
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (3): 352-358.DOI: 10.15541/jim20190397
Special Issue: 2020年环境材料论文精选(一)放射性元素去除; 【虚拟专辑】放射性污染物去除(2020~2021)
Previous Articles Next Articles
ZHANG Zhibin,ZHOU Runze,DONG Zhimin,CAO Xiaohong,LIU Yunhai( )
)
Received:2019-08-03
Revised:2019-09-22
Published:2020-03-20
Online:2019-10-23
About author:ZHANG Zhibin(1981-), male, associate professor. E-mail: zhbzhang@ecut.edu.cn
Supported by:CLC Number:
ZHANG Zhibin, ZHOU Runze, DONG Zhimin, CAO Xiaohong, LIU Yunhai. Adsorption of U(VI)-CO3/Ca-U(VI)-CO3 by Amidoxime-functionalized Hydrothermal Carbon[J]. Journal of Inorganic Materials, 2020, 35(3): 352-358.
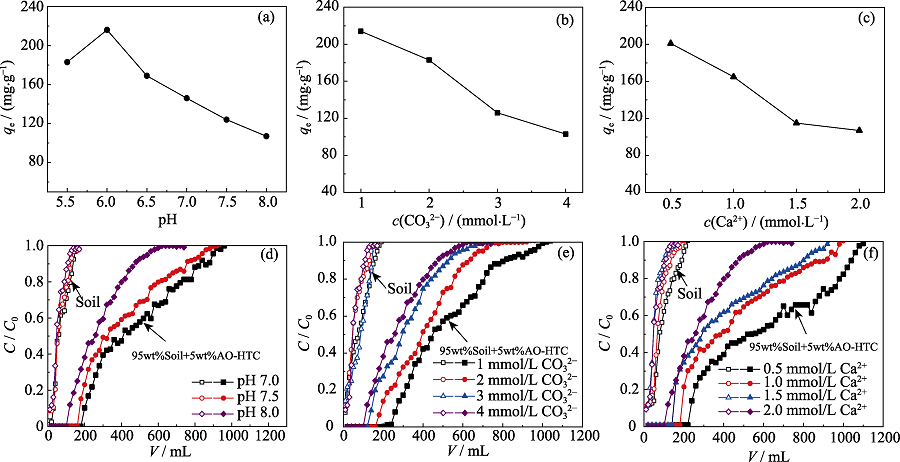
Fig. 3 Effects of pH (a) and CO32 (b) and Ca2+ concentration (c) on uranium adsorbed onto AO-HTC, breakthrough curves of soil column and AO-HTC column at different pH (d), CO32- (e) and Ca2+ concentration (f) (a) 1.0 mmol/L CO32-, 0.5 mmol/L Ca2+; (b) pH=6.0, 0.5 mmol/L Ca2+; (c) pH=6.0, 1.0 mmol/L CO32-; (d) 4.0 mmol/L CO32-, 2.0 mmol/L Ca2+; (e) pH=8.0, 2.0 mmol/L Ca2+; (f) pH=8.0, 4.0 mmol/L CO32-
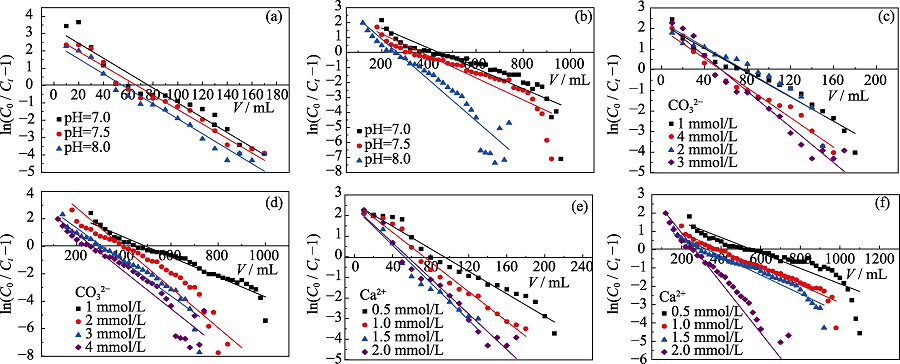
Fig. 5 Thomas kinetic plots for the adsorption of U(VI) on red soils (a, c, e) and AO-HTC columns (b, d, f) (a, b) Effect of pH; (c, d) Effect of carbonate; (e, f) Effect of calcium (a, b) 4.0 mmol/L CO32- , 2.0 mmol/L Ca2+; (c, d) pH=8.0, 2.0 mmol/L Ca2+; (e, f) pH=8.0, 4.0 mmol/L CO32-
| pH | CO32- /(mmol·L-1) | Ca2+ /(mmol·L-1) | Thomas model | Yoon-Nelson model | |||||
|---|---|---|---|---|---|---|---|---|---|
| KTh /(´10-3, mL·min-1·g-1) | qo /(´10-2, mg∙g-1) | R2 | KYN/min-1 | τ/min | R2 | ||||
| Soil column | 7.0 | 4 | 2 | 2.089 | 1.5 | 0.93 | 0.043 | 76 | 0.93 |
| 7.5 | 2.143 | 1.3 | 0.97 | 0.042 | 67 | 0.97 | |||
| 8.0 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 7.0 | 4 | 2 | 0.338 | 8.8 | 0.91 | 0.014 | 444 | 0.91 |
| 7.5 | 0.367 | 7.4 | 0.83 | 0.007 | 371 | 0.83 | |||
| 8.0 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.81 | |||
| Soil column | 8.0 | 1 | 2 | 1.475 | 1.5 | 0.95 | 0.029 | 75 | 0.95 |
| 2 | 1.797 | 1.1 | 0.96 | 0.036 | 55 | 0.96 | |||
| 3 | 1.509 | 1.5 | 0.87 | 0.031 | 76 | 0.87 | |||
| 4 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 8.0 | 1 | 2 | 0.364 | 9.8 | 0.95 | 0.007 | 492 | 0.95 |
| 2 | 0.727 | 7.8 | 0.90 | 0.015 | 392 | 0.90 | |||
| 3 | 0.619 | 6.1 | 0.92 | 0.012 | 307 | 0.92 | |||
| 4 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.91 | |||
| Soil column | 8.0 | 4 | 0.5 | 1.367 | 1.8 | 0.96 | 0.027 | 94 | 0.96 |
| 1.0 | 1.805 | 1.4 | 0.98 | 0.036 | 73 | 0.98 | |||
| 1.5 | 2.275 | 1.0 | 0.95 | 0.046 | 52 | 0.95 | |||
| 2.0 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 8.0 | 4 | 0.5 | 0.211 | 11.1 | 0.80 | 0.004 | 558 | 0.80 |
| 1.0 | 0.239 | 7.8 | 0.94 | 0.005 | 393 | 0.94 | |||
| 1.5 | 0.257 | 6.6 | 0.94 | 0.005 | 330 | 0.94 | |||
| 2.0 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.91 | |||
Table 1 Parameters of Thomas and Yoon-Nelson models
| pH | CO32- /(mmol·L-1) | Ca2+ /(mmol·L-1) | Thomas model | Yoon-Nelson model | |||||
