
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (7): 718-724.DOI: 10.15541/jim20200522
• RESEARCH ARTICLE • Previous Articles Next Articles
FU Yukun1( ), ZENG Min1, RAO Xianfa1, ZHONG Shengwen1, ZHANG Huijuan2, YAO Wenli1(
), ZENG Min1, RAO Xianfa1, ZHONG Shengwen1, ZHANG Huijuan2, YAO Wenli1( )
)
Received:2020-09-07
Revised:2021-01-04
Published:2021-07-20
Online:2021-01-25
Contact:
YAO Wenli, associate professor. E-mail:wenliyao@126.com
About author:FU Yukun(1996-), male, Master candidate. E-mail:475322904@qq.com
Supported by:CLC Number:
FU Yukun, ZENG Min, RAO Xianfa, ZHONG Shengwen, ZHANG Huijuan, YAO Wenli. Microwave-assisted Synthesis and Co, Al Co-modification of Ni-rich LiNi0.8Mn0.2O2 Materials for Li-ion Battery Electrode[J]. Journal of Inorganic Materials, 2021, 36(7): 718-724.
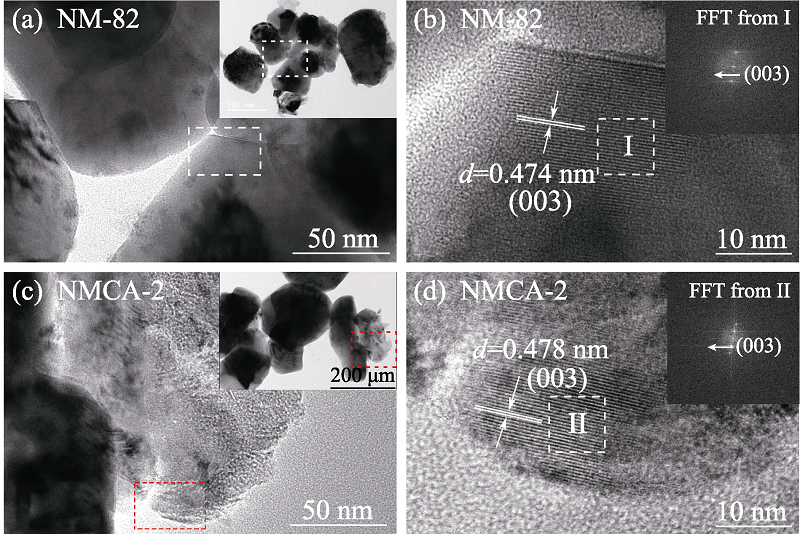
Fig. 4 (a,c) TEM images, (b,d)HR-TEM images and (insets in (b, d)) the corresponding FFT graphs of NM-82 and NMCA-2 (a, c) Magnified images of the square areas in the corresponding insets
| Sample | a/nm | c/nm | c/a | I(003)/I(104) |
|---|---|---|---|---|
| NM-82 | 0.2875 | 1.4232 | 4.9507 | 1.3197 |
| NMCA-0 | 0.2877 | 1.4229 | 4.9457 | 1.3945 |
| NMCA-2 | 0.2872 | 1.4243 | 4.9593 | 1.5888 |
Table S1 Parameters of Rietveld refinement for NM-82, NMCA-0 and NMCA-2
| Sample | a/nm | c/nm | c/a | I(003)/I(104) |
|---|---|---|---|---|
| NM-82 | 0.2875 | 1.4232 | 4.9507 | 1.3197 |
| NMCA-0 | 0.2877 | 1.4229 | 4.9457 | 1.3945 |
| NMCA-2 | 0.2872 | 1.4243 | 4.9593 | 1.5888 |
| Sample | 0.2C | 0.5C | 1C | 2C | 3C | 4C | 5C | 0.2C |
|---|---|---|---|---|---|---|---|---|
| NM-82 | 195.57 | 183.52 | 175.03 | 164.59 | 157.33 | 149.26 | 141.49 | 185.66 |
| NMCA-0 | 192.45 | 182.66 | 176.69 | 166.91 | 160.88 | 157.05 | 153.17 | 183.80 |
| NMCA-1 | 189.79 | 182.60 | 177.43 | 169.72 | 165.24 | 162.25 | 158.83 | 187.36 |
| NMCA-2 | 189.70 | 183.05 | 177.84 | 172.80 | 169.08 | 164.76 | 160.03 | 189.25 |
| NMCA-3 | 187.28 | 180.32 | 174.85 | 168.60 | 162.63 | 158.61 | 156.14 | 187.50 |
| NMCA-5 | 186.04 | 178.28 | 173.83 | 167.08 | 163.41 | 157.97 | 150.84 | 181.33 |
