
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (9): 972-986.DOI: 10.15541/jim20190568
Special Issue: 能源材料论文精选(一):锂离子电池(2020)
• REVIEW • Previous Articles Next Articles
BAI Xiangtao1( ),BAN Liqing2,ZHUANG Weidong2
),BAN Liqing2,ZHUANG Weidong2
Received:2019-11-07
Revised:2020-01-14
Published:2020-09-20
Online:2020-03-06
Supported by:CLC Number:
BAI Xiangtao,BAN Liqing,ZHUANG Weidong. Research Progress on Coating and Doping Modification of Nickel Rich Ternary Cathode Materials[J]. Journal of Inorganic Materials, 2020, 35(9): 972-986.
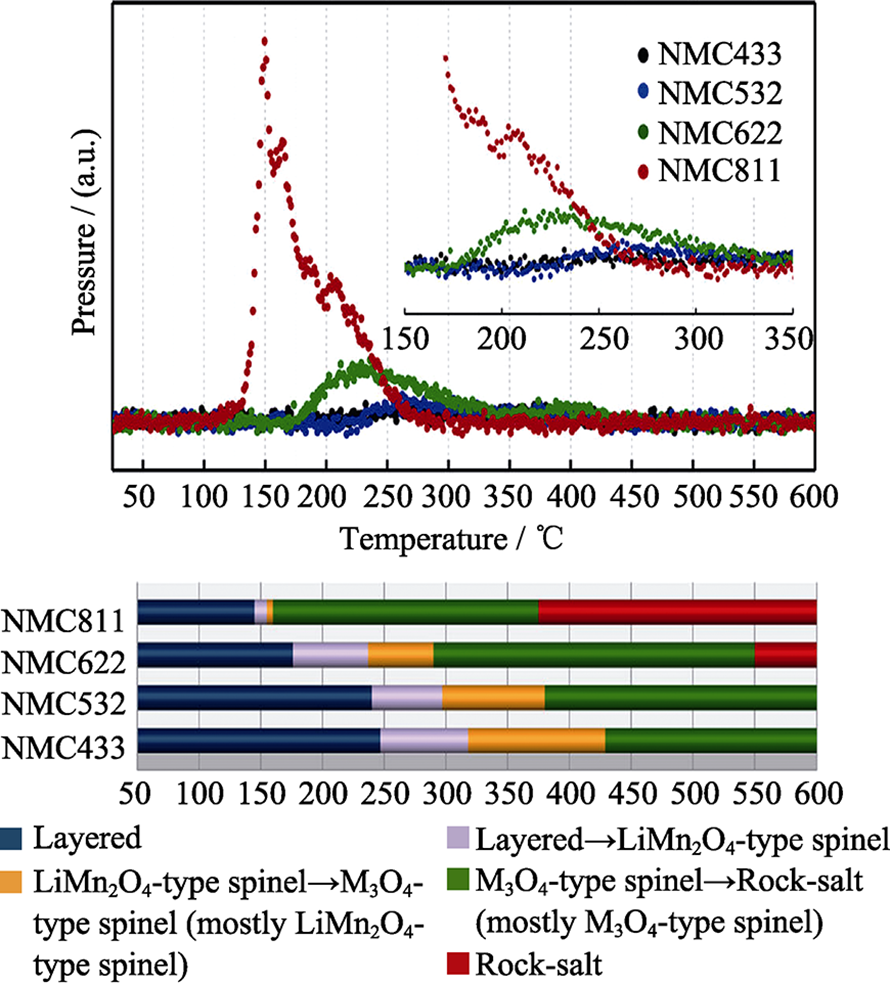
Fig. 1 Mass spectroscopy profiles for the oxygen (O2, m/z=32) collected simultaneously during measurement of TR-XRD (upper panel) and the corresponding temperature region of the phase transitions for NCM (lower panel) [19]
| Sample | Discharge capacity /(mAh·g-1) | |||||
|---|---|---|---|---|---|---|
| 0.1C /3 cycles | 1C /5 cycles | 2C /5 cycles | 5C /5 cycles | 10C /5 cycles | 0.1C /5 cycles* | |
| 0 | 196.8 | 168.9 | 155.8 | 131.8 | 94.5 | 184.6 |
| 1wt% | 213.9 | 185.5 | 170.2 | 148.1 | 121.6 | 207.2 |
| 2wt% | 203.8 | 161.1 | 147.9 | 122.6 | 92.1 | 195.7 |
Table 1 Discharge capacities of Li-ion batteries with different coating amounts[52]
| Sample | Discharge capacity /(mAh·g-1) | |||||
|---|---|---|---|---|---|---|
| 0.1C /3 cycles | 1C /5 cycles | 2C /5 cycles | 5C /5 cycles | 10C /5 cycles | 0.1C /5 cycles* | |
| 0 | 196.8 | 168.9 | 155.8 | 131.8 | 94.5 | 184.6 |
| 1wt% | 213.9 | 185.5 | 170.2 | 148.1 | 121.6 | 207.2 |
| 2wt% | 203.8 | 161.1 | 147.9 | 122.6 | 92.1 | 195.7 |

Fig. 6 Typical cycling performance of undoped and Mo-doped NCM523 at (a) 45 ℃ (4.3, 4.4, 4.5, and 4.6 V, C/3 rate) and (b) 30 ℃ (2.8-4.3 V, different rates)[96]

Fig. 7 Suggested mechanism for Ni2+ migration leading to a partial spinel nucleus[104] Red: oxygen atoms; Grey: Ni atoms; Violet: Mn atoms; Blue: cobalt atoms
| Sample | Cycling performance (1C@200*) | Rate performance | ||
|---|---|---|---|---|
| 3.0-4.4 V | 3.0-4.6 V | 16C/0.5C | ||
| Pristine | 75.59% | 72.99% | 64.94% | |
| Zr | 87.61% | 81.05% | 73.94% | |
| Zr/Ti | 94.20% | 91.71% | 79.57% | |
Table 2 Cycling performances and rate capabilities of NCM622 with different doping agents[104]
| Sample | Cycling performance (1C@200*) | Rate performance | ||
|---|---|---|---|---|
| 3.0-4.4 V | 3.0-4.6 V | 16C/0.5C | ||
| Pristine | 75.59% | 72.99% | 64.94% | |
| Zr | 87.61% | 81.05% | 73.94% | |
| Zr/Ti | 94.20% | 91.71% | 79.57% | |
| [1] |
CHOI N S, CHEN Z, FREUNBERGER S A,et al. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed., 2012,51(40):9994-10024.