|---|---|---|---|---|---|---|---|---|---|
| KTh /(´10-3, mL·min-1·g-1) | qo /(´10-2, mg∙g-1) | R2 | KYN/min-1 | τ/min | R2 | ||||
| Soil column | 7.0 | 4 | 2 | 2.089 | 1.5 | 0.93 | 0.043 | 76 | 0.93 |
| 7.5 | 2.143 | 1.3 | 0.97 | 0.042 | 67 | 0.97 | |||
| 8.0 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 7.0 | 4 | 2 | 0.338 | 8.8 | 0.91 | 0.014 | 444 | 0.91 |
| 7.5 | 0.367 | 7.4 | 0.83 | 0.007 | 371 | 0.83 | |||
| 8.0 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.81 | |||
| Soil column | 8.0 | 1 | 2 | 1.475 | 1.5 | 0.95 | 0.029 | 75 | 0.95 |
| 2 | 1.797 | 1.1 | 0.96 | 0.036 | 55 | 0.96 | |||
| 3 | 1.509 | 1.5 | 0.87 | 0.031 | 76 | 0.87 | |||
| 4 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 8.0 | 1 | 2 | 0.364 | 9.8 | 0.95 | 0.007 | 492 | 0.95 |
| 2 | 0.727 | 7.8 | 0.90 | 0.015 | 392 | 0.90 | |||
| 3 | 0.619 | 6.1 | 0.92 | 0.012 | 307 | 0.92 | |||
| 4 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.91 | |||
| Soil column | 8.0 | 4 | 0.5 | 1.367 | 1.8 | 0.96 | 0.027 | 94 | 0.96 |
| 1.0 | 1.805 | 1.4 | 0.98 | 0.036 | 73 | 0.98 | |||
| 1.5 | 2.275 | 1.0 | 0.95 | 0.046 | 52 | 0.95 | |||
| 2.0 | 2.161 | 1.1 | 0.96 | 0.043 | 55 | 0.96 | |||
| AO-HTC column | 8.0 | 4 | 0.5 | 0.211 | 11.1 | 0.80 | 0.004 | 558 | 0.80 |
| 1.0 | 0.239 | 7.8 | 0.94 | 0.005 | 393 | 0.94 | |||
| 1.5 | 0.257 | 6.6 | 0.94 | 0.005 | 330 | 0.94 | |||
| 2.0 | 0.683 | 5.3 | 0.91 | 0.014 | 266 | 0.91 | |||

Fig. 6 Yoon-Nelson kinetic plots for the adsorption of U(VI) on red soils (a, c, e) and AO-HTC columns (b, d, f) (a,b) Effect of pH; (c, d) Effect of calcium; (e, f) Effect of carbonate (a, b) 4.0 mmol/L CO32-, 2.0 mmol/L Ca2+; (c, d) pH=8.0, 2.0 mmol/L Ca2+; (e, f) pH=8.0, 4.0 mmol/L CO32-
| [1] | ZHANG Z B, DONG Z M, WANG X X , et al. Ordered mesoporous polymer-carbon composites containing amidoxime groups for uranium removal from aqueous solutions. Chemical Engineering Journal, 2018,341:208-217. |
| [2] | ZENG J Y, ZHANG H, SUI Y , et al. New amidoxime-based material TMP-g-AO for uranium adsorption under seawater conditions. Industrial & Engineering Chemistry Research, 2017,56(17):5021-5032. |
| [3] | LIU Y, CAO X, HUA R , et al. Selective adsorption of uranyl ion on ion-imprinted chitosan/PVA cross-linked hydrogel. Hydrometallurgy, 2010,104(2):150-155. |
| [4] | ZHANG Z, ZHANG H, QIU Y , et al. Study on adsorption of uranium by phosphorylated graphene oxide. Sci. Sin. Chim., 2018,48:1-12. |
| [5] | MAYES R T, GORKA J, DAI S . Impact of pore size on the sorption of uranyl under seawater conditions. Industrial & Engineering Chemistry Research, 2016,55(15):4339-4343. |
| [6] | LI J, WANG X, ZHAO G , et al. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev., 2018,47(7):2322-2356. |
| [7] | EBBS. Role of uranium speciation in the uptake and translocation of uranium by plants. Journal of Experimental Botany, 1998,49(324):1183-1190. |
| [8] | YAO Z . Review on remediation technologies of soil contaminated by heavy metals. Procedia Environmental Sciences, 2012,16(4):722-731. |
