
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (10): 1088-1098.DOI: 10.15541/jim20190572
Special Issue: 【虚拟专辑】钙钛矿材料(2020~2021)
Previous Articles Next Articles
YANG Dandan( ),LI Xiaoming(
),LI Xiaoming( ),MENG Cuifang,CHEN Jiaxin,ZENG Haibo
),MENG Cuifang,CHEN Jiaxin,ZENG Haibo
Received:2019-11-09
Revised:2020-02-04
Published:2020-10-20
Online:2020-09-23
About author:YANG Dandan (1989-), female, PhD candidate. E-mail: dandan.yang@njust.edu.cn
Supported by:CLC Number:
YANG Dandan, LI Xiaoming, MENG Cuifang, CHEN Jiaxin, ZENG Haibo. Research Progress on the Stability of CsPbX3 Nanocrystals[J]. Journal of Inorganic Materials, 2020, 35(10): 1088-1098.
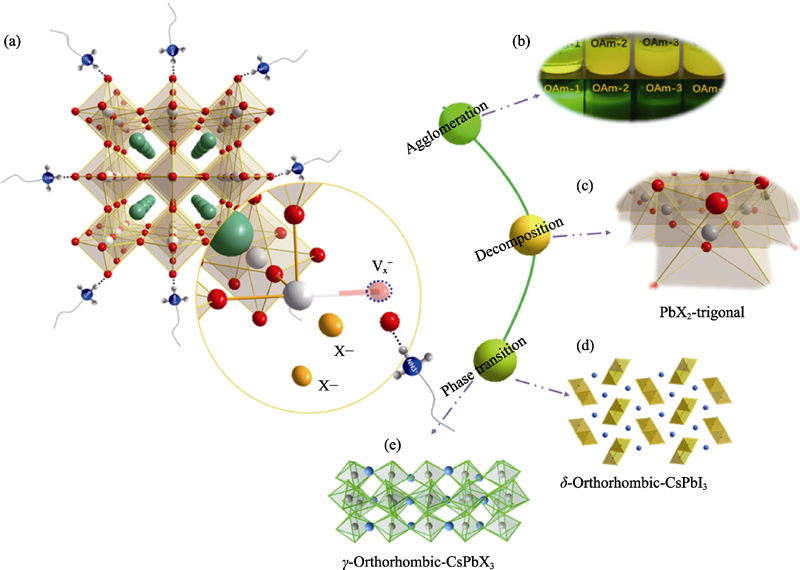
Fig. 2 (a) Schematic diagram of instability mechanism of CsPbX3 nanocrystals, (b) agglomeration and (c) decomposing product of CsPbX3 nanocrystals, and schematic diagram of (d) phase transition (non-perovskite phase) and (e) phase transition (perovskite phase)
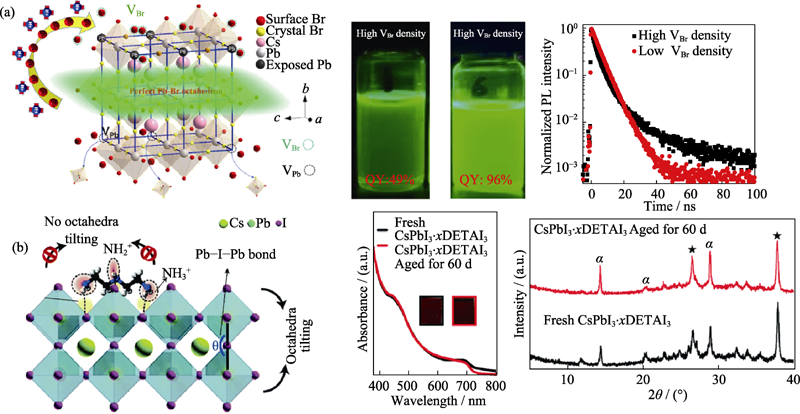
Fig. 3 (a) Passivation strategy based on hard Lewis acid ligands (left), images under 365 nm UV light (middle), and photoluminescence decay curves (right) of nanocrystals with high and low defect densities[25]; (b) Schematic diagram of DETAI3 surface passivation strategy (left), absorption curves (middle) and long-term phase stability (right) of CsPbI3?xDETAI3 thin films[29]
| Ligands | Treating agent | QY/% | Stability | Ref. |
|---|---|---|---|---|
| OA/OAm | DDDMAB | ~100 | 21 d | [ |
| OA/OAm | TOAB | 95 | - | [ |
| OA/OAm | DDAB | 96 | - | [ |
| OA/OAm | NH4BF4 | (95±2) | - | [ |
| OA/OAm | NH4SCN | (99±2) | - | [ |
| OA/OAm | Trimethylsilyl iodine | 85 | 105 d | [ |
| OA/OAm | Oxalic acid | 89 | - | [ |
Table 1 Surface passivation strategies of Lewis acid ligands
| Ligands | Treating agent | QY/% | Stability | Ref. |
|---|---|---|---|---|
| OA/OAm | DDDMAB | ~100 | 21 d | [ |
| OA/OAm | TOAB | 95 | - | [ |