Table S2 Specific discharge capacities of materials at different rates/(mAh·g-1)
| Sample | 0.2C | 0.5C | 1C | 2C | 3C | 4C | 5C | 0.2C |
|---|---|---|---|---|---|---|---|---|
| NM-82 | 195.57 | 183.52 | 175.03 | 164.59 | 157.33 | 149.26 | 141.49 | 185.66 |
| NMCA-0 | 192.45 | 182.66 | 176.69 | 166.91 | 160.88 | 157.05 | 153.17 | 183.80 |
| NMCA-1 | 189.79 | 182.60 | 177.43 | 169.72 | 165.24 | 162.25 | 158.83 | 187.36 |
| NMCA-2 | 189.70 | 183.05 | 177.84 | 172.80 | 169.08 | 164.76 | 160.03 | 189.25 |
| NMCA-3 | 187.28 | 180.32 | 174.85 | 168.60 | 162.63 | 158.61 | 156.14 | 187.50 |
| NMCA-5 | 186.04 | 178.28 | 173.83 | 167.08 | 163.41 | 157.97 | 150.84 | 181.33 |
| Sample | Cycle number | Rs/Ω | Rsf /Ω | Rct/Ω |
|---|---|---|---|---|
| NM-82 | 5 | 3.85 | 20.20 | 46.65 |
| 50 | 3.09 | 49.32 | 64.95 | |
| 100 | 7.27 | 94.06 | 138.40 | |
| NMCA-0 | 5 | 1.89 | 29.24 | 20.19 |
| 50 | 2.01 | 58.13 | 72.25 | |
| 100 | 4.04 | 82.55 | 114.90 | |
| NMCA-2 | 5 | 5.11 | 31.85 | 19.80 |
| 50 | 3.30 | 51.78 | 72.70 | |
| 100 | 4.08 | 67.32 | 67.88 |
Table S3 EIS fitting data of NM-82, NMCA-0 and NMCA-2
| Sample | Cycle number | Rs/Ω | Rsf /Ω | Rct/Ω |
|---|---|---|---|---|
| NM-82 | 5 | 3.85 | 20.20 | 46.65 |
| 50 | 3.09 | 49.32 | 64.95 | |
| 100 | 7.27 | 94.06 | 138.40 | |
| NMCA-0 | 5 | 1.89 | 29.24 | 20.19 |
| 50 | 2.01 | 58.13 | 72.25 | |
| 100 | 4.04 | 82.55 | 114.90 | |
| NMCA-2 | 5 | 5.11 | 31.85 | 19.80 |
| 50 | 3.30 | 51.78 | 72.70 | |
| 100 | 4.08 | 67.32 | 67.88 |
| [1] |
LIU J, ZHANG J G, YANG Z, et al. Materials science and materials chemistry for large scale electrochemical energy storage: from transportation to electrical grid. Adv. Funct. Mater., 2013,23(8):929-946.
DOI URL |
| [2] |
KE J, XIE K, HAN Y, et al. Morphology controlling of the high-voltage cathode materials with different co-solvents. J. Inorg. Mater., 2019,34(6):618-624.
DOI URL |
| [3] |
LI X, GE W, WANG H, et al. Research progress on the capacity fading mechanisms of high-nickel ternary layered oxide cathode materials. J. Inorg. Mater., 2017,32(2):113-121.
DOI URL |
| [4] |
MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun., 2020,11(1):1550.