| [9] | LANDSBERGER S, TAMALIS D . Leaching dynamics of uranium in a contaminated soil site. Journal of Radioanalytical & Nuclear Chemistry, 2013,296(1):319-341. |
| [10] | DING D X, LI S M, HU N , et al. Bioreduction of U(VI) in groundwater under anoxic conditions from a decommissioned in situ leaching uranium mine. Bioprocess & Biosystems Engineering, 2015,38(4):661-670. |
| [11] | OBIRI-NYARKO F, GRAJALES-MESA S J, MALINA G . An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere, 2014,111:243-259. |
| [12] | RICHARDSON J P, NICKLOW J W . In situ permeable reactive barriers for groundwater contamination. Soil & Sediment Contamination, 2002,11(2):241-268. |
| [13] | KORNILOVYCH B, WIREMAN M, UBALDINI S , et al. Uranium removal from groundwater by permeable reactive barrier with zero-valent iron and organic carbon mixtures: laboratory and field studies. Metals, 2018,8(6):408-419 |
| [14] | ZHANG W, GUO Y, PAN Z , et al. Remediation of uranium- contaminated groundwater using the permeable reactive barrier thchnique coupled with hydroxyapatite-coated quartz sands. Fresenius Environmental Bulletin, 2018,27(5):2703-2716. |
| [15] | LI Z J, WANG L, YUAN L Y , et al. Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite. Journal of Hazardous Materials, 2015,290:26-33. |
| [16] | ZHANG Z, LIU J, CAO X , et al. Comparison of U(VI) adsorption onto nanoscale zero-valent iron and red soil in the presence of U(VI)-CO3/Ca-U(VI)-CO3 complexes. Journal of Hazardous Materials, 2015,300:633-642. |
| [17] | WILTAFSKY M K, SCHMIDTLEIN B, ROTH F X . Estimates of the optimum dietary ratio of standardized ileal digestible valine to lysine for eight to twenty-five kilograms of body weight pigs. Journal of Animal Science, 2009,87(8):2544-2553. |
| [18] | LIU X, WU J, ZHANG S , et al. Amidoxime-functionalized hollow carbon spheres for efficient removal of uranium from wastewater. ACS Sustainable Chemistry & Engineering, 2019,7(12):10800-10807. |
| [19] | LADSHAW A P, DAS S, LIAO W P , et al. Experiments and modeling of uranium uptake by amidoxime-based adsorbent in the presence of other ions in simulated seawater. Industrial & Engineering Chemistry Research, 2016,55(15):4241-4248. |
| [20] | PANG H B, KUO L J, WAI C M , et al. Elution of uranium and transition metals from amidoxime-based polymer adsorbents for sequestering uranium from seawater. Industrial & Engineering Chemistry Research, 2016,55(15):4313-4320. |
| [21] | HAN B, ZHANG E, CHENG G , et al. Hydrothermal carbon superstructures enriched with carboxyl groups for highly efficient uranium removal. Chemical Engineering Journal, 2018,338:734-744. |
| [22] | ZHANG Z B, DONG Z M, DAI Y , et al. Amidoxime-functionalized hydrothermal carbon materials for uranium removal from aqueous solution. RSC Advances, 2016,6(104):102462-102471. |
| [23] | YABUSAKI S B, FANG Y, LONG P E , et al. Uranium removal from groundwater via in situ biostimulation: field-scale modeling of transport and biological processes. J. Contam. Hydrol., 2007,93(1-4):216-235. |