| OA/OAm | DDAB | 96 | - | [ |
| OA/OAm | NH4BF4 | (95±2) | - | [ |
| OA/OAm | NH4SCN | (99±2) | - | [ |
| OA/OAm | Trimethylsilyl iodine | 85 | 105 d | [ |
| OA/OAm | Oxalic acid | 89 | - | [ |
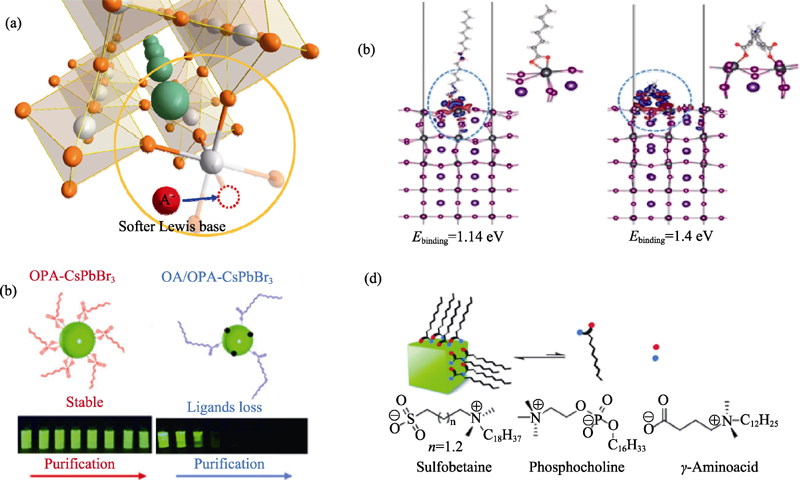
Fig. 4 (a) Schematic diagram of Lewis base surface passivation strategy for CsPbX3 nanocrystals; (b) Theoretical calculation of the binding energy of mono- and dicarboxylic acids on the surface of CsPbI3 nanocrystals[34]; (c) Schematic diagram of OPA and OAm-CsPbX3 surface passivation strategies and photos after multiple purifications[35]; (d) Surface passivation strategy with zwitterionic ligands (sulfobetaines, phosphocholines and γ-amino acids)[36]
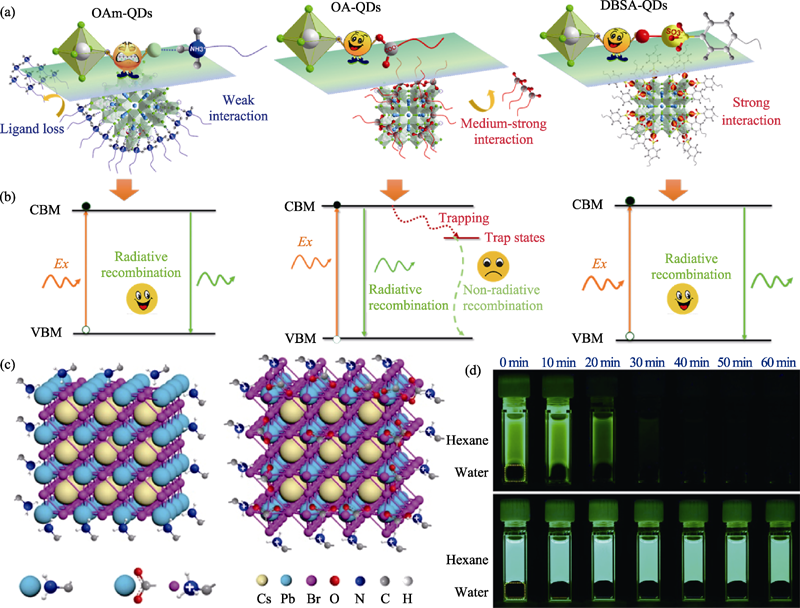
Fig. 5 (a) Passivation strategies with different ligands (OAm, OA and DBSA) on the surface of CsPbBr3 nanocrystals and (b) the corresponding exciton recombination processes[37]; (c) Schematic diagram of PbBrx-rich surface of OAm-CsPbBr3 nanocrystals (left) and Br-rich surface of OAm/OA-CsPbBr3 nanocrystals (right)[11]; (d) Photographs showing the resistance of different samples against water treatment of OAm/OA-CsPbBr3 nanocrystals (above) and OAm-CsPbBr3 nanocrystals (below)[11]
| Ligands | QY/% | Stability | Ref. |
|---|---|---|---|
| OAm | ~100 % | - | [ |
| OA | 70 | - | [ |
| IDA/OAm | 95 | 40 d | [ |
| OPA/TOPO | >90 | - | [ |
| Zwitterion | >90 | 28 d | [ |
| DBSA | 95 | 5 m | [ |
| TMPPA/OAm | 90 | 28 d | [ |
| OA/TOPO | 90 | - | [ |
| TDPA/OAm | 68 | - | [ |
| DA | 94.3 | 70 d | [ |
Table 2 Surface passivation strategies of Lewis base ligands
| Ligands | QY/% | Stability | Ref. |
|---|---|---|---|
| OAm | ~100 % | - | [ |
| OA | 70 | - | [ |
| IDA/OAm | 95 | 40 d | [ |
| OPA/TOPO | >90 | - | [ |
| Zwitterion | >90 | 28 d | [ |
| DBSA | 95 | 5 m | [ |
| TMPPA/OAm | 90 | 28 d | [ |
| OA/TOPO | 90 | - | [ |
| TDPA/OAm | 68 | - | [ |
| DA | 94.3 | 70 d | [ |
| Strategies | Characteristics | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|
