
Journal of Inorganic Materials ›› 2018, Vol. 33 ›› Issue (4): 434-440.DOI: 10.15541/jim20170222
• Orginal Article • Previous Articles Next Articles
WANG Hao1, LUO Yong-Chun1,2, DENG An-Qiang2, ZHAO Lei2, JIANG Wan-Ting1
Received:2017-05-04
Revised:2017-07-14
Published:2018-04-30
Online:2018-03-27
About author:WANG Hao. E-mail: 317003562@qq.com
Supported by:CLC Number:
WANG Hao, LUO Yong-Chun, DENG An-Qiang, ZHAO Lei, JIANG Wan-Ting. Annealing Temperature on Structural and Electrochemical Property of Mg-free La-Y-Ni Based A2B7-type Hydrogen Storage Alloys[J]. Journal of Inorganic Materials, 2018, 33(4): 434-440.
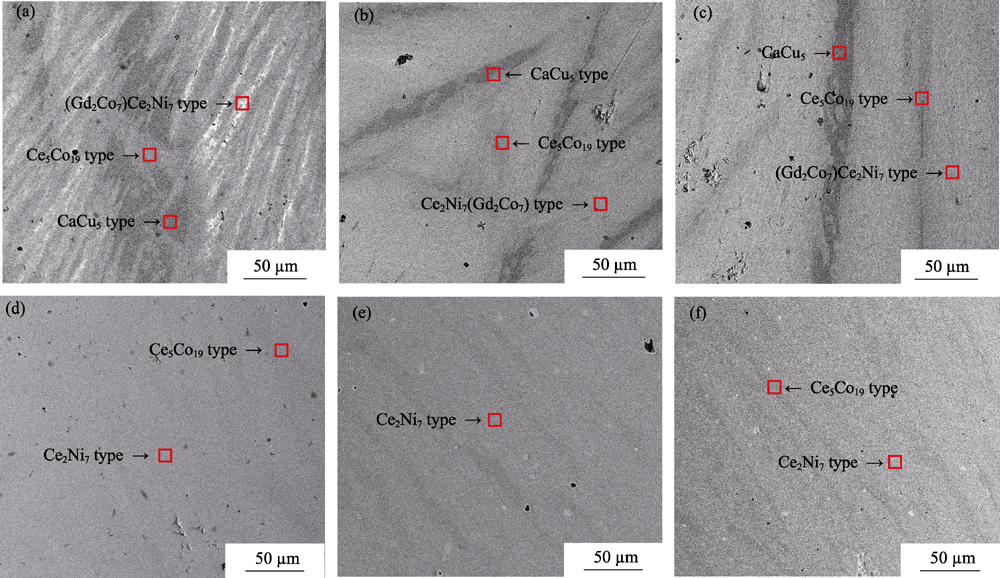
Fig. 1 Backscattered SEM images of the La0.33Y0.67Ni3.25Mn0.15Al0.1 alloys annealed at different temperatures (a) As cast; (b) 850℃; (c) 900℃; (d) 950℃; (e) 1000℃; (f) 1050℃
| Alloy | Phase area | Atomic ratios of different elements/at% | Stoichiometric B/A | ||||
|---|---|---|---|---|---|---|---|
| La | Y | Ni | Mn | Al | |||
| As-cast (ICP) As-cast | CaCu5-type | 5.3 | 16.7 | 69.9 | 4.4 | 3.7 | 3.54 |
| 3.4 | 14.1 | 76.0 | 4.2 | 2.3 | 4.71 | ||
| Ce2Ni7(Gd2Co7)-type | 4.6 | 15.0 | 74.4 | 3.8 | 2.2 | 3.42 | |
| Ce5Ni19-type | 3.8 | 15.4 | 74.5 | 3.7 | 3.0 | 4.08 | |
| 850℃ | CaCu5-type | 3.1 | 13.7 | 76.3 | 3.2 | 3.8 | 4.95 |
| Ce2Ni7(Gd2Co7)-type | 5.0 | 15.5 | 73.8 | 3.4 | 3.3 | 3.41 | |
| Ce5Ni19-type | 4.0 | 15.7 | 73.6 | 3.7 | 3.0 | 4.08 | |
| 900℃ | CaCu5-type | 3.1 | 13.8 | 75.0 | 3.8 | 3.6 | 5.06 |
| Ce2Ni7(Gd2Co7)-type | 5.5 | 16.2 | 71.4 | 3.7 | 2.5 | 3.58 | |
| Ce5Ni19-type | 4.3 | 16.1 | 72.1 | 3.5 | 3.4 | 3.76 | |
| 950℃ | Ce2Ni7-type | 6.2 | 16.0 | 71.4 | 3.3 | 3.1 | 3.41 |
| Ce5Co19-type | 5.1 | 15.8 | 72.1 | 4.0 | 3.0 | 3.85 | |
| 1000℃ | Ce2Ni7-type | 5.8 | 16.4 | 70.6 | 4.7 | 2.5 | 3.47 |
| Ce5Co19-type | 4.8 | 15.9 | 71.4 | 4.9 | 3.3 | 3.90 | |
| 1050℃ | Ce2Ni7-type | 5.2 | 16.5 | 72.0 | 4.5 | 2.6 | 3.51 |
| Ce5Co19-type | 4.6 | 16.2 | 72.1 | 4.6 | 2.5 | 3.81 | |
Table 1 Element compositions by ICP for the as-cast alloy and EDS results of the annealed alloys in phase regions
| Alloy | Phase area | Atomic ratios of different elements/at% | Stoichiometric B/A | ||||
|---|---|---|---|---|---|---|---|
| La | Y | Ni | Mn | Al | |||
| As-cast (ICP) As-cast | CaCu5-type | 5.3 | 16.7 | 69.9 | 4.4 | 3.7 | 3.54 |
| 3.4 | 14.1 | 76.0 | 4.2 | 2.3 | 4.71 | ||
| Ce2Ni7(Gd2Co7)-type | 4.6 | 15.0 | 74.4 | 3.8 | 2.2 | 3.42 | |
| Ce5Ni19-type | 3.8 | 15.4 | 74.5 | 3.7 | 3.0 | 4.08 | |