| [24] | OMIDI M H, AZAD F N, GHAEDI M , et al. Synthesis and characterization of Au-NPs supported on carbon nanotubes: application for the ultrasound assisted removal of radioactive UO2 2+ ions following complexation with Arsenazo III: spectrophotometric detection, optimization, isotherm and kinetic study. J. Colloid. Interface Sci., 2017,504:68-77. |
| [25] | ZHANG Z B, LIU Y H, CAO X H , et al. Sorption study of uranium on carbon spheres hydrothermal synthesized with glucose from aqueous solution. Journal of Radioanalytical and Nuclear Chemistry, 2013,295(3):1775-1782. |
| [26] | HUANG F, XU Y, LIAO S , et al. Preparation of amidoxime polyacrylonitrile chelating nanofibers and their application for adsorption of metal ions. Materials, 2013,6(3):969-980. |
| [27] | ZHAO Y, LI J, ZHAO L , et al. Synthesis of amidoxime-functionalized Fe3O4@SiO2 core-shell magnetic microspheres for highly efficient sorption of U(VI). Chemical Engineering Journal, 2014,235:275-283. |
| [28] | ZHANG Z B, LIU J, CAO X H , et al. Comparison of U(VI) adsorption onto nanoscale zero-valent iron and red soil in the presence of U(VI)-CO3/Ca-U(VI)-CO3 complexes. Journal of Hazardous Materials, 2015,300:633-642. |
| [29] | LIU J, ZHAO C, YUAN G , et al. Adsorption of U(VI) on a chitosan/ polyaniline composite in the presence of Ca/Mg-U(VI)-CO3 complexes. Hydrometallurgy, 2018,175:300-311. |
| [30] | LI X, ZHANG M, LIU Y , et al. Removal of U(VI) in aqueous solution by nanoscale zero-valent iron(nZVI). Water Quality Exposure & Health, 2013,5(1):31-40. |
| [31] | CERRATO J M, BARROWS C J, BLUE L Y , et al. Effect of Ca2+ and Zn2+ on UO2 dissolution rates. Environ. Sci. Technol., 2012,46(5):2731-2737. |
| [32] | KENNEDY D W, FREDRICKSON J K, BROOKS S C , et al. Inhibition of bacterial U(VI) reduction by calcium. Abstracts of the General Meeting of the American Society for Microbiology, 2003,37(9):1850-1858. |
| [33] | EL HAYEK E, TORRES C, RODRIGUEZ-FREIRE L , et al. Effect of calcium on the bioavailability of dissolved uranium(VI) in plant roots under circumneutral pH. Environ. Sci. Technol., 2018,52(22):13089-13098. |
| [34] | CHEN L F, YIN X B, YU Q , et al. Rapid and selective capture of perrhenate anion from simulated groundwater by a mesoporous silica-supported anion exchanger. Microporous Mesoporous Mat., 2019,274:155-162. |
| [35] | WANG X X, CHEN L, WANG L , et al. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci. China-Chem., 2019,62(8):933-967. |
| [36] | MAHMOUD M A . Adsorption of U(VI) ions from aqueous solution using silicon dioxide nanopowder. Journal of Saudi Chemical Society, 2018,22(2):229-238. |
| [37] | YAKOUT S M, METWALLY S S, EL-ZAKLA T . Uranium sorption onto activated carbon prepared from rice straw: competition with humic acids. Applied Surface Science, 2013,280(8):745-750. |
| [38] | ZHANG Z B, DONG Z M, WANG X X , et al. Synthesis of ultralight phosphorylated carbon aerogel for efficient removal of U(VI): batch and fixed-bed column studies. Chemical Engineering Journal, 2019,370:1376-1387. |