DOI URL |
| [2] |
LIU W, OH P, LIU X,et al. Nickel-rich layered lithium transition- metal oxide for high energy lithium-ion batteries. Angew. Chem. Int. Ed., 2015,54(15):4440-4457.
DOI URL |
| [3] |
SCROSATI B, GARCHE J. Lithium batteries: status, prospects and future. J. Power Sources, 2010,195(9):2419-2430.
DOI URL |
| [4] | 柏祥涛, 孙学义, 庄卫东, 等. 氢氧化物前驱体制备LiNi0.5Co0.2Mn0.3O2的机理. 电池, 2014,44(5):260-263. |
| [5] |
LEE K S, MYUNG S T, AMINE K,et al. Structural and electrochemical properties of layered Li[Ni1-2xCoxMnx]O2 ( x=0.1-0.3) positive electrode materials for Li-ion batteries. J. Electrochem. Soc., 2007,154(10):A971-A977.
DOI URL |
| [6] | JOUANNEAU S, MACNEIL D D, LU Z,et al. Morphology and safety of Li[NixCo1-2xMnx ]O2 (0≤x≤1/2). J. Electrochem. Soc., 2003,150(10):A1299-1304. |
| [7] |
MACNEIL D D, LU Z H, CHEN Z H,et al. A comparison of the electrode/electrolyte reaction at elevated temperatures for various Li-ion battery cathodes. J. Power Sources, 2002,108(1/2):8-14.
DOI URL |
| [8] | KIM J, LEE H, CHA H,et al. Nickel-rich cathodes: prospect and reality of Ni-rich cathode for commercialization. Adv. Energy Mater., 2018,8(6):1702028. |
| [9] | XIONG X H, WANG Z X, YUE P,et al. Washing effects on electrochemical performance and storage characteristics of LiNi0.8Co0.1Mn0.1O2 as cathode material for lithium-ion batteries. J. Power Sources, 2013,222:318-325. |
| [10] | XU S, WANG X, ZHANG W,et al. The effects of washing on LiNi0.83Co0.13Mn0.04O2 cathode materials. J. Solid State Ionics, 2019,334:105-110. |
| [11] |
LI J, CHEN B R, ZHOU H M. Effects of washing and heat-treatment on structure and electrochemical charge/discharge property of LiNi0.8Co0.15Al0.05O2 powder. J. Inorg. Mater., 2016,31(7):773-778.
DOI URL |
| [12] |
XU S Z, LUO G F, JACOBS R,et al. Ab initio modeling of electrolyte molecule ethylene carbonate decomposition reaction on Li(Ni, Mn, Co)O2 cathode surface. ACS Appl. Mater. Interfaces, 2017,9:20545-20553.
URL PMID |
| [13] | MYUNG S T, IZUMI K, KOMABA S,et al. Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium-ion batteries. Chem. Mater., 2005,17(14):3695-3704. |
| [14] | YU H J, QIAN Y M, OTANI M,et al. Study of the lithium/nickel ions exchange in the layered LiNi0.42Mn0.42Co0.16O2 cathode material for lithium ion batteries: experimental and first-principles calculations. Energy Environ. Sci., 2014,7:1068-1078. |
| [15] | AURBACH D. Electrode-solution interactions in Li-ion batteries: a short summary and new insights. J. Power Sources, 2003, 119-121:497-503. |
| [16] | MYUNG S T, AMINE K, SUN Y K. Surface modification of cathode materials from nano- to microscale for rechargeable lithium- ion batteries. J. Mater. Chem., 2010,20:7074-7095. |
| [17] | YOON W S, HANSON J, MCBREEN J,et al. A study on the newly observed intermediate structures during the thermal decomposition of nickel-based layered cathode materials using time-resolved XRD. Electrochem.Commun., 2006,8:859-862. |
| [18] |
KONISHI H, YUASA T, YOSHIKAWA M. Thermal stability of Li1-yNixMn(1-x)/2Co(1-x)/2O2 layer-structured cathode materials used in Li-ion batteries. J. Power Sources, 2011,196:6884-6888.
DOI URL |
| [19] |
BAK S M, HU E Y, ZHOU Y N,et al. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces, 2014,6(24):22594-22601.
URL PMID |
| [20] | JUNG S K, GWON H, HONG J,et al. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater., 2014,4:1300787. |
| [21] | 邵奕嘉, 黄斌, 刘全兵, 等. 三元镍钴锰正极材料的制备及改性. 化学进展, 2018,30(4):410-419. |
| [22] | MANTHIRAM A, KNIGHT J C, MYUNG S T,et al. Nickel-rich and lithium-rich layered oxide cathodes: progress and perspectives. Adv. Energy Mater., 2016,6(1):1501010. |
| [23] | CHEN Z, CHAO D, LIN J,et al. Recent progress in surface coating of layered LiNixCoyMnzO2 for lithium-ion batteries. Mater. Res. Bull., 2017,96(4):491-502. |
| [24] |
AURBACH D. The electrochemical behavior of lithium salt solutions of γ-butyrolactone with noble metal electrodes J. Electrochem. Soc., 1989,136(4):906-913.
