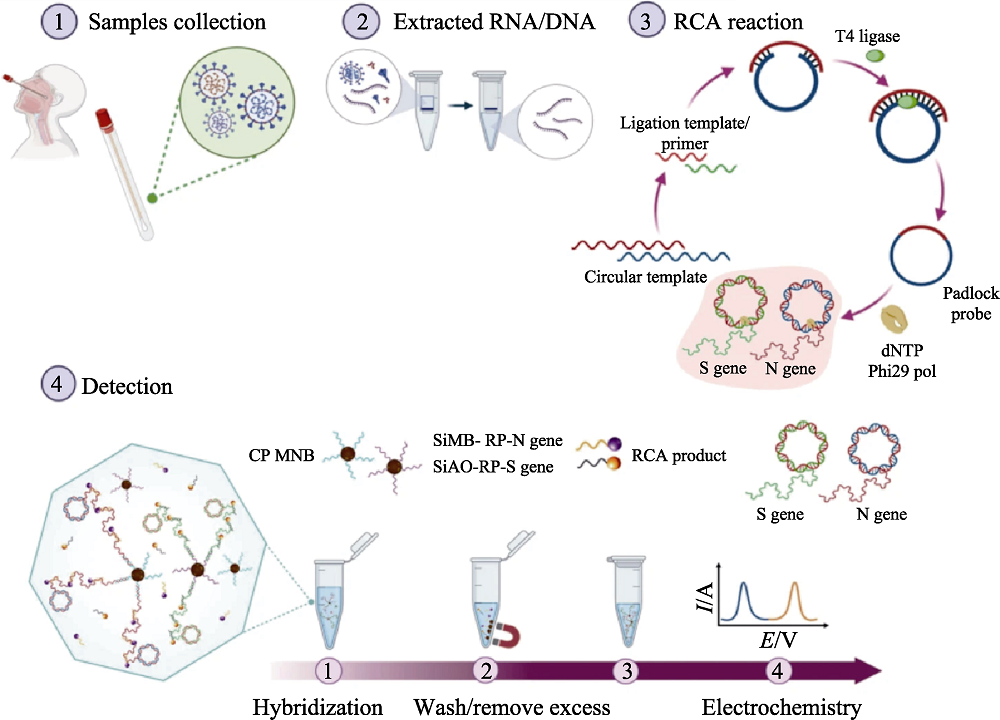
Fig. 9 Medmaterials for biosensing[111] Scheme illustrating detection workflow of SARS-CoV-2 using the electrochemical biosensor RCA: Rolling circle amplification; CP MNB: Capture probe-conjugated magnetic bead particle; SiMB: Silica with a redox-dye layer; RP: Reporter probe
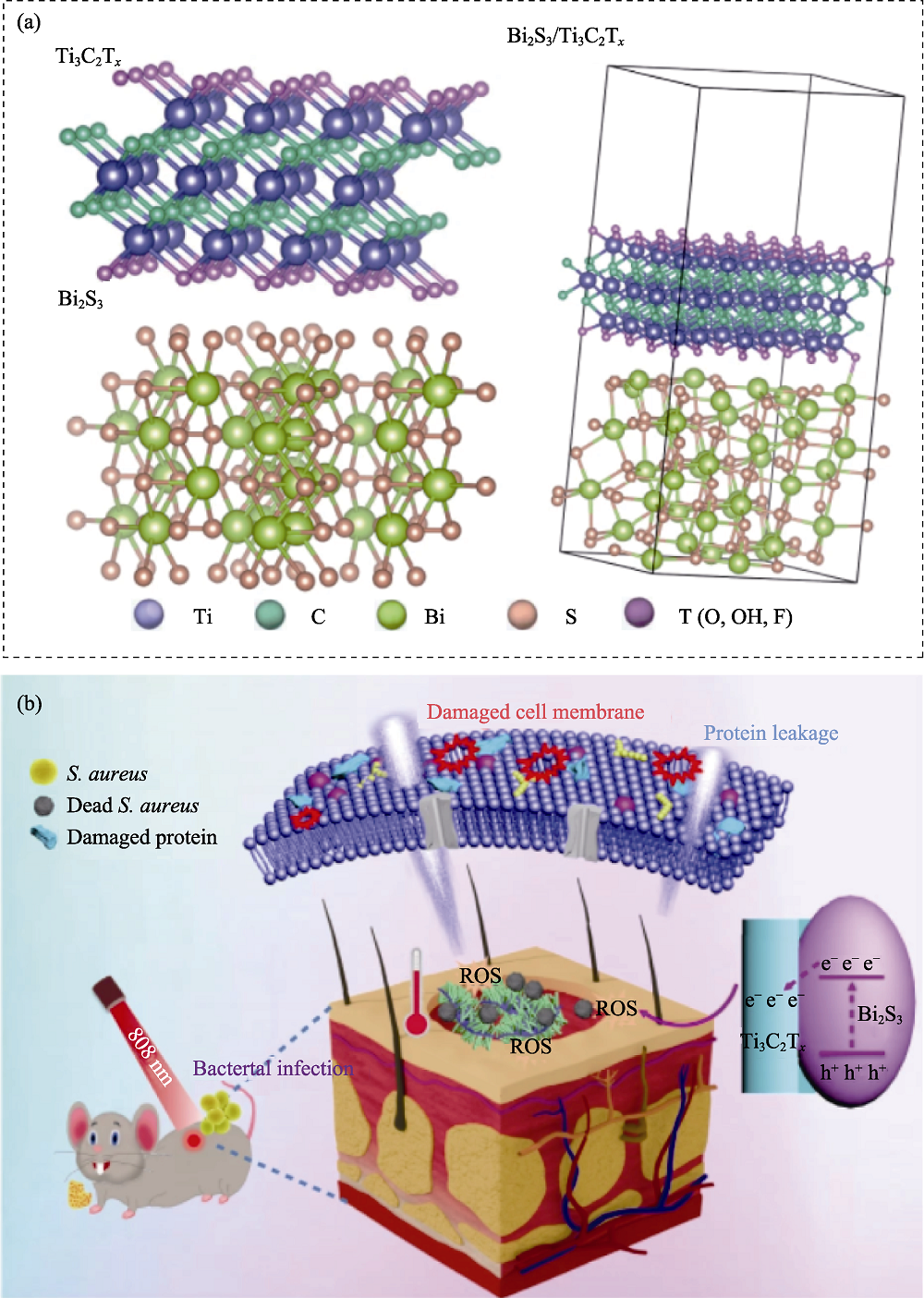
Fig. 10 Medmaterials for antibacterial applications[118] (a) Crystal structure of Ti3C2/Bi2S3; (b) Schematic illustration of the antibacterial mechanism of Ti3C2/Bi2S3 under 808 nm laser irradiation
| [1] |
MALEKJAHANI A, SINDHWANI S, SYED A M, et al. Engineering steps for mobile point-of-care diagnostic devices. Accounts of Chemical Research, 2019, 52(9): 2406-2414.
DOI PMID |
| [2] |
HE H, LIU L, MORIN E E, et al. Survey of clinical translation of cancer nanomedicines—lessons learned from successes and failures. Accounts of Chemical Research, 2019, 52(9): 2445-2461.
DOI URL |
| [3] | HUANG H, FENG W, CHEN Y, et al. Inorganic nanoparticles in clinical trials and translations. Nano Today, 2020, 35: 100972-24. |
| [4] |
HUANG H, FENG W, CHEN Y. Two-dimensional biomaterials: material science, biological effect and biomedical engineering applications. Chemical Society Reviews, 2021, 50(20): 11381-11485.
DOI PMID |
| [5] |
PELAZ B, ALEXIOU C H, ALVAREZ-PUEBLA R A, et al. Diverse applications of nanomedicine. ACS Nano, 2017, 11(3): 2313-2381.
DOI PMID |
| [6] |
BURDA C, CHEN X, NARAYANAN R, et al. Chemistry and properties of nanocrystals of different shapes. Chemical Reviews, 2005, 105(4): 1025-1102.
PMID |
| [7] |
XIANG H, CHEN Y. Materdicine: interdiscipline of materials and medicine. VIEW, 2020, 1(3): 20200016-29.
DOI URL |
| [8] |
SHI J J, KANTOFF P W, WOOSTER R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nature Reviews Cancer, 2017, 17(1): 20-37.