DOI URL |
| [5] | 邱世涛, 钟盛文, 李婷婷, 等. Cu掺杂LiNi0.6Co0.2Mn0.2O2的电化学性能. 有色金属科学与工程, 2018,9(5):25-29. |
| [6] |
LI M, JUN L. Cobalt in lithium-ion batteries. Science, 2020,367(6481):979-980.
DOI URL |
| [7] |
LI W, ERICKSON E M, MANTHIRAM A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy, 2020,5(1):26-34.
DOI URL |
| [8] |
ZHAO E, FANG L, CHEN M, et al. New insight into Li/Ni disorder in layered cathode materials for lithium ion batteries: a joint study of neutron diffraction, electrochemical kinetic analysis and first-principles calculations. J. Mater. Chem. A, 2017,5(4):1679-1686.
DOI URL |
| [9] |
WU F, LIU N, CHEN L, et al. Improving the reversibility of the H2-H3 phase transitions for layered Ni-rich oxide cathode towards retarded structural transition and enhanced cycle stability. Nano Energy, 2019,59:50-57.
DOI URL |
| [10] | 孔祥泽, 李东林, 王子匀, 等. 钨掺杂对锂离子电池LiNiO2正极材料性能的影响. 无机化学学报, 2019,35(7):1169-1175. |
| [11] |
YAO W, LIU Y, LI D, et al. Synergistically enhanced electrochemical performance of Ni-rich cathode materials for lithium-ion batteries by K and Ti co-modification. J. Phys. Chem. C, 2020,124(4):2346-2356.
DOI URL |
| [12] |
DIXIT M, MARKOVSKY B, SCHIPPER F, et al. The origin of structural degradation during cycling and low thermal stability of Ni-rich layered transition metal-based electrode materials. J. Phys. Chem. C, 2017,121:22628.
DOI URL |
| [13] | 钟盛文, 张华军, 姚文俐, 等. LiNi0.5Mn0.5-xCoxO2(0≤x≤0.12) 正极材料的制备及其电化学性能. 材料研究学报, 2018,32(7):9-16. |
| [14] |
TORNHEIM A, SHARIFI-ASL S, GARCIA J C, et al. Effect of electrolyte composition on rock salt surface degradation in NMC cathodes during high-voltage potentiostatic holds. Nano Energy, 2019,55:216-225.
DOI URL |
| [15] |
MATSUMOTO K, KUZUO R, TAKEYA K, et al. Effects of CO2 in air on Li deintercalation from LiNi1-x-yCoxAlyO2. J. Power Sources, 1999, 81-82:558-561.
DOI URL |
| [16] |
ZHANG S S, FAN X, WANG C. Enhanced electrochemical performance of Ni-rich layered cathode materials by using LiPF6 as a cathode additive. ChemElectroChem, 2019,6(5):1536-1541.
DOI URL |
| [17] |
HATSUKADE T, SCHIELE A, HARTMANN P, et al. Origin of carbon dioxide evolved during cycling of nickel-rich layered NCM cathodes. ACS Appl. Mater. Interfaces, 2018,10:38892-38899.
DOI URL |
| [18] |
YAO W, DAI Q, LIU Y, et al. Microwave-assisted synthesis of Co3O4 sheets for reversible Li storage: regulation of structure and performance. ChemElectroChem, 2017,4(5):1236-1242.
DOI URL |
| [19] |
LIU Y, YAO W, LEI C, et al. Ni-rich oxide LiNi0.85Co0.05Mn0.1O2 for lithium ion battery: effect of microwave radiation on its morphology and electrochemical property. J. Electrochem. Soc., 2019,166(8):A1300-A1309.
DOI URL |
| [20] |
YOSHIO M, NOGUCHI H, ITOH J I, et al. Preparation and properties of LiCoyMnxNi1-x-yO2 as a cathode for lithium ion batteries. J. Power Sources, 2000,90(2):176-181.
DOI URL |
| [21] |
LI Z, ZHANG J, GAO R, et al. Unveiling the role of Co in improving the high-rate capability and cycling performance of layered Na0.7Mn0.7Ni0.3-xCoxO2 cathode materials for sodium-ion batteries. ACS Appl. Mater. Interfaces, 2016,8(24):15439-15448.