DOI URL |
| [25] |
EDSTROM K, GUSTAFSSON T, THOMAS J. The cathode- electrolyte interface in the Li-ion battery. Electrochim. Acta, 2004,50(2/3):397-403.
DOI URL |
| [26] | AURBACH D. Identification of surface films formed on lithium surfaces in γ-butyrolactone solutions II. contaminated solutions. J. Electrochem. Soc., 1989,136(6):1611-1614. |
| [27] |
LIN F, MARKUS I M, NORDLUND D,et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun., 2014,5:3529.
DOI URL PMID |
| [28] | LI J, LIU H, XIA J,et al. The impact of electrolyte additives and upper cut-off voltage on the formation of a rocksalt surface layer in LiNi0.8Mn0.1Co0.1O2 electrodes. J. Electrochem. Soc., 2017,164(4):A655-A665. |
| [29] |
SHI Y, ZHANG M H, QIAN D N,et al. Ultrathin Al2O3 coatings for improved cycling performance and thermal stability of LiNi0.5Co0.2Mn0.3O2 cathode material. Electrochim. Acta, 2016,203(10):154-161.
DOI URL |
| [30] |
NEUDECK S, STRAUSS F, GARCIA G,et al. Room temperature, liquid-phase Al2O3 surface coating approach for Ni-rich layered oxide cathode material. Chem. Commun., 2019,55:2174-2177.
DOI URL |
| [31] | VETTER J, NOVAK P, WAGNER M R,et al. Ageing mechanisms in lithium-ion batteries. J. Power Sources, 2005,147(1/2):269-281. |
| [32] | AURBACH D, MARKOVSKY B, SALITRA G,et al. Review on electrode-electrolyte solution interactions, related to cathode materials for Li-ion batteries. J. Power Sources, 2007,165(2):491-499. |
| [33] | LIANG L W, HU G R, JIANG F,et al. Electrochemical behaviours of SiO2-coated LiNi0.8Co0.1Mn0.1O2 cathode materials by a novel modification method. J. Alloys Compd., 2016,657:570-581. |
| [34] |
GAN Z G, HU G R, PENG Z D,et al. Surface modification of LiNi0.8Co0.1Mn0.1O2 by WO3 as a cathode material for LIB. Appl. Surf. Sci., 2019,481:1228-1238.
DOI URL |
| [35] | LI Y P, YAN G J, LUO L M,et al. Enhanced electrochemical performance of LiNi0.4Co0.2Mn0.4O2 cathode materials via Y2O3 coating. Mater. Res. Express, 2019,6(10):105533. |
| [36] | LOGHAVI M M, MOHAMMADI-MANESH H, EQRA R. LiNi0.8Co0.15Al0.05O2 coated by chromium oxide as a cathode material for lithium-ion batteries. J Solid State Electrochem., 2019,23(8):2569-2578. |
| [37] |
MYUNG S T, IZUMI K, KOMABA S,et al. Functionality of oxide coating for Li[Li0.05Ni0.4Co0.15Mn0.4]O2 as positive electrode materials for lithium-ion secondary batteries. J. Phys. Chem. C, 2007,111(10):4061-4067.
DOI URL |
| [38] | ZUO D X, WANG C P, TIAN G L,et al. Comparative study of Al2O3, SiO2 and TiO2-coated LiNi0.6Co0.2Mn0.2O2 electrode prepared by hydrolysis coating technology. J. Electrochem. Sci. Eng., 2019,9(2):85-97. |
| [39] | ZHU W C, HUANG X, LIU T T,et al. Ultrathin Al2O3 coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced cycleability at extended voltage ranges. Coatings, 2019,9(2):92. |
| [40] |
NEUDECK S, MAZILKIN A, REITZ C,et al. Effect of low-temperature Al2O3 ALD coating on Ni-rich layered oxide composite cathode on the long-term cycling performance of lithium- ion batteries. Sci. Rep., 2019,9:5328.
DOI URL PMID |
| [41] | CUI X L, AI L, MAO L P,et al. Enhanced electrochemical properties of LiNi0.6Co0.2Mn0.2O2 cathode material by the diffusional Al2O3 coating layer. Ionics, 2019,25(2):411-419. |
| [42] |
YANG K, FAN L Z, GUO J,et al. Significant improvement of electrochemical properties of AlF3-coated LiNi0.5Co0.2Mn0.3O2 cathode materials. Electrochim. Acta, 2012,63:363-368.
DOI URL |
| [43] | LEE S H, YOON C S, AMINE K,et al. Improvement of long-term cycling performance of Li[Ni0.8Co0.15Al0.05]O2 by AlF3 coating. J. Power Sources, 2013,234:201-207. |
| [44] |
CHO Y, OH P, CHO J. A new type of protective surface layer for high-capacity Ni-based cathode materials: nanoscaled surface pillaring layer. Nano Lett., 2013,13(3):1145-1152.
URL PMID |
| [45] | HAN B L, LU X C. Effect of nano-sized CeF3 on microstructure, mechanical, high temperature friction and corrosion behavior of Ni-W composite coatings. Surf. Coat. Technol., 2009,203(23):3656-3660. |
| [46] | KUMAR D A, SELVASEKARAPANDIAN S, NITHYA H,et al. Structural and conductivity analysis on cerium fluoride nanoparticles prepared by sonication assisted method. Solid State Sci., 2012,14(5):626-634. |
| [47] | KUMAR D A, SELVASEKARAPANDIAN S, NITHYA H,et al. Influence of substrate temperature on CeF3 thin films prepared by thermal evaporation. Mater. Chem. Phys., 2014,143(2):765-772. |
| [48] |
XIE Y, GAO D, ZHANG L L,et al. CeF3-modified LiNi1/3Co1/3Mn1/3O2 cathode material for high-voltage Li-ion batteries. Ceram. Int., 2016,42(13):14587-14594.