| [1] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [2] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [3] | HONG Peiping, LIANG Long, WU Lian, MA Yingkang, PANG Hao. Structure Regulation of ZIF-67 and Adsorption Properties for Chlortetracycline Hydrochloride [J]. Journal of Inorganic Materials, 2025, 40(4): 388-396. |
| [4] | WU Guangyu, SHU Song, ZHANG Hongwei, LI Jianjun. Enhanced Styrene Adsorption by Grafted Lactone-based Activated Carbon [J]. Journal of Inorganic Materials, 2024, 39(4): 390-398. |
| [5] | XIE Tian, SONG Erhong. Effect of Elastic Strains on Adsorption Energies of C, H and O on Transition Metal Oxides [J]. Journal of Inorganic Materials, 2024, 39(11): 1292-1300. |
| [6] | CHAO Shaofei, XUE Yanhui, WU Qiong, WU Fufa, MUHAMMAD Sufyan Javed, ZHANG Wei. Efficient Potassium Storage through Ti-O-H-O Electron Fast Track of MXene Heterojunction [J]. Journal of Inorganic Materials, 2024, 39(11): 1212-1220. |
| [7] | XU Zhou, LIU Yuxuan, CHI Junlin, ZHANG Tingting, WANG Shuyue, LI Wei, MA Chunhui, LUO Sha, LIU Shouxin. Horseshoe-shaped Hollow Porous Carbon: Synthesis by Hydrothermal Carbonization with Dual-template and Electrochemical Property [J]. Journal of Inorganic Materials, 2023, 38(8): 954-962. |
| [8] | MA Xiaosen, ZHANG Lichen, LIU Yanchao, WANG Quanhua, ZHENG Jiajun, LI Ruifeng. 13X@SiO2: Synthesis and Toluene Adsorption [J]. Journal of Inorganic Materials, 2023, 38(5): 537-543. |
| [9] | GUO Chunxia, CHEN Weidong, YAN Shufang, ZHAO Xueping, YANG Ao, MA Wen. Adsorption of Arsenate in Water by Zirconia-halloysite Nanotube Material [J]. Journal of Inorganic Materials, 2023, 38(5): 529-536. |
| [10] | WANG Shiyi, FENG Aihu, LI Xiaoyan, YU Yun. Pb (II) Adsorption Process of Fe3O4 Supported Ti3C2Tx [J]. Journal of Inorganic Materials, 2023, 38(5): 521-528. |
| [11] | YU Yefan, XU Ling, NI Zhongbing, SHI Dongjian, CHEN Mingqing. Prussian Blue Modified Biochar: Preparation and Adsorption of Ammonia Nitrogen from Sewage [J]. Journal of Inorganic Materials, 2023, 38(2): 205-212. |
| [12] | LING Jie, ZHOU Anning, WANG Wenzhen, JIA Xinyu, MA Mengdan. Effect of Cu/Mg Ratio on CO2 Adsorption Performance of Cu/Mg-MOF-74 [J]. Journal of Inorganic Materials, 2023, 38(12): 1379-1386. |
| [13] | TANG Ya, SUN Shengrui, FAN Jia, YANG Qingfeng, DONG Manjiang, KOU Jiahui, LIU Yangqiao. PEI Modified Hydrated Calcium Silicate Derived from Fly Ash and Its adsorption for Removal of Cu (II) and Catalytic Degradation of Organic Pollutants [J]. Journal of Inorganic Materials, 2023, 38(11): 1281-1291. |
| [14] | DAI Jieyan, FENG Aihu, MI Le, YU Yang, CUI Yuanyuan, YU Yun. Adsorption Mechanism of NaY Zeolite Molecular Adsorber Coating on Typical Space Contaminations [J]. Journal of Inorganic Materials, 2023, 38(10): 1237-1244. |
| [15] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, HUANG Weiqiu, CHEN Ruoyu. Mesoporous Organic-inorganic Hybrid Siliceous Hollow Spheres: Synthesis and VOCs Adsorption [J]. Journal of Inorganic Materials, 2022, 37(9): 991-1000. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||