DOI URL |
| [22] |
BAI X, BAN L, ZHUANG W. Research progress on coating and doping modification of nickel rich ternary cathode materials. J. Inorg. Mater., 2020,35(9):972-986.
DOI URL |
| [23] |
LI Y C, XIANG W, WU Z G, et al. Construction of homogeneously Al3+ doped Ni rich Ni-Co-Mn cathode with high stable cycling performance and storage stability via scalable continuous precipitation. Electrochim. Acta, 2018,291:84-94.
DOI URL |
| [24] |
PARK K J, CHOI M J, MAGLIA F, et al. High-capacity concentration gradient Li[Ni0.865Co0.120Al0.015]O2 cathode for lithium-ion batteries. Adv. Energy Mater., 2018,8(19):1703612.
DOI URL |
| [25] |
ZHAO W G, ZOU L F, JIA H P, et al. Optimized Al doping improves both interphase stability and bulk structural integrity of Ni-rich NMC cathode materials. ACS Appl. Energy Mater., 2020,3(4):3369-3377.
DOI URL |
| [26] | 郭乾坤, 黄吉丽, 周苗苗, 等. 单晶LiNi0.83Co0.1Mn0.07O2正极材料的合成及电化学性能. 有色金属科学与工程, 2020,11(4):23-28. |
| [27] |
XIE Z, ZHANG Y, YUAN A, et al. Effects of lithium excess and SnO2 surface coating on the electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material for lithium-ion batteries. J. Alloys Compd., 2019,787:429-439.
DOI URL |
| [28] |
LI J, LIU Y, YAO W, et al. Li2TiO3 and Li2ZrO3 co-modification LiNi0.8Co0.1Mn0.1O2 cathode material with improved high-voltage cycling performance for lithium-ion batteries. Solid State Ionics, 2020,349:115292.
DOI URL |
| [1] | WANG Yang, FAN Guangxin, LIU Pei, YIN Jinpei, LIU Baozhong, ZHU Linjian, LUO Chengguo. Microscopic Mechanism of K+ Doping on Performance of Lithium Manganese Cathode for Li-ion Battery [J]. Journal of Inorganic Materials, 2022, 37(9): 1023-1029. |
| [2] | Yong LI, Wei-Xin HE, Xin-Yue ZHENG, Sheng-Lan YU, Hai-Tong LI, Hong-Yi LI, Rong ZHANG, Yu WANG. Prussian Blue Cathode Materials for Aqueous Sodium-ion Batteries:Preparation and Electrochemical Performance [J]. Journal of Inorganic Materials, 2019, 34(4): 365-372. |
| [3] | WANG Qi, PENG Da-Chun, MA Qian, HE Yue-De, LIU Hong-Bo. Synthesis and Electrochemical Property of Carbon Coated LiFePO4 Nanoplates [J]. Journal of Inorganic Materials, 2018, 33(12): 1349-1354. |
| [4] | MA Guo-Qiang, WEN Zhao-Yin, WANG Qing-Song, JIN Jun, WU Xiang-Wei, ZHANG Jing-Chao. Effects of CeO2 Nano-crystal on Electrochemical Properties of Lithium/Sulfur Batteries [J]. Journal of Inorganic Materials, 2015, 30(9): 913-918. |
| [5] | QIU Cai-Xia, YUAN Zhong-Zhi, LIU Ling, CHENG Si-Jie, LIU Jin-Cheng. Preparation and Characterization of Ge4+-doping Li4Ti5O12 Anode Material for Li-ion Battery and Its Electrochemical Properties [J]. Journal of Inorganic Materials, 2013, 28(7): 727-732. |
| [6] | HUA Ning, WANG Chen-Yun, KANG Xue-Ya, Tuerdi, HAN Ying. Synthesis and Electrochemical Characterizations of Zinc-doped LiFePO4/C by Carbothermal Reduction [J]. Journal of Inorganic Materials, 2010, 25(8): 887-892. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||