DOI PMID |
| [9] |
IRBY D, DU C, LI F. Lipid-drug conjugate for enhancing drug delivery. Molecular Pharmaceutics, 2017, 14(5): 1325-1338.
DOI PMID |
| [10] |
MURA S, NICOLAS J, COUVREUR P. Stimuli-responsive nanocarriers for drug delivery. Nature Materials, 2013, 12(11): 991-1003.
DOI PMID |
| [11] |
NICOLAS J, MURA S, BRAMBILLA D, et al. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chemical Society Reviews, 2013, 42(3): 1147-1235.
DOI PMID |
| [12] |
KUNJACHAN S, EHLING J, STORM G, et al. Noninvasive imaging of nanomedicines and nanotheranostics: principles, progress, and prospects. Chemical Reviews, 2015, 115(19): 10907-10937.
DOI PMID |
| [13] |
CHEN H M, ZHANG W Z, ZHU G Z, et al. Rethinking cancer nanotheranostics. Nature Reviews Materials, 2017, 2(7): 17024-18.
DOI URL |
| [14] |
WANG C, HUANG W, ZHOU Y, et al. 3D printing of bone tissue engineering scaffolds. Bioactive Materials, 2020, 5(1): 82-91.
DOI PMID |
| [15] | KAUR B, KUMAR S, KAUSHIK B K. Recent advancements in optical biosensors for cancer detection. Biosensors and Bioelectronics, 2022, 197: 113805-11. |
| [16] |
HUH A J, KWON Y J. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. Journal of Controlled Release, 2011, 156(2): 128-145.
DOI URL |
| [17] |
DUAN Y, LIU B. Recent advances of optical imaging in the second near-infrared window. Advanced Materials, 2018, 30(47): 1802394-19.
DOI URL |
| [18] |
HUANG L Y, ZHU S, CUI R, et al. Noninvasive in vivo imaging in the second near-infrared window by inorganic nanoparticle-based fluorescent probes. Analytical Chemistry, 2019, 92(1): 535-542.
DOI URL |
| [19] |
LIN H, GAO S, DAI C, et al. A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. Journal of the American Chemical Society, 2017, 139(45): 16235-16247.
DOI PMID |
| [20] |
YANG Q, HU Z, ZHU S, et al. Donor engineering for NIR-II molecular fluorophores with enhanced fluorescent performance. Journal of the American Chemical Society, 2018, 140(5): 1715-1724.
DOI PMID |
| [21] |
YANG H C, LI R F, ZHANG Y J, et al. Colloidal alloyed quantum dots with enhanced photoluminescence quantum yield in the NIR-II window. Journal of the American Chemical Society, 2021, 143(6): 2601-2607.
DOI PMID |
| [22] | FAN Y, WANG S, ZHANG F. Optical multiplexed bioassays for improved biomedical diagnostics. Angewandte Chemie International Edition, 2019, 131(38): 13342-13353. |
| [23] |
WELSHER K, LIU Z, SHERLOCK SP, et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature Nanotechnology, 2009, 4(11): 773-780.
DOI PMID |
| [24] |
CHANG B, LI D, REN Y, et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nature Biomedical Engineering, 2022, 6(6): 629-639.
DOI URL |
| [25] |
WANG Q, LIANG Z, LI F, et al. Dynamically switchable magnetic resonance imaging contrast agents. Exploration, 2021, 1(2): 20210009-8.
DOI URL |
| [26] |
KIM D, KIM J, PARK Y I, et al. Recent development of inorganic nanoparticles for biomedical imaging. ACS Central Science, 2018, 4(3): 324-336.
DOI PMID |
| [27] |
XU Z, LIU C, ZHAO S, et al. Molecular sensors for NMR-based detection. Chemical Reviews, 2018, 119(1): 195-230.
DOI URL |
| [28] |
CHO M H, SHIN S H, PARK S H, et al. Targeted, stimuli-responsive, and theranostic 19F magnetic resonance imaging probes. Bioconjugate Chemistry, 2019, 30(10): 2502-2518.
DOI URL |
| [29] |
ZHAO X, DUAN G, WU K, et al. Intelligent metamaterials based on nonlinearity for magnetic resonance imaging. Advanced Materials, 2019, 31(49): 1905461-7.
