
Journal of Inorganic Materials ›› 2016, Vol. 31 ›› Issue (11): 1191-1197.DOI: 10.15541/jim20160019
• Orginal Article • Previous Articles Next Articles
WU Mei-Jian1, GAO Zhen-Yuan2, YUAN Jing2, ZHAO Kun-Feng2, CAI Ting2, YANG Ling2, ZHANG Tao2, HE Dan-Nong1, 2
Received:2016-01-07
Revised:2016-04-14
Published:2016-11-10
Online:2016-10-25
About author:WU Mei-Jian. E-mail: wumj1989@163.com
CLC Number:
WU Mei-Jian, GAO Zhen-Yuan, YUAN Jing, ZHAO Kun-Feng, CAI Ting, YANG Ling, ZHANG Tao, HE Dan-Nong. Hydrothermal Fabrication and Catalytic Performance of Chromium Oxide for Low-concentration NO Oxidation at Ambient Temperature[J]. Journal of Inorganic Materials, 2016, 31(11): 1191-1197.
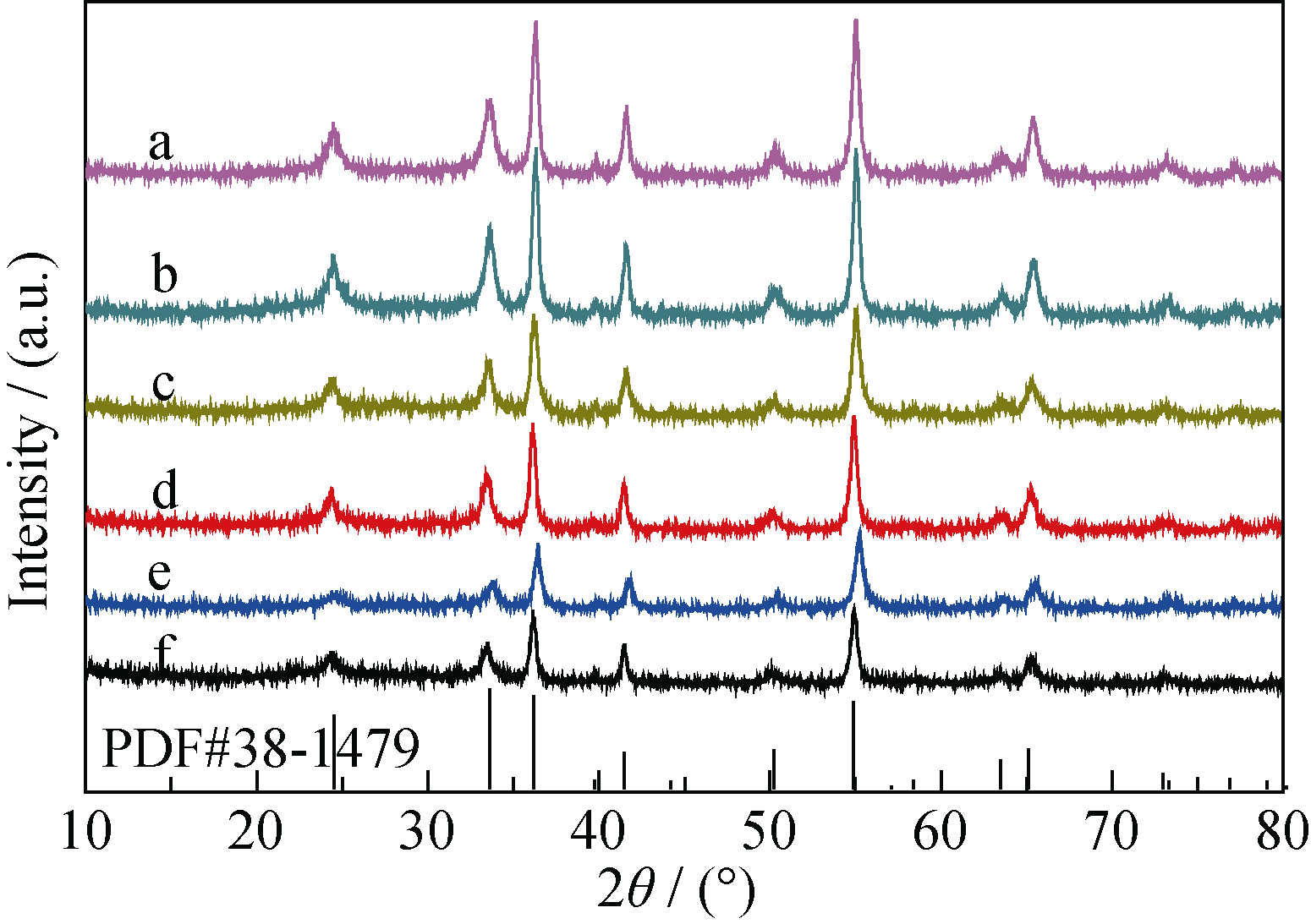
Fig. 1 XRD patterns of catalyst synthesized at various hydrothermal temperatures(a) Cr-160; (b) Cr-140; (c) Cr-120; (d) Cr-100U; (e) Cr-100; (f) Cr-80
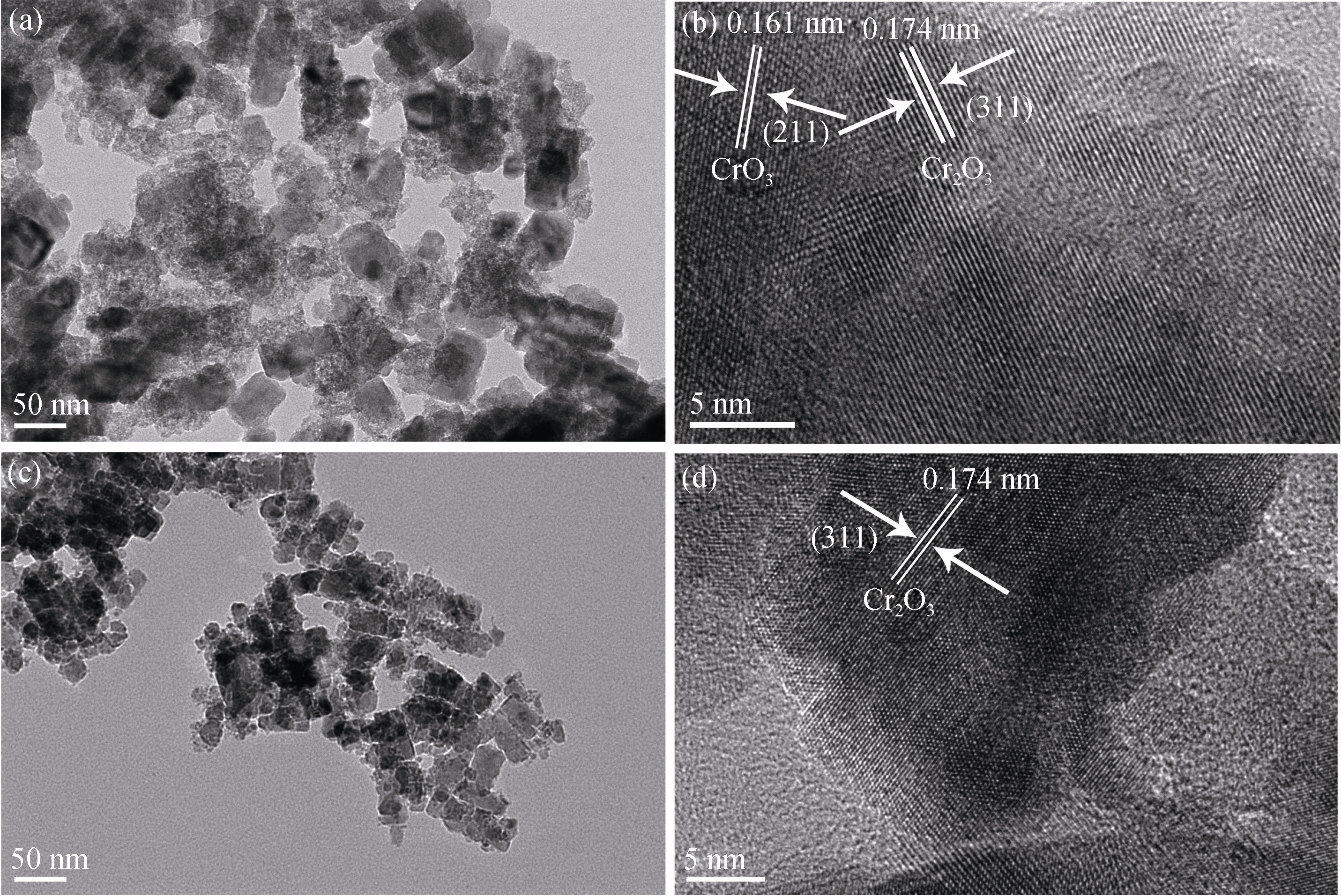
Fig. 2 TEM (a, c) and HETEM (b, d) images of the chromium oxide catalysts synthesized at different hydrothermal temperatures(a) Cr-100; (b) Cr-100; (c) Cr-160; (d) Cr-160

Fig. 3 XPS spectra of Cr2p of fresh samples prepared at different hydrothermal temperatures and catalyst after NO removal test(a) Cr-100; (b) Cr-100U; (c) Cr-80; (d) Cr-160