| Lewis acid ligands | Quaternary ammonium salt | Large steric hindrance | High QY* | Instable | [ |
| Lewis base ligands | Carboxylic acids | Hard base, weak acid | Simple synthesis | Instable, low QY | [ |
| Phosphoric acid | Soft alkali, moderately strong acid | High QY, stable | TOPO assisted dissolution | [ | |
| Zwitterionic ligands | Surfactant | High QY, stable | Complex process | [ | |
| Sulfonic acid | Soft alkali, strong acid | High QY, stable | High temperature | [ | |
| Neutral ligands | Lone pair electrons | High QY, stable | Room temperature | [ | |
Table 3 Characteristics, advantages and disadvantages of different passivation strategies
| Strategies | Characteristics | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|
| Lewis acid ligands | Quaternary ammonium salt | Large steric hindrance | High QY* | Instable | [ |
| Lewis base ligands | Carboxylic acids | Hard base, weak acid | Simple synthesis | Instable, low QY | [ |
| Phosphoric acid | Soft alkali, moderately strong acid | High QY, stable | TOPO assisted dissolution | [ | |
| Zwitterionic ligands | Surfactant | High QY, stable | Complex process | [ | |
| Sulfonic acid | Soft alkali, strong acid | High QY, stable | High temperature | [ | |
| Neutral ligands | Lone pair electrons | High QY, stable | Room temperature | [ | |
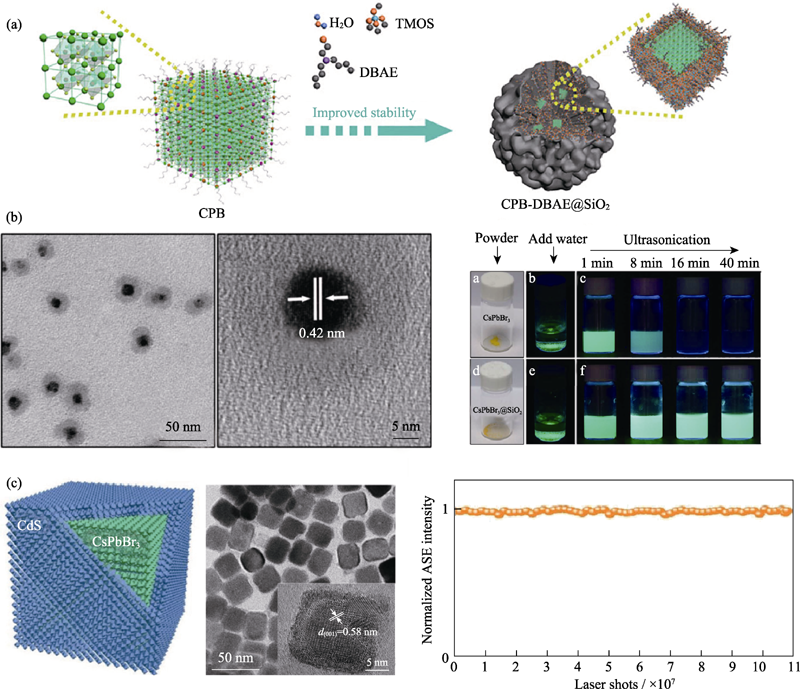
Fig. 6 (a) Schematic diagram of CPB-DBAE@SiO2 preparation process[51]; (b) Transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) images of CsPbBr3@SiO2 nanocrystals and photographs of water stability[60]; (c) Schematic representation (left), HRTEM image (middle), and plot of emission intensity under continuous pulsed laser irradiation (right) of CsPbBr3/CdS nanocrystals[67]
| [1] |
KOJIMA A, TESHIMA K, SHIRAI Y , et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. Journal of the American Chemical Society, 2009,131(17):6050-6051.