DOI URL |
| [49] | SONG H G, KIM S B, PARK Y J. Enhanced electrochemical properties of Li[Ni0.5Co0.2Mn0.3]O2 cathode by surface coating using LaF3 and MgF2. J. Electroceram., 2012,29(2):163-169. |
| [50] | DAI S C, YAN G J, WANG L,et al. Enhanced electrochemical performance and thermal properties of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material via CaF2 coating. J. Electroanal. Chem., 2019,847:113197. |
| [51] |
LIM Y J, LEE S M, LIM H,et al. Amorphous Li-Zr-O layer coating on the surface of high-Ni cathode materials for lithium ion batteries. Electrochim. Acta, 2018,282:311-316.
DOI URL |
| [52] |
LIU S J, WU H, HUANG L,et al. Synthesis of Li2Si2O5-coated LiNi0.6Co0.2Mn0.2O2 cathode materials with enhanced high-voltage electrochemical properties for lithium-ion batteries. J. Alloys Compd., 2016,674:447-454.
DOI URL |
| [53] | JO C H, CHO D H, NOH H J,et al. An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid derived Li3PO4 nanolayer. Nano Res., 2015,8(5):1464-1479. |
| [54] | ZHU J, LI Y J, XUE L L,et al. Enhanced electrochemical performance of Li3PO4 modified Li[Ni0.8Co0.1Mn0.1]O2 cathode material via lithium-reactive coating. J. Alloys Compd., 2019,773:112-120. |
| [55] | FAN Q L, YANG S D, LIU J,et al. Mixed-conducting interlayer boosting the electrochemical performance of Ni-rich layered oxide cathode materials for lithium ion batteries. J. Power Sources, 2019,421:91-99. |
| [56] | BAN L Q, YIN Y P, ZHUANG W D,et al. Electrochemical performance improvement of Li1.2[Mn0.54Ni0.13Co0.13]O2 cathode material by sulfur incorporation. Electrochim. Acta, 2015,180:218-226. |
| [57] |
HUANG Y Q, HUANG Y H, HU X L. Enhanced electrochemical performance of LiNi0.8Co0.15Al0.05O2 by nanoscale surface modification with Co3O4. Electrochim. Acta, 2017,231:294-299.
DOI URL |
| [58] | XU S, DU C Y, XU X,et al. A mild surface washing method using protonated polyaniline for Ni-rich LiNi0.8Co0.1Mn0.1O2 material of lithium ion batteries. Electrochim. Acta, 2017,248:534-540. |
| [59] | HE X S, HAN G K, LOU S F,et al. Improved electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material by coating of graphene nanodots. J. Electrochem. Soc., 2019,166(6):A1038-A1044. |
| [60] |
YANG H, WU K P, HU G R,et al. Design and synthesis of double- functional polymer composite layer coating to enhance the electrochemical performance of the Ni-rich cathode at the upper cutoff voltage. ACS Appl. Mater. Interfaces, 2019,11:8556-8566.
URL PMID |
| [61] |
GAN Q M, QIN N, ZHU Y H,et al. Polyvinylpyrrolidone-induced uniform surface-conductive polymer coating endows Ni-rich LiNi0.8Co0.1Mn0.1O2 with enhanced cyclability for lithium-ion batteries. ACS Appl. Mater. Interfaces, 2019,11(13):12594-12604.
URL PMID |
| [62] | 尹艳萍, 庄卫东, 王忠, 等. 一种锂离子电池用含镍层状正极材料/碳复合材料及其制备方法. 中国, CN108232153A. 2018.06.29. |
| [63] | 王忠, 庄卫东, 尹艳萍, 等. 一种石墨烯改性锂镍钴锰氧化物正极材料及其制备方法. 中国, CN109244448A. 2019.01.18. |
| [64] | 王忠, 庄卫东, 尹艳萍, 等. 一种纳米碳材料改性锂镍钴锰氧化物正极材料及制备方法. 中国, CN109473642A. 2019.03.15. |
| [65] |
ZHANG S S, CHEN J, WANG C S. Elemental sulfur as a cathode additive for enhanced rate capability of layered lithium transition metal oxides. J. Electrochem. Soc., 2019,166(4):A487-A492.
DOI URL |
| [66] | ZHANG S S, FAN X L, WANG C S. Enhanced electrochemical performance of Ni-rich layered cathode materials by using LiPF6 as a cathode additive. ChemElectroChem, 2019,6(5):1536-1541. |
| [67] | LI J, DOWNIE L E, MA L,et al. Study of the failure mechanisms of LiNi0.8Mn0.1Co0.1O2 cathode material for lithium ion batteries. J. Electrochem. Soc., 2015,162(7):A1401-A1408. |
| [68] |
LIU H, WOLF M, KARKI K,et al. Intergranular cracking as a major cause of long-term capacity fading of layered cathodes. Nano Lett., 2017,17(6):3452-3457.
DOI URL PMID |
| [69] |
ZHAO E Y, CHEN M M, HU Z B,et al. Improved cycle stability of high-capacity Ni-rich LiNi0.8Mn0.1Co0.1O2 at high cut-off voltage by Li2SiO3 coating. J. Power Sources, 2017,343:345-353.