| 850℃ | CaCu5-type | 3.1 | 13.7 | 76.3 | 3.2 | 3.8 | 4.95 |
| Ce2Ni7(Gd2Co7)-type | 5.0 | 15.5 | 73.8 | 3.4 | 3.3 | 3.41 | |
| Ce5Ni19-type | 4.0 | 15.7 | 73.6 | 3.7 | 3.0 | 4.08 | |
| 900℃ | CaCu5-type | 3.1 | 13.8 | 75.0 | 3.8 | 3.6 | 5.06 |
| Ce2Ni7(Gd2Co7)-type | 5.5 | 16.2 | 71.4 | 3.7 | 2.5 | 3.58 | |
| Ce5Ni19-type | 4.3 | 16.1 | 72.1 | 3.5 | 3.4 | 3.76 | |
| 950℃ | Ce2Ni7-type | 6.2 | 16.0 | 71.4 | 3.3 | 3.1 | 3.41 |
| Ce5Co19-type | 5.1 | 15.8 | 72.1 | 4.0 | 3.0 | 3.85 | |
| 1000℃ | Ce2Ni7-type | 5.8 | 16.4 | 70.6 | 4.7 | 2.5 | 3.47 |
| Ce5Co19-type | 4.8 | 15.9 | 71.4 | 4.9 | 3.3 | 3.90 | |
| 1050℃ | Ce2Ni7-type | 5.2 | 16.5 | 72.0 | 4.5 | 2.6 | 3.51 |
| Ce5Co19-type | 4.6 | 16.2 | 72.1 | 4.6 | 2.5 | 3.81 | |

Fig. 2 XRD patterns of La0.33Y0.67Ni3.25Mn0.15Al0.1 alloys annealed at different temperatures (a), (b) and Rietveld refinement pattern of the alloy annealed at 950℃ (c)
| Alloy | Na | Cmax/(mAh•g-1) | (H/M)/wt% | S100/% | HRD900/% | I0/(mA•g-1) | D0/ (×10-10, cm2•s-1) | Icorr /(mA•g-1) | |
|---|---|---|---|---|---|---|---|---|---|
| 60/(mA•g-1) | 100/(mA•g-1) | ||||||||
| As-cast | 3 | 299.2 | 252.4 | 1.07 | 77.7 | 78.8 | 330.9 | 1.18 | 6.82 |
| 850℃ | 3 | 347.2 | 303.5 | 1.10 | 78.2 | 80.3 | 278.6 | 1.57 | 6.19 |
| 900℃ | 3 | 353.3 | 318.5 | 1.21 | 78.3 | 82.3 | 229.9 | 1.82 | 6.14 |
| 950℃ | 3 | 371.5 | 365.2 | 1.35 | 88.9 | 83.4 | 243.5 | 2.68 | 5.01 |
| 1000℃ | 5 | 370.4 | 358.6 | 1.34 | 86.5 | 80.6 | 269.1 | 2.56 | 5.12 |
| 1050℃ | 6 | 365.2 | 356.7 | 1.28 | 81.5 | 76.4 | 321.5 | 2.32 | 5.23 |
Table 2 Hydrogen storage and electrochemical properties of La0.33Y0.67Ni3.25Mn0.15Al0.1 alloy electrodes annealed at different temperatures
| Alloy | Na | Cmax/(mAh•g-1) | (H/M)/wt% | S100/% | HRD900/% | I0/(mA•g-1) | D0/ (×10-10, cm2•s-1) | Icorr /(mA•g-1) | |
|---|---|---|---|---|---|---|---|---|---|
| 60/(mA•g-1) | 100/(mA•g-1) | ||||||||
| As-cast | 3 | 299.2 | 252.4 | 1.07 | 77.7 | 78.8 | 330.9 | 1.18 | 6.82 |
| 850℃ | 3 | 347.2 | 303.5 | 1.10 | 78.2 | 80.3 | 278.6 | 1.57 | 6.19 |
| 900℃ | 3 | 353.3 | 318.5 | 1.21 | 78.3 | 82.3 | 229.9 | 1.82 | 6.14 |
| 950℃ | 3 | 371.5 | 365.2 | 1.35 | 88.9 | 83.4 | 243.5 | 2.68 | 5.01 |
| 1000℃ | 5 | 370.4 | 358.6 | 1.34 | 86.5 | 80.6 | 269.1 | 2.56 | 5.12 |
| 1050℃ | 6 | 365.2 | 356.7 | 1.28 | 81.5 | 76.4 | 321.5 | 2.32 | 5.23 |
| [1] | KADIR K, SAKAI T, UEHARA I.Synthesis and structure determination of a new series of hydrogen storage alloys RMg2Ni9 (R=La, Ce, Pr, Nd, Sm and Gd) built from MgNi2 Laves-type layers alternating with AB5 layers. J. Alloys Compd., 1997. 257(1/2): 115-121. |