DOI URL PMID |
| [2] |
SCHMIDT L C, PERTEGAS A, GONZALEZ-CARRERO S , et al. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. Journal of the American Chemical Society, 2014,136(3):850-853.
DOI URL PMID |
| [3] |
PROTESESCU L, YAKUNIN S, BODNARCHUK M I , et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Letters, 2015,15(6):3692-3696.
DOI URL PMID |
| [4] | DOU L, LAI M, KLEY C S , et al. Spatially resolved multicolor CsPbX3 nanowire heterojunctions via anion exchange. Proceedings of the National Academy of Sciences of the United States of America, 2017,114(28):7216-7221. |
| [5] |
ALMEIDA G, INFANTE I, MANNA L . Resurfacing halide perovskite nanocrystals. Science, 2019,364(6443):833-834.
DOI URL PMID |
| [6] | LI X, WU Y, ZHANG S , et al. CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Advanced Functional Materials, 2016,26(15):2435-2445. |
| [7] |
DE ROO J, IBANEZ M, GEIREGAT P , et al. Highly dynamic ligand binding and light absorption coefficient of cesium lead bromide perovskite nanocrystals. ACS Nano, 2016,10(2):2071-2081.
DOI URL PMID |
| [8] |
RAVI V K, SANTRA P K, JOSHI N , et al. Origin of the substitution mechanism for the binding of organic ligands on the surface of CsPbBr3 perovskite nanocubes. The Journal of Physical Chemistry Letters, 2017,8(20):4988-4994.
DOI URL PMID |
| [9] | IMRAN M, IJAZ P, GOLDONI L , et al. Simultaneous cationic and anionic ligand exchange for colloidally stable CsPbBr3 nanocrystals. ACS Energy Letters, 2019,4(4):819-824. |
| [10] |
NENON D P, PRESSLER K, KANG J , et al. Design principles for trap-free CsPbX3 nanocrystals: enumerating and eliminating surface halide vacancies with softer Lewis bases. Journal of the American Chemical Society, 2018,140(50):17760-17772.
DOI URL PMID |
| [11] |
ZHONG Q, CAO M, XU Y , et al. L-type ligand-assisted acid-free synthesis of CsPbBr3 nanocrystals with near-unity photoluminescence quantum yield and high stability. Nano Letters, 2019,19(6):4151-4157.
DOI URL PMID |
| [12] |
WEI Y, CHENG Z, LIN J . An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chemical Society Reviews, 2019,48(1):310-350.
DOI URL PMID |
| [13] |
ZHAO Y, ZHU K . Organic-inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chemical Society Reviews, 2016,45(3):655-689.