DOI URL |
| [30] |
DELCASSIAN D, LUZHANSKY I, SPANOUDAKI V, et al. Magnetic retrieval of encapsulated beta cell transplants from diabetic mice using dual-function MRI visible and retrievable microcapsules. Advanced Materials, 2020, 32(16): 1904502-10.
DOI URL |
| [31] |
ZHANG J, YUAN Y, GAO M, et al. Carbon dots as a new class of diamagnetic chemical exchange saturation transfer (diacest) MRI contrast agents. Angewandte Chemie International Edition, 2019, 58(29): 9871-9875.
DOI URL |
| [32] |
ZHANG P, HOU Y, ZENG J, et al. Coordinatively unsaturated Fe3+ based activatable probes for enhanced MRI and therapy of tumors. Angewandte Chemie International Edition, 2019, 58(32): 11088- 11096.
DOI URL |
| [33] |
DAI C, CHEN Y, JING X X, et al. Two-dimensional tantalum carbide (MXenes) composite nanosheets for multiple imaging- guided photothermal tumor ablation. ACS Nano, 2017, 11(12): 12696-12712.
DOI URL |
| [34] |
DAI C, LIN H, XU G, et al. Biocompatible 2D titanium carbide (MXenes) composite nanosheets for pH-responsive MRI-guided tumor hyperthermia. Chemistry of Materials, 2017, 29(20): 8637-8652.
DOI URL |
| [35] |
LIU Z, LIN H, ZHAO M L, et al. 2D superparamagnetic tantalum carbide composite MXenes for efficient breast-cancer theranostics. Theranostics, 2018, 8(6): 1648-1664.
DOI PMID |
| [36] |
LIU Z, ZHAO M L, LIN H, et al. 2D magnetic titanium carbide MXene for cancer theranostics. Journal of Materials Chemistry B, 2018, 6(21): 3541-3548.
DOI PMID |
| [37] | CAO Y, WU T, ZHANG K, et al. Engineered exosome-mediated near-infrared-II region V2C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano, 2019, 13(2): 1499-1510. |
| [38] |
BAR-ZION A, NOURMAHNAD A, MITTELSTEIN D R, et al. Acoustically triggered mechanotherapy using genetically encoded gas vesicles. Nature Nanotechnology, 2021, 16(12): 1403-1412.
DOI URL |
| [39] |
LIU Y, BHATTARAI P, DAI Z, et al. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chemical Society Reviews, 2019, 48(7): 2053-2108.
DOI URL |
| [40] |
LI X, CAI Z, JIANG L P, et al. Metal-ligand coordination nanomaterials for biomedical imaging. Bioconjugate Chemistry, 2019, 31(2): 332-339.
DOI URL |
| [41] |
MENG Z, ZHOU X, SHE J, et al. Ultrasound-responsive conversion of microbubbles to nanoparticles to enable background-free in vivo photoacoustic imaging. Nano Letters, 2019, 19(11): 8109-8117.
DOI URL |
| [42] |
CHEN Y S, ZHAO Y, YOON S J, et al. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nature Nanotechnology, 2019, 14(5): 465-472.
DOI URL |
| [43] |
KIM J, CHHOUR P, HSU J, et al. Use of nanoparticle contrast agents for cell tracking with computed tomography. Bioconjugate Chemistry, 2017, 28(6): 1581-1597.
DOI PMID |
| [44] |
ZHOU B G, YIN H H, DONG C H, et al. Biodegradable and excretable 2D W1.33C i-MXene with vacancy ordering for theory- oriented cancer nanotheranostics in near-infrared biowindow. Advanced Science, 2021, 8(24): 2101043-13.
DOI URL |
| [45] |
XU J, SHI R, CHEN G, et al. All-in-one theranostic nanomedicine with ultrabright second near-infrared emission for tumor- modulated bioimaging and chemodynamic/photodynamic therapy. ACS Nano, 2020, 14(8): 9613-9625.
DOI URL |
| [46] |
JUNG H S, VERWILST P, SHARMA A, et al. Organic molecule- based photothermal agents: an expanding photothermal therapy universe. Chemical Society Reviews, 2018, 47(7): 2280-2297.