| [2] | KOHNO T, YOSHIDA H, KAWASHIMA F,et al. Hydrogen storage properties of new ternary system alloys: La2MgNi9, La5Mg2Ni23, La3MgNi14. J. Alloys Compd., 2000, 311(2): L5-L7. |
| [3] | LIU Y F, PAN H G, GAO M,et al. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries. J. Mater. Chem., 2011, 21(13): 4743-4755. |
| [4] | LIU J J, HAN S M, LI Y,et al. Phase structures and electrochemical properties of La-Mg-Ni based hydrogen storage alloys with superlattice structure. Int. J. Hydrogen Energy, 2016, 41(44): 20261-20275. |
| [5] | YASUOKA S, MAGARI Y, MURATA T,et al. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sources, 2006, 156(2): 662-666. |
| [6] | DENYS V R., RIABOV A B, YARTYS V A,et al. Mg substitution effect on the hydrogenation behaviour, thermodynamic and structural properties of the La2Ni7-H(D)2 system. J. Solid State Chem., 2008, 181(4): 812-821. |
| [7] | GAL L, CHARBONNIER V, ZHANG J, et al. Optimization of the La substitution by Mg in the La2Ni7 hydride-forming system for use as negative electrode in Ni-MH battery. Int. J. Hydrogen Energy, 2015, 40(47): 17017-17020. |
| [8] | MONNIER J, CHEN H, JOIRET S, et al. Identification of a new pseudo-binary hydroxide during calendar corrosion of (La, Mg)2Ni7-type hydrogen storage alloys for nickel-metal hydride batteries. J. Power Sources, 2014, 266: 162-169. |
| [9] | BADDOUR R H, MEYER L, PEREIRA RAMOS J P,et al. An electrochemical study of new La1-xCexY2Ni9(0≤x≤1) hydrogen storage alloys. Electrochim. Acta, 2001, 46(15): 2385-2393. |
| [10] | BEREZOVETS V V, DENYS R V, RYABOV O B,et al. Hydrides of substituted derivatives based on the YNi3 compound. Mater. Sci., 2007, 43(4): 499-507. |
| [11] | CHARBONNIER V, MONNIER J, ZHANG J,et al. Relationship between H2 sorption properties and aqueous corrosion mechanisms in A2Ni7 hydride forming alloys (A = Y, Gd or Sm). J. Power Sources, 2016, 326: 146-155. |
| [12] | YAN H Z, XIONG W, WANG L,et al. Investigations on AB3-, A2B7- and A5B19-type La-Y-Ni system hydrogen storage alloys. Int. J. Hydrogen Energy, 2017, 42(4): 2257-2264. |
| [13] | 长崎诚三, 平林真. 二元合金状态相图集. 北京: 冶金工业出版社, 2004: 197. |
| [14] | SUBRAMANIAN P R, SMITH J F.Thermodynamics of formation of Y-Ni alloys.Metallurgical Transactions B, 1985, 16B: 577-584. |
| [15] | YOUNG R A. The Rietvld Method.London: Oxford University Press, 1995. |