DOI URL |
| [70] | PENG Z J, YANG G W, LI F Q,et al. Improving the cathode properties of Ni-rich LiNi0.6Co0.2Mn0.2O2 at high voltages under 5C by Li2SiO3 coating and Si4+ doping. J. Alloys Compd., 2018,762:827-834. |
| [71] | LEE S W, KIM M S, JEONG J H,et al. Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted Sol-Gel method: improved thermal stability and high-voltage performance. J. Power Sources, 2017,360:206-214. |
| [72] | KIM S B, LEE K J, CHOI W J,et al. Preparation and cycle performance at high temperature for Li[Ni0.5Co0.2Mn0.3]O2 coated with LiFePO4. J. Solid State Electrochem., 2010,14:919-922. |
| [73] |
WU Z Z, JI S P, LIU T C,et al. Aligned Li+ tunnels in core-shell Li(NixMnyCoz)O2@LiFePO4 enhances its high voltage cycling stability as Li-ion battery cathode. Nano Lett., 2016,16(10):6357-6363.
URL PMID |
| [74] | ZHU L, YAN T F, JIA D,et al. LiFePO4-coated LiNi0.5Co0.2Mn0.3O2 cathode materials with improved high voltage electrochemical performance and enhanced safety for lithium ion pouch cells. J. Electrochem. Soc., 2019,166(3):A5437-A5444. |
| [75] | KIM W S, KIM S B, JANG I C,et al. Remarkable improvement in cell safety for Li[Ni0.5Co0.2Mn0.3]O2 coated with LiFePO4. J. Alloys Compd., 2010,492(1/2):L87-L90. |
| [76] | DIAO R J, NAYAKA G P, ZHU C Y,et al. CePO4 coated LiNi0.6Co0.2Mn0.2O2 as cathode material and its electrochemical performance. Int. J. Electrochem. Sci., 2019,14:8070-8079. |
| [77] |
TONG H, DONG P Y, ZHANG J F,et al. Cathode material LiNi0.8Co0.1Mn0.1O2/LaPO4 with high electrochemical performance for lithium-ion batteries. J. Alloys Compd., 2018,764:44-50.
DOI URL |
| [78] | LIU W M, HU G R, DU K,et al. Surface coating of LiNi0.8Co0.15Al0.05O2 with LiCoO2 by a molten salt method. Surf. Coat. Tech., 2013,216:267-272. |
| [79] |
LIU W M, HU G R, DU K, et al. Synthesis and characterization of LiCoO2-coated LiNi0.8Co0.15Al0.05O2 cathode materials. Mater. Lett., 2012,83:11-13.
DOI URL |
| [80] | LIU W M, HU G R, DU K,et al. Enhanced storage property of LiNi0.8Co0.15Al0.05O2 coated with LiCoO2. J. Power Sources, 2013,230:201-206. |
| [81] |
YAN P F, ZHENG J M, GU M,et al. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun., 2017,8:14101.
DOI URL PMID |
| [82] |
HASHIGAMI S, KATO Y, YOSHIMI K,et al. Influence of lithium silicate coating on retarding crack formation in LiNi0.5Co0.2Mn0.3O2 cathode particles. Electrochim. Acta, 2018,291:304-310.
DOI URL |
| [83] |
DU M L, YANG P, HE W X,et al. Enhanced high-voltage cycling stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode coated with Li2O-2B2O3. J. Alloys Compd., 2019,805:991-998.
DOI URL |
| [84] | HASHIGAMI S, YOSHIMI K, KATO Y,et al. Improvement of cycleability and rate-capability of LiNi0.5Co0.2Mn0.3O2 cathode materials coated with lithium boron oxide by an antisolvent precipitation method. ChemistrySelect, 2019,4(29):8676-8681. |
| [85] | ZHANG J Y, CAO Y, OU X,et al. Constituting the NASICON type solid electrolyte coated material forming anti-high voltage system to enhance the high cut-off voltage performance of LiNi0.6Co0.2Mn0.2O2 via charge attracts electrostatic assembly. J. Power Sources, 2019,436:226722. |
| [86] |
PARK K, PARK J H, HONG S G,et al. Enhancement in the electrochemical performance of zirconium/phosphate bi-functional coatings on LiNi0.8Co0.15Mn0.05O2 by the removal of Li residuals. Phys. Chem. Chem. Phys., 2016,18:29076-29085.
URL PMID |
| [87] | HE X S, XU X, WANG L G,et al. Enhanced electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material via Li2TiO3 nanoparticles coating. J. Electrochem. Soc., 2019,166(2):A143-A150. |
| [88] |
YANG J, YU Z Y, YANG B,et al. Electrochemical characterization of Cr8O21 modified LiNi0.5Co0.2Mn0.3O2 cathode material. Electrochim. Acta, 2018,266:342-347.
DOI URL |
| [89] |
GAO T P, WONG K W, FUNG K Y,et al. A rational three-step calcination strategy for synthesizing high-quality LiNi0.5Mn0.3Co0.2O2 cathode materials: the key role of suppressing Li2O formation. Electrochim. Acta, 2018,288:153-164.
DOI URL |
| [90] | BHUVANESWARI D, BABU G, KALAISELVI N. Effect of surface modifiers in improving the electrochemical behavior of LiNi0.4Mn0.4Co0.2O2 cathode. Electrochim. Acta, 2013,109:684-693. |
| [91] | WANG D, LI X H, WANG Z X,et al. Role of zirconium dopant on the structure and high voltage electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Electrochim. Acta, 2016,188:48-56. |
| [92] | POUILLERIE C, CROGUENNEC L, BIENSAN P,et al. Synthesis and characterization of new LiNi1-yMgyO2 positive electrode materials for lithium-ion batteries. J. Electrochem. Soc., 2000,147(6):2061-2069. |
| [93] | XIE Q, LI W D, MANTHIRAM A. A Mg-doped high-nickel layered oxide cathode enabling safer, high-energy-density Li-ion batteries. Chem. Mater., 2019,31(3):938-946. |
| [94] |
POUILLERIE C, CROGUENNEC L, DELMAS C. The LixNi1-yMgyO2 (y=0.05, 0.10) system: structural modifications observed upon cycling. Solid State Ionics, 2000,132(1/2):15-29.