DOI URL PMID |
| [14] | ZHOU Y, ZHAO Y . Chemical stability and instability of inorganic halide perovskites. Energy & Environmental Science, 2019,12(5):1495-1511. |
| [15] | DUTTA A, PRADHAN N . Phase-stable red-emitting CsPbI3 nanocrystals: successes and challenges. ACS Energy Letters, 2019,4(3):709-719. |
| [16] | LI F, LIU Y, WANG H , et al. Postsynthetic surface trap removal of CsPbX3(X=Cl, Br, or I) quantum dots via a ZnX2/hexane solution toward an enhanced luminescence quantum yield. Chemistry of Materials, 2018,30(23):8546-8554. |
| [17] | SUN J K, HUANG S, LIU X Z , et al. Polar solvent induced lattice distortion of cubic CsPbI3 nanocubes and hierarchical self-assembly into orthorhombic single-crystalline nanowires. Journal of the American Chemical Society, 2018,140(37):11705-11715. |
| [18] |
HOU Y, ZHOU Z R, WEN T Y , et al. Enhanced moisture stability of metal halide perovskite solar cells based on sulfur-oleylamine surface modification. Nanoscale Horizons, 2019,4(1):208-213.
DOI URL PMID |
| [19] |
ZHOU W, SUI F, ZHONG G , et al. Lattice dynamics and thermal stability of cubic-phase CsPbI3 quantum dots. The Journal of Physical Chemistry Letters, 2018,9(17):4915-4920.
DOI URL PMID |
| [20] |
RUAN L, SHEN W, WANG A , et al. Stable and conductive lead halide perovskites facilitated by X-type ligands. Nanoscale, 2017,9(21):7252-7259.
DOI URL PMID |
| [21] | YANG D, LI X, ZENG H . Surface chemistry of all inorganic halide perovskite nanocrystals: passivation mechanism and stability . Advanced Materials Interfaces, 2018,5(8):1701662. |
| [22] |
KANG J, WANG L W . High defect tolerance in lead halide perovskite CsPbBr3. The Journal of Physical Chemistry Letters, 2017,8(2):489-493.
DOI URL PMID |
| [23] |
RAN C, XU J, GAO W , et al. Defects in metal triiodide perovskite materials towards high-performance solar cells: origin, impact, characterization, and engineering. Chemical Society Reviews, 2018,47(12):4581-4610.
DOI URL PMID |
| [24] |
LI X, CAO F, YU D , et al. All inorganic halide perovskites nanosystem: synthesis, structural features, optical properties and optoelectronic applications. Small, 2017,13(9):1603996.
DOI URL |
| [25] |
YANG D, LI X, WU Y , et al. Surface halogen compensation for robust performance enhancements of CsPbX3 perovskite quantum dots. Advanced Optical Materials, 2019,7(11):1900276.
DOI URL |
| [26] |
WU H, ZHANG Y, LU M , et al. Surface ligand modification of cesium lead bromide nanocrystals for improved light-emitting performance. Nanoscale, 2018,10(9):4173-4178.
DOI URL PMID |
| [27] | AHMED T, SETH S, SAMANTA A . Boosting the photoluminescence of CsPbX3 (X=Cl, Br, I) perovskite nanocrystals covering a wide wavelength range by postsynthetic treatment with tetrafluoroborate salts. Chemistry of Materials, 2018,30(11):3633-3637. |
| [28] |
KOSCHER B A, SWABECK J K, BRONSTEIN N D , et al. Essentially trap-free CsPbBr3 colloidal nanocrystals by postsynthetic thiocyanate surface treatment. Journal of the American Chemical Society, 2017,139(19):6566-6569.
DOI URL PMID |
| [29] | DING X, CHEN H, WU Y , et al. Triple cation additive NH 3+C 2H 4NH 2+C2H4NH 3+-induced phase-stable inorganic α-CsPbI3 perovskite films for use in solar cells. Journal of Materials Chemistry A , 2018,6(37):18258-18266. |
| [30] |
PAN J, QUAN L N, ZHAO Y , et al. Highly efficient perovskite quantum dot light-emitting diodes by surface engineering. Advanced Materials, 2016,28(39):8718-8725.
DOI URL PMID |
| [31] | ZHU J, ZHU Y, HUANG J , et al. Synthesis of CsPbBr3 perovskite nanocrystals with the sole ligand of protonated (3-aminopropyl) triethoxysilane. Journal of Materials Chemistry C, 2019,7(24):7201-7206. |
| [32] | WANG S, SHEN X, ZHANG Y , et al. Oxalic acid enabled emission enhancement and continuous extraction of chloride from cesium lead chloride/bromide perovskite nanocrystals. Small, 2019,15(34):1901828. |
| [33] | YASSITEPE E, YANG Z, VOZNYY O , et al. Amine-free synthesis of cesium lead halide perovskite quantum dots for efficient light- emitting diodes. Advanced Functional Materials, 2016,26(47):8757-8763. |
| [34] | PAN J, SHANG Y, YIN J , et al. Bidentate ligand-passivated CsPbI3 perovskite nanocrystals for stable near-unity photoluminescence quantum yield and efficient red light-emitting diodes. Journal of the American Chemical Society, 2018,140(2):562-565. |
| [35] |
TAN Y, ZOU Y, WU L , et al. Highly luminescent and stable perovskite nanocrystals with octylphosphonic acid as a ligand for efficient light-emitting diodes. ACS Applied Materials & Interfaces, 2018,10(4):3784-3792.