| [16] | YAMAMOTO T, INUI H, YAMAGUCHI M,et al. Microstructures and hydrogen absorption/desorption properties of La-Ni alloys in the composition range of La-(77.8-83.2)at%Ni. Acta Materialia, 1997, 45(12): 5213-5218. |
| [17] | XIONG W, YAN H Z, WANG L,et al. Effects of annealing temperature on the structure and properties of the LaY2Ni10Mn0.5 hydrogen storage alloy. Int. J. Hydrogen Energy, 2017, 42(22): 15319-15327. |
| [18] | LUO G, HU X C, LI S L,et al. Hydrogen storage properties of La1-xYxNi5-yAly Alloys. Rare Metal Materials and Engineering, 2012, 41(10): 1693-1699. |
| [19] | LIU J J, LI Y, HAN D,et al. Electrochemical performance and capacity degradation mechanism of single-phase La-Mg-Ni-based hydrogen storage alloys. J. Power Sources, 2015, 300: 77-86. |
| [20] | ZHANG Q, CHEN Z, LI Y,et al. Comparative investigations on hydrogen absorption-desorption properties of Sm-Mg-Ni compounds: the effect of [SmNi5]/[SmMgNi4] unit ratio. J. Phys. Chem. C, 2015, 119(9): 4719-4727. |
| [21] | NOTTEN P H L, HOKKELING P. Double phase hydride forming compounds: a new class of highly electrocatalytic materials.J. Electrochem. Soc., 1991, 138(7): 1877-1885. |
| [22] | ZHENG G, POPOV B N, WHITE R E.Electrochemical determination of the diffusion coefficient of hydrogen through an LaNi4.25Al0.75 electrode in alkaline aqueous solution. J. Electrochem. Soc., 1995, 142(8): 2695-2698. |
| [1] | MU Haojie, ZHANG Yuanjiang, YU Bin, FU Xiumei, ZHOU Shibin, LI Xiaodong. Preparation and Properties of ZrO2 Doped Y2O3-MgO Nanocomposite Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 281-289. |
| [2] | FAN Wugang, CAO Xiong, ZHOU Xiang, LI Ling, ZHAO Guannan, ZHANG Zhaoquan. Anticorrosion Performance of 8YSZ Ceramics in Simulated Aqueous Environment of Pressurized Water Reactor [J]. Journal of Inorganic Materials, 2024, 39(7): 803-809. |
| [3] | CHEN Qian, SU Haijun, JIANG Hao, SHEN Zhonglin, YU Minghui, ZHANG Zhuo. Progress of Ultra-high Temperature Oxide Ceramics: Laser Additive Manufacturing and Microstructure Evolution [J]. Journal of Inorganic Materials, 2024, 39(7): 741-753. |
| [4] | JIANG Lingyi, PANG Shengyang, YANG Chao, ZHANG Yue, HU Chenglong, TANG Sufang. Preparation and Oxidation Behaviors of C/SiC-BN Composites [J]. Journal of Inorganic Materials, 2024, 39(7): 779-786. |
| [5] | ZHENG Yawen, ZHANG Cuiping, ZHANG Ruijie, XIA Qian, RU Hongqiang. Fabrication of Boron Carbide Ceramic Composites by Boronic Acid Carbothermal Reduction and Silicon Infiltration Reaction Sintering [J]. Journal of Inorganic Materials, 2024, 39(6): 707-714. |