DOI URL |
| [47] | WANG H, CHANG J, SHI M, et al. A dual-targeted organic photothermal agent for enhanced photothermal therapy. Angewandte Chemie International Edition, 2019, 131(4): 1069-1073. |
| [48] |
YU Z, HU W, ZHAO H, et al. Generating new cross-relaxation pathways by coating prussian blue on NaNdF4 to fabricate enhanced photothermal agents. Angewandte Chemie International Edition, 2019, 58(25): 8536-8540.
DOI URL |
| [49] |
JIANG Y, LI J, ZHEN X, et al. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: a comparative study. Advanced Materials, 2018, 30(14): 1705980-7.
DOI URL |
| [50] | ZHEN X, XIE C, PU K. Temperature-correlated afterglow of a semiconducting polymer nanococktail for imaging-guided photothermal therapy. Angewandte Chemie International Edition, 2018, 130(15): 4002-4006. |
| [51] |
SMITH A M, MANCINI M C, NIE S. Second window for in vivo imaging. Nature Nanotechnology, 2009, 4(11): 710-711.
DOI URL |
| [52] |
ZHOU J, JIANG Y, HOU S, et al. Compact plasmonic blackbody for cancer theranosis in the near-infrared II window. ACS Nano, 2018, 12(3): 2643-2651.
DOI PMID |
| [53] |
CHENG Y, YANG F, XIANG G, et al. Ultrathin tellurium oxide/ ammonium tungsten bronze nanoribbon for multimodality imaging and second near-infrared region photothermal therapy. Nano Letters, 2019, 19(2): 1179-1189.
DOI URL |
| [54] |
CUI J, JIANG R, GUO C, et al. Fluorine grafted Cu7S4-Au heterodimers for multimodal imaging guided photothermal therapy with high penetration depth. Journal of the American Chemical Society, 2018, 140(18): 5890-5894.
DOI URL |
| [55] |
SUN S, SONG Y, CHEN J, et al. NIR-I and NIR-II irradiation tumor ablation using NbS2 nanosheets as the photothermal agent. Nanoscale, 2021, 13(43): 18300-18310.
DOI URL |
| [56] |
XIANG H J, CHEN Y. Energy-converting nanomedicine. Small, 2019, 15(13): 1805339-31.
DOI URL |
| [57] |
HU H, QIAN X Q, CHEN Y. Microalgae-enabled photosynthetic alleviation of tumor hypoxia for enhanced nanotherapies. Science Bulletin, 2020, 65(22): 1869-1871.
DOI URL |
| [58] |
CHEN B D, XIANG H J, PAN S S, et al. Advanced theragenerative biomaterials with therapeutic and regeneration multifunctionality. Advanced Functional Materials, 2020, 30(34): 2002621-27.
DOI URL |
| [59] |
FENG W, CHEN Y. Chemoreactive nanomedicine. Journal of Materials Chemistry B, 2020, 8(31): 6753-6764.
DOI PMID |
| [60] |
LUCKY S S, SOO K C, ZHANG Y. Nanoparticles in photodynamic therapy. Chemical Reviews, 2015, 115(4): 1990-2042.
DOI PMID |
| [61] | CHANG M, FENG W, DING L, et al. Persistent luminescence phosphor as in-vivo light source for tumoral cyanobacterial photosynthetic oxygenation and photodynamic therapy. Bioactive Materials, 2022, 10: 131-144. |
| [62] | ZHOU L, DONG C, DING L, et al. Targeting ferroptosis synergistically sensitizes apoptotic sonodynamic anti-tumor nanotherapy. Nano Today, 2021, 39: 101212-12. |
| [63] |
SHEN Y, CHEN L, GUAN X, et al. Tailoring chemoimmunostimulant bioscaffolds for inhibiting tumor growth and metastasis after incomplete microwave ablation. ACS Nano, 2021, 15(12): 20414-20429.
DOI PMID |
| [64] |
MELLMAN I, COUKOS G, DRANOFF G. Cancer immunotherapy comes of age. Nature, 2011, 480(7378): 480-489.
DOI URL |
| [65] |
RIBAS A, WOLCHOK J D. Cancer immunotherapy using checkpoint blockade. Science, 2018, 359(6382): 1350-1355.
DOI URL |
| [66] |
MAHONEY K M, RENNERT P D, FREEMAN G J. Combination cancer immunotherapy and new immunomodulatory targets. Nature Reviews Drug Discovery, 2015, 14(8): 561-584.