DOI URL PMID |
| [36] |
KRIEG F, OCHSENBEIN S T, YAKUNIN S , et al. Colloidal CsPbX3(X=Cl, Br, I) nanocrystals 2.0: zwitterionic capping ligands for improved durability and stability. ACS Energy Letters, 2018,3(3):641-646.
DOI URL PMID |
| [37] | YANG D, LI X, ZHOU W , et al. CsPbBr3 quantum dots 2.0: benzenesulfonic acid equivalent ligand awakens complete purification. Advanced Materials, 2019,31(30):1900767. |
| [38] |
WANG C, CHESMAN A S, JASIENIAK J J . Stabilizing the cubic perovskite phase of CsPbI3 nanocrystals by using an alkyl phosphinic acid. Chemical Communications, 2016,53(1):232-235.
DOI URL PMID |
| [39] |
ALMEIDA G, ASHTON O J, GOLDONI L , et al. The phosphine oxide route toward lead halide perovskite nanocrystals. Journal of the American Chemical Society, 2018,140(44):14878-14886.
DOI URL PMID |
| [40] |
XUAN T, YANG X, LOU S , et al. Highly stable CsPbBr3 quantum dots coated with alkyl phosphate for white light-emitting diodes. Nanoscale, 2017,9(40):15286-15290.
DOI URL PMID |
| [41] | YAN D, SHI T, ZANG Z , et al. Ultrastable CsPbBr3 perovskite quantum dot and their enhanced amplified spontaneous emission by surface ligand modification. Small, 2019,15(23):1901173. |
| [42] |
WU L Z, ZHONG Q X, YANG D , et al. Improving the stability and size tunability of cesium lead halide perovskite nanocrystals using trioctylphosphine oxide as the capping ligand. Langmuir, 2017,33(44):12689-12696.
DOI URL PMID |
| [43] |
HOU S, GUO Y, TANG Y , et al. Synthesis and stabilization of colloidal perovskite nanocrystals by multidentate polymer micelles. ACS Applied Materials & Interfaces, 2017,9(22):18417-18422.
DOI URL PMID |
| [44] |
HAI J, LI H, ZHAO Y , et al. Designing of blue, green, and red CsPbX3 perovskite-codoped flexible films with water resistant property and elimination of anion-exchange for tunable white light emission. Chemical Communications, 2017,53(39):5400-5403.
DOI URL PMID |
| [45] |
MEYNS M, PERALVAREZ M, HEUER-JUNGEMAN A , et al. Polymer-enhanced stability of inorganic perovskite nanocrystals and their application in color conversion LEDs. ACS Applied Materials & Interfaces, 2016,8(30):19579-19586.
DOI URL PMID |
| [46] |
RAJA S N, BEKENSTEIN Y, KOC M A , et al. Encapsulation of perovskite nanocrystals into macroscale polymer matrices: enhanced stability and polarization. ACS Applied Materials & Interfaces, 2016,8(51):35523-35533.
DOI URL PMID |
| [47] |
LOIUDICE A, SARIS S, OVEISI E , et al. CsPbBr3 QD/AlOx inorganic nanocomposites with exceptional stability in water, light, and heat. Angewandte Chemie-International Edition, 2017,56(36):10696-10701.
DOI URL PMID |
| [48] | LI Z J, HOFMAN E, LI J , et al. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Advanced Functional Materials, 2018,28(1):1704288. |
| [49] | HU Z, LIU Z, BIAN Y , et al. Enhanced two-photon-pumped emission from in situ synthesized nonblinking CsPbBr3/SiO2 nanocrystals with excellent stability. Advanced Optical Materials, 2018,6(3):1700997. |
| [50] |
LI Z, KONG L, HUANG S , et al. Highly luminescent and ultrastable CsPbBr3 perovskite quantum dots incorporated into a silica/ alumina monolith. Angewandte Chemie International Edition, 2017,56(28):8134-8138.