| [6] | XUE Yifan, LI Weijie, ZHANG Zhongwei, PANG Xu, LIU Yu. Process Control of PyC Interphases Microstructure and Uniformity in Carbon Fiber Cloth [J]. Journal of Inorganic Materials, 2024, 39(4): 399-408. |
| [7] | SUN Chuan, HE Pengfei, HU Zhenfeng, WANG Rong, XING Yue, ZHANG Zhibin, LI Jinglong, WAN Chunlei, LIANG Xiubing. SiC-based Ceramic Materials Incorporating GNPs Array: Preparation and Mechanical Characterization [J]. Journal of Inorganic Materials, 2024, 39(3): 267-273. |
| [8] | ZHENG Jiaqian, LU Xiao, LU Yajie, WANG Yingjun, WANG Zhen, LU Jianxi. Functional Bioadaptability in Medical Bioceramics: Biological Mechanism and Application [J]. Journal of Inorganic Materials, 2024, 39(1): 1-16. |
| [9] | HE Danqi, WEI Mingxu, LIU Ruizhi, TANG Zhixin, ZHAI Pengcheng, ZHAO Wenyu. Heavy-Fermion YbAl3 Materials: One-step Synthesis and Enhanced Thermoelectric Performance [J]. Journal of Inorganic Materials, 2023, 38(5): 577-582. |
| [10] | WU Shuang, GOU Yanzi, WANG Yongshou, SONG Quzhi, ZHANG Qingyu, WANG Yingde. Effect of Heat Treatment on Composition, Microstructure and Mechanical Property of Domestic KD-SA SiC Fibers [J]. Journal of Inorganic Materials, 2023, 38(5): 569-576. |
| [11] | XIE Jiaye, LI Liwen, ZHU Qiang. Contrastive Study on in Vitro Antibacterial Property and Biocompatibility of Three Clinical Pulp Capping Agents [J]. Journal of Inorganic Materials, 2023, 38(12): 1449-1456. |
| [12] | LI Jianbo, TIAN Zhen, JIANG Quanwei, YU Lifeng, KANG Huijun, CAO Zhiqiang, WANG Tongmin. Effects of Different Element Doping on Microstructure and Thermoelectric Properties of CaTiO3 [J]. Journal of Inorganic Materials, 2023, 38(12): 1396-1404. |
| [13] | WU Dongjiang, ZHAO Ziyuan, YU Xuexin, MA Guangyi, YOU Zhulin, REN Guanhui, NIU Fangyong. Direct Additive Manufacturing of Al2O3-TiCp Composite Ceramics by Laser Directed Energy Deposition [J]. Journal of Inorganic Materials, 2023, 38(10): 1183-1192. |
| [14] | ZHANG Ye, ZENG Yuping. Progress of Porous Silicon Nitride Ceramics Prepared via Self-propagating High Temperature Synthesis [J]. Journal of Inorganic Materials, 2022, 37(8): 853-864. |
| [15] | XIA Qian, SUN Shihao, ZHAO Yiliang, ZHANG Cuiping, RU Hongqiang, WANG Wei, YUE Xinyan. Effect of Boron Carbide Particle Size Distribution on the Microstructure and Properties of Reaction Bonded Boron Carbide Ceramic Composites by Silicon Infiltration [J]. Journal of Inorganic Materials, 2022, 37(6): 636-642. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||