DOI PMID |
| [67] |
VANNEMAN M, DRANOFF G. Combining immunotherapy and targeted therapies in cancer treatment. Nature Reviews Cancer, 2012, 12(4): 237-251.
DOI PMID |
| [68] |
SANG W, ZHANG Z, DAI Y, et al. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chemical Society Reviews, 2019, 48(14): 3771-3810.
DOI PMID |
| [69] |
SHIELDS IV CW, WANG L W, EVANS M A, et al. Materials for immunotherapy. Advanced Materials, 2020, 32(13): 1901633-56.
DOI URL |
| [70] |
NAM J, SON S, PARK K S, et al. Cancer nanomedicine for combination cancer immunotherapy. Nature Reviews Materials, 2019, 4(6): 398-414.
DOI |
| [71] | SONG W, MUSETTI S N, HUANG L. Nanomaterials for cancer immunotherapy. Biomaterials, 2017, 148: 16-30. |
| [72] |
CHEUNG A S, MOONEY D J. Engineered materials for cancer immunotherapy. Nano Today, 2015, 10(4): 511-531.
PMID |
| [73] |
LIU Y, WANG L, SONG Q, et al. Intrapleural nano-immunotherapy promotes innate and adaptive immune responses to enhance anti- PD-L1 therapy for malignant pleural effusion. Nature Nanotechnology, 2022, 17(2): 206-216.
DOI URL |
| [74] |
ZHENG Y, LI X, DONG C, et al. Ultrasound-augmented nanocatalytic ferroptosis reverses chemotherapeutic resistance and induces synergistic tumor nanotherapy. Advanced Functional Materials, 2022, 32(4): 2107529-17.
DOI URL |
| [75] |
XU W, DONG C, HU H, et al. Engineering Janus chemoreactive nanosonosensitizers for bilaterally augmented sonodynamic and chemodynamic cancer nanotherapy. Advanced Functional Materials, 2021, 31(37): 2103134-13.
DOI URL |
| [76] | XIANG H, YOU C, LIU W, et al. Chemotherapy-enabled /augmented cascade catalytic tumor-oxidative nanotherapy. Biomaterials, 2021, 277: 121071-12. |
| [77] |
WANG H X, LI M, LEE C M, et al. CRISPR/Cas9-based genome editing for disease modeling and therapy: challenges and opportunities for nonviral delivery. Chemical Reviews, 2017, 117(15): 9874-9906.
DOI URL |
| [78] |
LYU Y, HE S S, LI J C, et al. A photolabile semiconducting polymer nanotransducer for near-infrared regulation of CRISPR/ Cas9 gene editing. Angewandte Chemie International Edition, 2019, 58(50): 18197-18201.
DOI URL |
| [79] |
CHENG Q, WEI T, FARBIAK L, et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nature Nanotechnology, 2020, 15(4): 313-320.
DOI PMID |
| [80] |
YIN H, ZHOU B, DONG C, et al. CRISPR/Cas9-2D silicene gene-editing nanosystem for remote NIR-II-induced tumor microenvironment reprogramming and augmented photonic tumor ablation. Advanced Functional Materials, 2021, 31(50): 2107093-12.
DOI URL |
| [81] |
YIN H, SUN L, PU Y, et al. Ultrasound-controlled CRISPR/Cas9 system augments sonodynamic therapy of hepatocellular carcinoma. ACS Central Science, 2021, 7(12): 2049-2062.
DOI PMID |
| [82] |
LI M, MA H, HAN F, et al. Microbially catalyzed biomaterials for bone regeneration. Advanced Materials, 2021, 33(49): 2104829-13.
DOI URL |
| [83] | ZHOU Y, WU C, CHANG J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Materials Today, 2019, 24: 41-56. |
| [84] |
LI T, CHANG J, ZHU Y, et al. 3D printing of bioinspired biomaterials for tissue regeneration. Advanced Healthcare Materials, 2020, 9(23): 2000208-17.
DOI URL |
| [85] | STEVENS M M. Biomaterials for bone tissue engineering. Materials Today, 2008, 11(5): 18-25. |
| [86] |
DISCHER D E, MOONEY D J, ZANDSTRA P W. Growth factors, matrices, and forces combine and control stem cells. Science, 2009, 324(5935): 1673-1677.