DOI URL PMID |
| [51] | LI L, ZHANG Z, CHEN Y , et al. Sustainable and self-enhanced electrochemiluminescent ternary suprastructures derived from CsPbBr3 perovskite quantum dots. Advanced Functional Materials, 2019,29(32):1902533. |
| [52] |
WU H, LIN S, WANG R , et al. Water-stable and ion exchange-free inorganic perovskite quantum dots encapsulated in solid paraffin and their application in light emitting diodes. Nanoscale, 2019,11(12):5557-5563.
DOI URL PMID |
| [53] |
WANG B, ZHANG C, HUANG S , et al. Postsynthesis phase transformation for CsPbBr3/Rb4PbBr6 core/shell nanocrystals with exceptional photostability. ACS Applied Materials & Interfaces, 2018,10(27):23303-23310.
DOI URL PMID |
| [54] | WEI Y, XIAO H, XIE Z , et al. Highly luminescent lead halide perovskite quantum dots in hierarchical CaF2 matrices with enhanced stability as phosphors for white light-emitting diodes. Advanced Optical Materials, 2018,6(11):1701343. |
| [55] | VELDHUIS S A, NG Y F, AHMAD R , et al. Crown ethers enable room-temperature synthesis of CsPbBr3 quantum dots for light- emitting diodes. ACS Energy Letters, 2018,3(3):526-531. |
| [56] |
CHEN Y, YU M, YE S , et al. All-inorganic CsPbBr3 perovskite quantum dots embedded in dual-mesoporous silica with moisture resistance for two-photon-pumped plasmonic nanolasers. Nanoscale, 2018,10(14):6704-6711.
DOI URL PMID |
| [57] | HAO J, QU X, QIU L , et al. One-step loading on natural mineral halloysite nanotube: an effective way to enhance the stability of perovskite CsPbX3(X=Cl, Br, I) quantum dots. Advanced Optical Materials, 2019,7(4):1801323. |
| [58] |
WU L, HU H, XU Y , et al. From nonluminescent Cs4PbX6(X=Cl, Br, I) nanocrystals to highly luminescent CsPbX3 nanocrystals: water-triggered transformation through a CsX-stripping mechanism. Nano Letters, 2017,17(9):5799-5804.
DOI URL PMID |
| [59] |
HU H, WU L, TAN Y , et al. Interfacial synthesis of highly stable CsPbX3/oxide Janus nanoparticles. Journal of the American Chemical Society, 2018,140(1):406-412.
DOI URL PMID |
| [60] |
ZHONG Q, CAO M, HU H , et al. One-pot synthesis of highly stable CsPbBr3@SiO2 core-shell nanoparticles. ACS Nano, 2018,12(8):8579-8587.
DOI URL PMID |
| [61] |
ZHANG X, LU M, ZHANG Y , et al. PbS capped CsPbI3 nanocrystals for efficient and stable light-emitting devices using p-i-n structures. ACS Central Science, 2018,4(10):1352-1359.
DOI URL PMID |
| [62] |
ZHANG D, ZHOU W, LIU Q , et al. CH3NH3PbBr3 perovskite nanocrystals encapsulated in lanthanide metal-organic frameworks as a photoluminescence converter for anti-counterfeiting. ACS Applied Materials & Interfaces, 2018,10(33):27875-27884.
DOI URL PMID |
| [63] |
ZHANG C, WANG B, LI W , et al. Conversion of invisible metal-organic frameworks to luminescent perovskite nanocrystals for confidential information encryption and decryption. Nature Communications, 2017,8(1):1138.
DOI URL PMID |
| [64] |
ZHANG D, XU Y, LIU Q , et al. Encapsulation of CH3NH3PbBr3 perovskite quantum dots in MOF-5 microcrystals as a stable platform for temperature and aqueous heavy metal ion detection. Inorganic Chemistry, 2018,57(8):4613-4619.
DOI URL PMID |
| [65] |
ZHANG Q, YIN Y . All-inorganic metal halide perovskite nanocrystals: opportunities and challenges . ACS Central Science, 2018,4(6):668-679.
DOI URL PMID |
| [66] | CHEN W, HAO J, HU W , et al. Enhanced stability and tunable photoluminescence in perovskite CsPbX3/ZnS quantum dot heterostructure. Small, 2017,13(21):1604085. |
| [67] |
TANG X, YANG J, LI S , et al. Single halide perovskite/semiconductor core/shell quantum dots with ultrastability and nonblinking properties. Advanced Science, 2019,6(18):1900412.