DOI URL |
| [95] |
HUANG B, LIN X H, WANG Z X,et al. Synthesis of Mg-doped LiNi0.8Co0.15Al0.05O2 oxide and its electrochemical behavior in high-voltage lithium-ion batteries. Ceram. Int., 2014,40(8):13223-13230.
DOI URL |
| [96] |
BREUER O, CHAKRABORTY A, LIU J,et al. Understanding the role of minor molybdenum doping in LiNi0.5Co0.2Mn0.3O2 electrodes: from structural and surface analyses and theoretical modeling to practical electrochemical cells. ACS Appl. Mater. Interfaces, 2018,10(35):29608-29621.
DOI URL PMID |
| [97] | WOO S U, PARK B C, YOON C S,et al. Improvement of electrochemical performances of Li[Ni0.8Co0.1Mn0.1]O2 cathode materials by fluorine substitution. J. Electrochem. Soc., 2007,154(7):A649-A655. |
| [98] | LI C L, KAN W H, XIE H L,et al. Inducing favorable cation antisite by doping halogen in Ni-rich layered cathode with ultrahigh stability. Adv. Sci., 2019,6(4):1801406. |
| [99] | LI X, XIE Z W, LIU W J,et al. Effects of fluorine doping on structure, surface chemistry, and electrochemical performance of LiNi0.8Co0.15Al0.05O2. Electrochim. Acta, 2015,174:1122-1130. |
| [100] |
LÜ C J, YANG J, PENG Y,et al. 1D Nb-doped LiNi1/3Co1/3Mn1/3O2 nanostructures as excellent cathodes for Li-ion battery. Electrochim. Acta, 2019,297:258-266.
DOI URL |
| [101] | YANG Z G, XIANG W, WU Z G,et al. Effect of niobium doping on the structure and electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Ceram. Int., 2017,43(4):3866-3872. |
| [102] | WU J F, LIU H G, YE X H,et al. Effect of Nb doping on electrochemical properties of LiNi1/3Co1/3Mn1/3O2 at high cutoff voltage for lithium-ion battery. J. Alloys Compd., 2015,644:223-227. |
| [103] | BREGER J, MENG Y S, HINUMA Y,et al. Effect of high voltage on the structure and electrochemistry of LiNi0.5Mn0.5O2: a joint experimental and theoretical study. Chem. Mater., 2006,18(20):4768-4781. |
| [104] | SCHIPPER F, DIXIT M, KOVACHEVA D,et al. Stabilizing nickel-rich layered cathode materials by a high-charge cation doping strategy: zirconium-doped LiNi0.6Co0.2Mn0.2O2. J. Mater. Chem. A, 2016,4:16073-16084. |
| [105] |
DONG S D, ZHOU Y, HAI C X,et al. Ultrathin CeO2 coating for improved cycling and rate performance of Ni-rich layered LiNi0.7Co0.2Mn0.1O2 cathode materials. Ceram. Int., 2019,45(1):144-152.
DOI URL |
| [106] |
CHEN Y X, LI Y J, LI W,et al. High-voltage electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material via the synergetic modification of the Zr/Ti elements. Electrochim. Acta, 2018,281:48-59.
DOI URL |
| [107] | DU R, BI Y, YANG W,et al. Improved cyclic stability of LiNi0.8Co0.1Mn0.1O2 via Ti substitution with a cut-off potential of 4.5 V. Ceram. Int., 2015,41(5):7133-7139. |
| [108] | PARK S H, OH S W, SUN, Y K. Synthesis and structural characterization of layered Li[Ni1/3+xCo1/3Mn1/3-2xMox]O2 cathode materials by ultrasonic spray pyrolysis. J. Power Sources, 2005,146(1/2):622-625. |
| [109] | YANG J, XIA Y Y. Suppressing the phase transition of the layered Ni-rich oxide cathode during high-voltage cycling by introducing low-content Li2MnO3. ACS Appl. Mater. Interfaces, 2016,8(2):1297-1308. |
| [110] |
BAK S M, NAM K W, CHANG W,et al. Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode materials. Chem. Mater., 2013,25(3):337-351.
DOI URL |
| [111] |
MIN K, SEO S W, SONG Y Y,et al. A first-principles study of the preventive effects of Al and Mg doping on the degradation in LiNi0.8Co0.1Mn0.1O2 cathode materials. Phys. Chem. Chem. Phys., 2017,19:1762-1769.
URL PMID |
| [112] |
DIXIT M, MARKOVSKY B, AURBACH D,et al. Unraveling the effects of Al doping on the electrochemical properties of LiNi0.5Co0.2Mn0.3O2 using first principles. J. Electrochem. Soc., 2017,164(1):A6359-A6365.
DOI URL |
| [113] | XIAO P H, DENG Z Q, MANTHIRAM A,et al. Calculations of oxygen stability in lithium-rich layered cathodes. J. Phys. Chem. C, 2012,116(44):23201-23204. |
| [114] | AURBACH D, SRUR-LAVI O, GHANTY C,et al. Studies of aluminum-doped LiNi0.5Co0.2Mn0.3O2: electrochemical behavior, aging, structural transformations, and thermal characteristics. J. Electrochem. Soc., 2015,162(6):A1014-A1027. |
| [115] | KIM U H, JUN D W, PARK K J,et al. Pushing the limit of layered transition metal oxide cathodes for high-energy density rechargeable Li ion batteries. Energy Environ. Sci., 2018,11:1271-1279. |
| [116] |
KONISHI H, YOSHIKAWA M, HIRANO T. The effect of thermal stability for high-Ni-content layer-structured cathode materials, LiNi0.8Mn0.1-xCo0.1MoxO2 (x=0, 0.02, 0.04). J. Power Sources, 2013,244:23-28.