DOI URL |
| [87] |
GEIGER B, SPATZ J P, BERSHADSKY A D. Environmental sensing through focal adhesions. Nature Reviews Molecular Cell Biology, 2009, 10(1): 21-33.
DOI PMID |
| [88] |
WANG L, YANG Q, HUO M, et al. Engineering single-atomic iron-catalyst-integrated 3D-printed bioscaffolds for osteosarcoma destruction with antibacterial and bone defect regeneration bioactivity. Advanced Materials, 2021, 33(31): 2100150-12.
DOI URL |
| [89] |
SOMMARIVA M, LE NOCI V, BIANCHI F, et al. The lung microbiota: role in maintaining pulmonary immune homeostasis and its implications in cancer development and therapy. Cellular and Molecular Life Sciences, 2020, 77(14): 2739-2749.
DOI PMID |
| [90] |
YANG W, PETERS J I, WILLIAMS R O. Inhaled nanoparticles-a current review. International Journal of Pharmaceutics, 2008, 356(1): 239-247.
DOI URL |
| [91] |
MYERSON J W, PATEL P N, RUBEY K M, et al. Supramolecular arrangement of protein in nanoparticle structures predicts nanoparticle tropism for neutrophils in acute lung inflammation. Nature Nanotechnology, 2022, 17(1): 86-97.
DOI URL |
| [92] | VASARMIDI E, TSITOURA E, SPANDIDOS D A, et al. Pulmonary fibrosis in the aftermath of the COVID-19 era. Experimental and Therapeutic Medicine, 2020, 20(3): 2557-2560. |
| [93] |
CRISAN-DABIJA R, PAVEL C A, POPA I V, et al. “A chain only as strong as its weakest link”: an up-to-date literature review on the bidirectional interaction of pulmonary fibrosis and COVID-19. Journal of Proteome Research, 2020, 19(11): 4327-4338.
DOI URL |
| [94] |
LEDERER D J, MARTINEZ F J. Idiopathic pulmonary fibrosis. New England Journal of Medicine, 2018, 378(19): 1811-1823.
DOI URL |
| [95] |
RICHELDI L, COLLARD H R, JONES M G. Idiopathic pulmonary fibrosis. Lancet, 2017, 389(10082): 1941-1952.
DOI PMID |
| [96] | MERKT W, BUENO M, MORA AL, et al. Senotherapeutics: targeting senescence in idiopathic pulmonary fibrosis. Seminars in Cell & Developmental Biology, 2020, 101: 104-110. |
| [97] | MALSIN E S, KAMP D W. The mitochondria in lung fibrosis: Friend or foe? Translational Research, 2018, 202: 1-23. |
| [98] |
YU G, TZOUVELEKIS A, WANG R, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nature Medicine, 2018, 24(1): 39-49.
DOI PMID |
| [99] | SALEH J, PEYSSONNAUX C, SINGH K K, et al. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion, 2020, 54: 1-7. |
| [100] |
HUANG T, ZHANG T, JIANG X, et al. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Science Advances, 2021, 7(40): eabj0534-15.
DOI URL |
| [101] |
FENG W, HAN X G, HU H, et al. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nature Communications, 2021, 12(1): 2203-16.
DOI PMID |
| [102] |
WANG K, ZHANG Y, MAO W, et al. Engineering ultrasmall ferroptosis-targeting and reactive oxygen/nitrogen species- scavenging nanozyme for alleviating acute kidney injury. Advanced Functional Materials, 2022, 32(10): 2109221-13.
DOI URL |
| [103] |
TANG Z, HUO M, JU Y, et al. Nanoprotection against retinal pigment epithelium degeneration via ferroptosis inhibition. Small Methods, 2021, 5(12): 2100848-14.
DOI URL |
| [104] |
WU J, YUK H, SARRAFIAN TIFFANY L, et al. An off-the-shelf bioadhesive patch for sutureless repair of gastrointestinal defects. Science Translational Medicine, 14(630): eabh2857-13.
DOI URL |
| [105] |
KIM S J, CHOI S J, JANG J S, et al. Innovative nanosensor for disease diagnosis. Accounts of Chemical Research, 2017, 50(7): 1587-1596.