DOI URL PMID |
| [1] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [2] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [3] | PAN Zesheng, YOU Yaping, ZHENG Ya, CHEN Haijie, WANG Lianjun, JIANG Wan. Stability of Phosphors for White LED Excitable by Violet Light [J]. Journal of Inorganic Materials, 2025, 40(3): 314-322. |
| [4] | QU Mujing, ZHANG Shulan, ZHU Mengmeng, DING Haojie, DUAN Jiaxin, DAI Henglong, ZHOU Guohong, LI Huili. CsPbBr3@MIL-53 Nanocomposite Phosphors: Synthesis, Properties and Applications in White LEDs [J]. Journal of Inorganic Materials, 2024, 39(9): 1035-1043. |
| [5] | MIAO Xin, YAN Shiqiang, WEI Jindou, WU Chao, FAN Wenhao, CHEN Shaoping. Interface Layer of Te-based Thermoelectric Device: Abnormal Growth and Interface Stability [J]. Journal of Inorganic Materials, 2024, 39(8): 903-910. |
| [6] | CHEN Tian, LUO Yuan, ZHU Liu, GUO Xueyi, YANG Ying. Organic-inorganic Co-addition to Improve Mechanical Bending and Environmental Stability of Flexible Perovskite Solar Cells [J]. Journal of Inorganic Materials, 2024, 39(5): 477-484. |
| [7] | YANG Bo, LÜ Gongxuan, MA Jiantai. Electrocatalytic Water Splitting over Nickel Iron Hydroxide-cobalt Phosphide Composite Electrode [J]. Journal of Inorganic Materials, 2024, 39(4): 374-382. |
| [8] | LIU Song, ZHANG Faqiang, LUO Jin, LIU Zhifu. 0.9BaTiO3-0.1Bi(Mg1/2Ti1/2)O3 Ferroelectric Thin Films: Preparation and Energy Storage [J]. Journal of Inorganic Materials, 2024, 39(3): 291-298. |
| [9] | ZHANG Yuchen, LU Zhiyao, HE Xiaodong, SONG Guangping, ZHU Chuncheng, ZHENG Yongting, BAI Yuelei. Predictions of Phase Stability and Properties of S-group Elements Containing MAX Borides [J]. Journal of Inorganic Materials, 2024, 39(2): 225-232. |
| [10] | WANG Yu, XIONG Hao, HUANG Xiaokun, JIANG Linqin, WU Bo, LI Jiansheng, YANG Aijun. Regulation of Low-dose Stannous Iso-octanoate for Two-step Prepared Sn-Pb Alloyed Perovskite Solar Cells [J]. Journal of Inorganic Materials, 2024, 39(12): 1339-1347. |
| [11] | ZHOU Yunkai, DIAO Yaqi, WANG Minglei, ZHANG Yanhui, WANG Limin. First-principles Calculation Study of the Oxidation Resistance of PANI Modified Ti3C2(OH)2 [J]. Journal of Inorganic Materials, 2024, 39(10): 1151-1158. |
| [12] | FANG Wanli, SHEN Lili, LI Haiyan, CHEN Xinyu, CHEN Zongqi, SHOU Chunhui, ZHAO Bin, YANG Songwang. Effect of Film Formation Processes of NiOx Mesoporous Layer on Performance of Perovskite Solar Cells with Carbon Electrodes [J]. Journal of Inorganic Materials, 2023, 38(9): 1103-1109. |
| [13] | CHEN Yu, LIN Puan, CAI Bing, ZHANG Wenhua. Research Progress of Inorganic Hole Transport Materials in Perovskite Solar Cells [J]. Journal of Inorganic Materials, 2023, 38(9): 991-1004. |
| [14] | HU Zhongliang, FU Yuntian, JIANG Meng, WANG Lianjun, JIANG Wan. Thermal Stability of Nb/Mg3SbBi Interface [J]. Journal of Inorganic Materials, 2023, 38(8): 931-937. |
| [15] | LIU Jian, WANG Lingkun, XU Baoliang, ZHAO Qian, WANG Yaoxuan, DING Yi, ZHANG Shengtai, DUAN Tao. Nd-doped ZrSiO4 Ceramics: Synthesis in Molten Salt at Low Temperature, Phase Evolution and Chemical Stability [J]. Journal of Inorganic Materials, 2023, 38(8): 910-916. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||