DOI URL |
| [117] |
LIU Q, ZHAO Z K, WU F,et al. The effects of molybdenum doping on LiNi0.6Co0.2Mn0.2O2 cathode material. Solid State Ionics, 2019,337:107-114.
DOI URL |
| [118] |
WEIGEL T, SCHIPPER F, ERICKSON E M,et al. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett., 2019,4(2):508-516.
DOI URL |
| [119] | XIANG W, ZHU C Q, ZHANG J,et al. Synergistic coupling effect of sodium and fluorine co-substitution on enhancing rate capability and cycling performance of Ni-rich cathode for lithium ion battery. J. Alloys Compd., 2019,786:56-64. |
| [120] | HU G, ZHANG M, LIANG L,et al. Mg-Al-B co-substitution LiNi0.5Co0.2Mn0.3O2 cathode materials with improved cycling performance for lithium-ion battery under high cutoff voltage. Electrochim. Acta, 2016,190:264-275. |
| [121] | CHANG S H, CHEN Y X, LI Y J,et al. Improvement of the high-voltage electrochemical properties of Li[Ni0.5Co0.2Mn0.3]O2@ZrO2 cathode materials with liquid phase modification. J. Alloys Compd., 2019,781:496-503. |
| [122] | LI X, ZHANG K J, WANG M S,et al. Dual functions of zirconium modification on improving the electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2. Sustain. Energ. Fuels, 2018,2:413-421. |
| [123] |
HE T, LU Y, SU Y F,et al. Sufficient utilization of zirconium ions to improve the structure and surface properties of nickel-rich cathode materials for lithium-ion batteries. ChemSusChem, 2018,11(10):1639-1648.
DOI URL PMID |
| [124] |
SUN Y K, KIM D H, YOON C S,et al. A novel cathode material with a concentration-gradient for high-energy and safe lithium- ion batteries. Adv. Funct. Mater., 2010,20(3):485-491.
DOI URL |
| [125] |
XU X, JIAN J Y, XIANG L Z,et al. Enhancing high-voltage performances of nickel-based cathode material via aluminum and progressive concentration gradient modification. Electrochim. Acta, 2019,317:459-467.
DOI URL |
| [126] |
SHI J L, QI R, ZHANG X D,et al. High-thermal- and air-stability cathode material with concentration-gradient buffer for Li-ion batteries. ACS Appl. Mater. Interfaces, 2017,9(49):42829-42835.
URL PMID |
| [127] |
TANG M J, YANG J, CHEN N T,et al. Overall structural modification of a layered Ni-rich cathode for enhanced cycling stability and rate capability at high voltage. J. Mater. Chem. A, 2019,7:6080-6089.
DOI URL |
| [128] |
RAN Q W, ZHAO H Y, WANG Q,et al. Dual functions of gradient phosphate polyanion doping on improving the electrochemical performance of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode at high cut-off voltage and high temperature. Electrochim. Acta, 2019,299:971-978.
DOI URL |
| [129] |
ZHANG M L, ZHAO H Y, TAN M,et al. Yttrium modified Ni-rich LiNi0.8Co0.1Mn0.1O2 with enhanced electrochemical performance as high energy density cathode material at 4.5 V high voltage. J. Alloys Compd., 2019,774:82-92.
DOI URL |
| [130] |
TANG W J, CHEN Z X, XIONG F,et al. An effective etching- induced coating strategy to shield LiNi0.8Co0.1Mn0.1O2 electrode materials by LiAlO2. J. Power Sources, 2019,412:246-254.
DOI URL |
| [131] |
YU H F, LI Y G, HU Y J,et al. 110th anniversary: concurrently coating and doping high-valence vanadium in nickel-rich lithiated oxides for high-rate and stable lithium-ion batteries. Ind. Eng. Chem. Res., 2019,58(10):4108-4115.
DOI URL |
| [132] |
CHEN Y X, LI Y J, TANG S Y,et al. Enhanced electrochemical properties of the Cd-modified LiNi0.6Co0.2Mn0.2O2 cathode materials at high cut-off voltage. J. Power Sources, 2018,395:403-413.
DOI URL |
| [133] |
KONG D F, HU J T, CHEN Z F,et al. Ti-gradient doping to stabilize layered surface structure for high performance high-Ni oxide cathode of Li-ion battery. Adv. Energy Mater., 2019,9:1901756.
DOI URL |
| [134] |
HAN B, XU S, ZHAO S,et al. Enhancing the structural stability of Ni-rich layered oxide cathodes with a preformed Zr-concentrated defective nanolayer. ACS Appl. Mater. Interfaces, 2018,10(46):39599-39607.
DOI URL PMID |
| [135] |
SCHIPPER F, BOUZAGLO H, DIXIT M,et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries. Adv. Energy Mater., 2018,8(4):1701682.
DOI URL |
| [136] |
MENG K, WANG Z X, GUO H J,et al. Improving the cycling performance of LiNi0.8Co0.1Mn0.1O2 by surface coating with Li2TiO3. Electrochim. Acta, 2016,211:822-831.
DOI URL |
| [137] |
WU F, LI Q, CHEN L,et al. Use of Ce to reinforce the interface of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium-ion batteries under high operating voltage. ChemSusChem, 2019,12(4):935-943.
DOI URL PMID |
| [138] |
ZHAN X W, GAO S, CHENG Y T. Influence of annealing atmosphere on Li2ZrO3-coated LiNi0.6Co0.2Mn0.2O2 and its high-voltage cycling performance. Electrochim. Acta, 2019,300:36-44.