DOI URL |
| [106] |
TANG Z M, KONG N, ZHANG X C, et al. A materials-science perspective on tackling COVID-19. Nature Reviews Materials, 2020, 5(11): 847-860.
DOI URL |
| [107] |
FAROKHZAD N, TAO W. Materials chemistry-enabled platforms in detecting sexually transmitted infections: progress towards point-of-care tests. Trends in Chemistry, 2021, 3(7): 589-602.
DOI URL |
| [108] |
GONG M M, SINTON D. Turning the page: advancing paper-based microfluidics for broad diagnostic application. Chemical Reviews, 2017, 117(12): 8447-8480.
DOI PMID |
| [109] |
HUANG X, LIU Y, YUNG B, et al. Nanotechnology-enhanced no-wash biosensors for in vitro diagnostics of cancer. ACS Nano, 2017, 11(6): 5238-5292.
DOI URL |
| [110] |
WANG C, QI B, LIN M, et al. Continuous monitoring of deep-tissue haemodynamics with stretchable ultrasonic phased arrays. Nature Biomedical Engineering, 2021, 5(7): 749-758.
DOI PMID |
| [111] |
CHAIBUN T, PUENPA J, NGAMDEE T, et al. Rapid electrochemical detection of coronavirus SARS-Cov-2. Nature Communications, 2021, 12(1): 802-10.
DOI PMID |
| [112] |
GATES B. Responding to COVID-19-a once-in-a-century pandemic? New England Journal of Medicine, 2020, 382(18): 1677-1679.
DOI URL |
| [113] |
LAURING A S, FRYDMAN J, ANDINO R. The role of mutational robustness in RNA virus evolution. Nature Reviews Microbiology, 2013, 11(5): 327-336.
DOI PMID |
| [114] |
SEO G, LEE G, KIM M J, et al. Rapid detection of COVID-19 causative virus (SARS-Cov-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano, 2020, 14(4): 5135-5142.
DOI PMID |
| [115] |
XIAO G, HE J, CHEN X, et al. A wearable, cotton thread/paper- based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose, 2019, 26(7): 4553-4562.
DOI URL |
| [116] |
LI T, CHEN L, YANG X, et al. A flexible pressure sensor based on an MXene-textile network structure. Journal of Materials Chemistry C, 2019, 7(4): 1022-1027.
DOI |
| [117] |
KALELKAR P P, RIDDICK M, GARCIA A J. Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nature Reviews Materials, 2022, 7(1): 39-54.
DOI URL |
| [118] |
LI J F, LI Z Y, LIU X M, et al. Interfacial engineering of Bi2S3/Ti3C2Tx MXene based on work function for rapid photo- excited bacteria-killing. Nature Communications, 2021, 12(1): 1224-10.
DOI URL |
| [119] |
DURÁN N, DURÁN M, DE JESUS MB, et al. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine: Nanotechnology, Biology and Medicine, 2016, 12(3): 789-799.
DOI URL |
| [120] |
PANÁČEK A, KVÍTEK L, SMÉKALOVÁ M, et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nature Nanotechnology, 2018, 13(1): 65-71.
DOI PMID |
| [121] |
STABRYLA L M, JOHNSTON K A, DIEMLER N A, et al. Role of bacterial motility in differential resistance mechanisms of silver nanoparticles and silver ions. Nature Nanotechnology, 2021, 16(9): 996-1003.
DOI URL |
| [122] |
LI B, WANG W, SONG W, et al. Antiviral and anti-inflammatory treatment with multifunctional alveolar macrophage-like nanoparticles in a surrogate mouse model of COVID-19. Advanced Science, 2021, 8(13): 2003556-14.
DOI URL |
| [123] |
VALLIERES C, HOOK A L, HE Y, et al. Discovery of (meth)acrylate polymers that resist colonization by fungi associated with pathogenesis and biodeterioration. Science Advances, 2020, 6(23): eaba6574-12.
DOI URL |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | CHEN Xi, YUAN Yuan, TAN Yeqiang, LIU Changsheng. Strategic Study on the Development of Inorganic Non-metallic Biomaterials [J]. Journal of Inorganic Materials, 2025, 40(5): 449-456. |
| [8] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [9] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [10] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [11] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [12] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [13] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [14] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [15] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||