DOI URL |
| [139] | 李宁, 李文进, 张宇宙, 等. 一种梯度高镍正极材料及其制备方法和锂离子电池. 中国, CN107528060A. 2017.12.29. |
| [140] |
WANG D, LI X H, WANG Z X,et al. Co-modification of LiNi0.5Co0.2Mn0.3O2 cathode materials with zirconium substitution and surface polypyrrole coating: towards superior high voltage electrochemical performances for lithium ion batteries. Electrochim. Acta, 2016,196:101-109.
DOI URL |
| [141] |
YANG H P, WU H H, GE M Y,et al. Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium- ion batteries. Adv. Funct. Mater., 2019,29(13):1808825.
DOI URL |
| [142] |
RAN Q W, ZHAO H Y, SHU X H,et al. Enhancing the electrochemical performance of Ni-rich layered oxide cathodes by combination of the gradient doping and dual-conductive layers coating. ACS Appl. Energy Mater., 2019,2(5):3120-3130.
DOI URL |
| [143] |
SUN S M, LIU T, NIU Q H,et al. Improvement of superior cycle performance of LiNi0.8Co0.15Al0.05O2 cathode for lithium-ion batteries by multiple compound modifications. J. Electroanal. Chem., 2019,838:178-185.
DOI URL |
| [144] | 任志敏, 王振尧, 高敏, 等. 一种磷镁协同掺杂改性的富锂锰基正极材料及其制备方法和锂离子电池. 中国, CN107591534A. 2018.01.16. |
| [145] |
XU G L, LIU Q, LAU K K S, et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy, 2019,4:484-494.
DOI URL |
| [1] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [2] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [3] | YU Yiping, XIAO Peng, ZHAO Changhao, XU Mengdi, YAO Lidong, LI Wei, WANG Song. Ablation Behavior of High-temperature Laminated Ta/Ta0.5Hf0.5C Cermets under High-frequency Plasma Wind Tunnel Test [J]. Journal of Inorganic Materials, 2025, 40(7): 790-798. |
| [4] | TANG Ying, LI Jie, XIANG Huaicheng, FANG Weishuang, LIN Huixing, YANG Junfeng, FANG Liang. Rattling Effect: A New Mechanism Affecting the Resonant Frequency Temperature Coefficient of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 656-666. |
| [5] | ZHOU Yangyang, ZHANG Yanyan, YU Ziyi, FU Zhengqian, XU Fangfang, LIANG Ruihong, ZHOU Zhiyong. Enhancement of Piezoelectric Properties in CaBi4Ti4O15-based Ceramics through Bi3+ Self-doping Strategy [J]. Journal of Inorganic Materials, 2025, 40(6): 719-728. |
| [6] | SUN Yuxuan, WANG Zheng, SHI Xue, SHI Ying, DU Wentong, MAN Zhenyong, ZHENG Liaoying, LI Guorong. Defect Dipole Thermal-stability to the Electro-mechanical Properties of Fe Doped PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(5): 545-551. |
| [7] | AN Ran, LIN Si, GUO Shigang, ZHANG Chong, ZHU Shun, HAN Yingchao. Iron-doped Nano-hydroxyapatite: Preparation and Ultraviolet Absorption Performance [J]. Journal of Inorganic Materials, 2025, 40(5): 457-465. |
| [8] | PAN Yuzhou, HE Fajian, XU Lulu, DAI Shixun. Broadband 3 μm Mid-infrared Emission in Dy3+/Yb3+ Co-doped Tellurite Glass under 980 nm LD Excitation [J]. Journal of Inorganic Materials, 2025, 40(5): 521-528. |
| [9] | QU Jifa, WANG Xu, ZHANG Weixuan, ZHANG Kangzhe, XIONG Yongheng, TAN Wenyi. Enhanced Sulfur-resistance for Solid Oxide Fuel Cells Anode via Doping Modification of NaYTiO4 [J]. Journal of Inorganic Materials, 2025, 40(5): 489-496. |
| [10] | GUO Ziyu, ZHU Yunzhou, WANG Li, CHEN Jian, LI Hong, HUANG Zhengren. Effect of Zn2+ Catalyst on Microporous Structure of Porous Carbon Prepared from Phenolic Resin/Ethylene Glycol [J]. Journal of Inorganic Materials, 2025, 40(5): 466-472. |
| [11] | LIANG Ruihui, ZHONG Xin, HONG Du, HUANG Liping, NIU Yaran, ZHENG Xuebin. High-temperature Water Vapor Corrosion Behaviors of Environmental Barrier Coatings with Yb2O3-modified Silicon Bond Layer [J]. Journal of Inorganic Materials, 2025, 40(4): 425-432. |
| [12] | MU Haojie, ZHANG Yuanjiang, YU Bin, FU Xiumei, ZHOU Shibin, LI Xiaodong. Preparation and Properties of ZrO2 Doped Y2O3-MgO Nanocomposite Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 281-289. |
| [13] | HOU Jiaqi, CHEN Ruicong, ZENG Yaoying, ZHOU Lei, ZHANG Jiaping, FU Qiangang. Thermal Shock and Ablation Resistance of SiC Coating Repaired by Gaseous Silicon Infiltration [J]. Journal of Inorganic Materials, 2025, 40(2): 168-176. |
| [14] | FENG Guanzheng, YANG Jian, ZHOU Du, CHEN Qiming, XU Wentao, ZHOU Youfu. Mechanism for Hydrothermal-carbothermal Synthesis of AlN Nanopowders [J]. Journal of Inorganic Materials, 2025, 40(1): 104-110. |
| [15] | ZHANG Li, GUAN Haoyang, ZHENG Qining, HONG Zhiliang, WANG Jiaxuan, XING Ning, LI Mei, LIU Yongsheng, ZHANG Chengyu. Creep Properties and Damage Mechanisms of SiCf/SiC-SiYBC Prepared by Melt Infiltration [J]. Journal of Inorganic Materials, 2025, 40(1): 23-30. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||