| Samples | Cr6+/Cr3+ | Cr/O | N/Cr | dXRD/nm |
|---|---|---|---|---|
| Cr-80 | 0.37 | 0.22 | - | 26.4 |
| Cr-100 | 0.64 | 0.32 | 0 | 20.7 |
| Cr-100U | 0.52 | 0.34 | 0.23 | 22.0 |
| Cr-160 | 0.23 | 0.19 | - | 37.6 |
Table 1 Properties and XPS result of fresh samples prepared at different hydrothermal temperatures and catalyst after NO removal test
| Samples | Cr6+/Cr3+ | Cr/O | N/Cr | dXRD/nm |
|---|---|---|---|---|
| Cr-80 | 0.37 | 0.22 | - | 26.4 |
| Cr-100 | 0.64 | 0.32 | 0 | 20.7 |
| Cr-100U | 0.52 | 0.34 | 0.23 | 22.0 |
| Cr-160 | 0.23 | 0.19 | - | 37.6 |
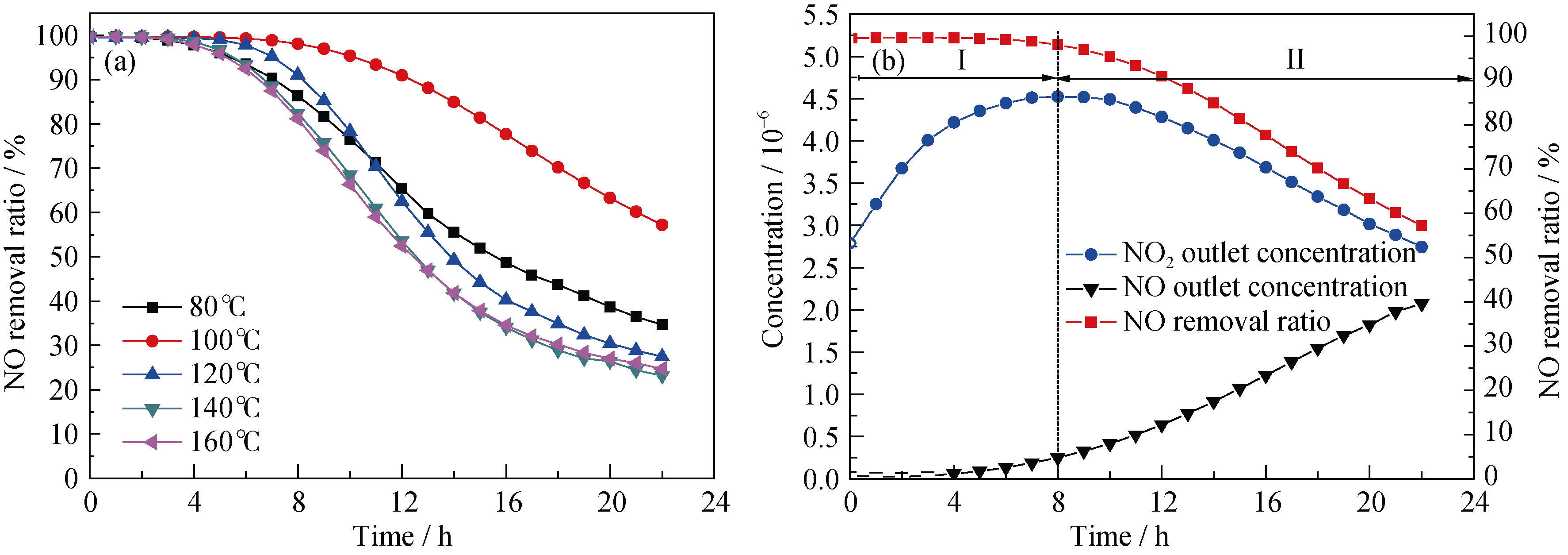
Fig. 6 (a) The stability tests of NO oxidation at ambient temperature over chromic catalysts and (b) NO2 concentration catalyzed by Cr-100 catalyst(Reaction conditions: mass of catalyst = 0.25 g, total flow rate =500 mL/min, GHSV = 120000 mL/(g·h), NO concentration=5×10-6, temperature=30℃)
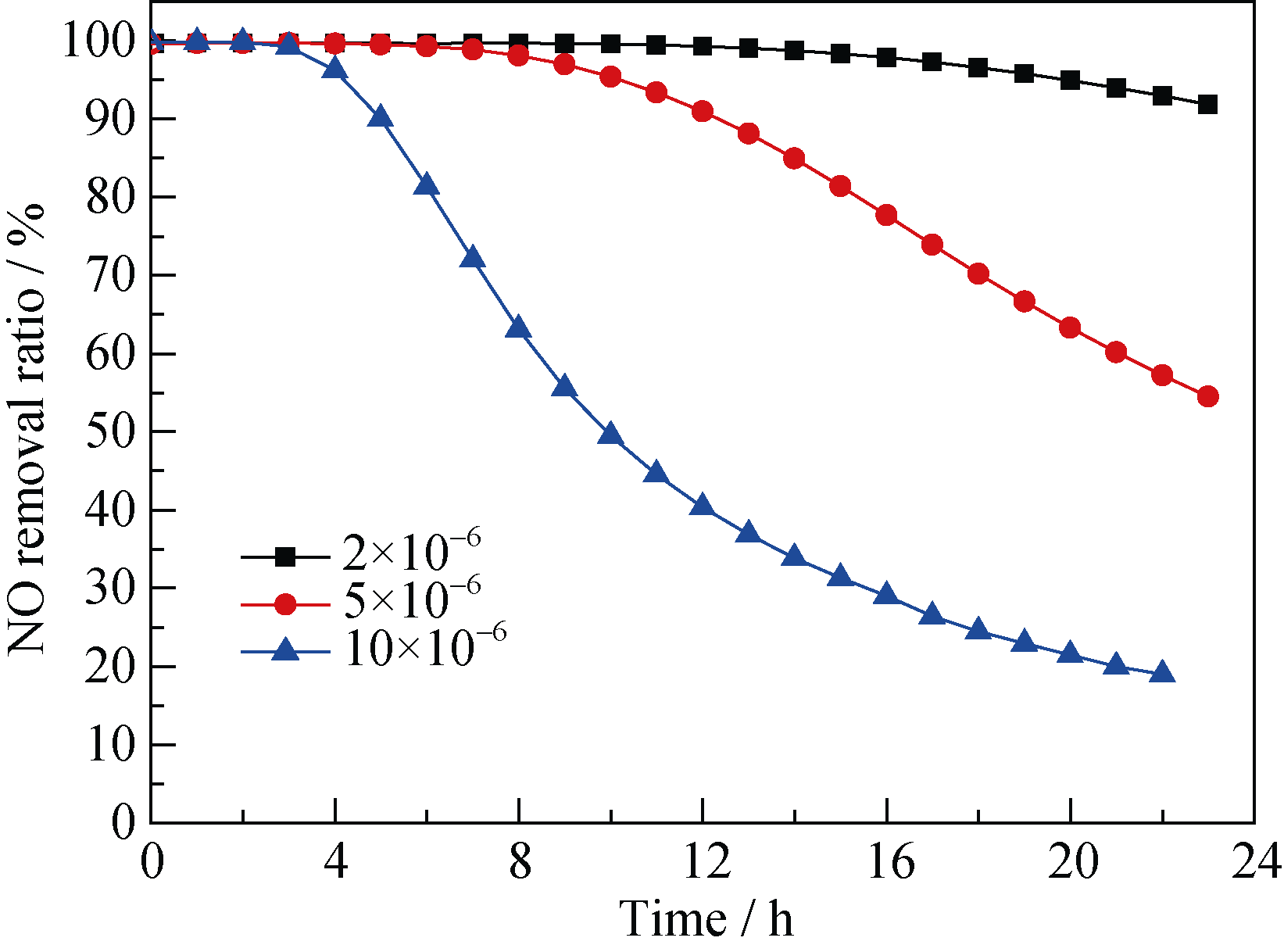
Fig. 7 Effect of different initial NO concentrations on NO removal over the Cr-100 catalyst(Reaction conditions: mass of Cr-100 catalyst = 0.25 g, total flow rate = 500 mL/min, GHSV = 120000 mL/(g·h), NO concentration = 2×10-6, 5×10-6, 10×10-6, temperature=30℃)
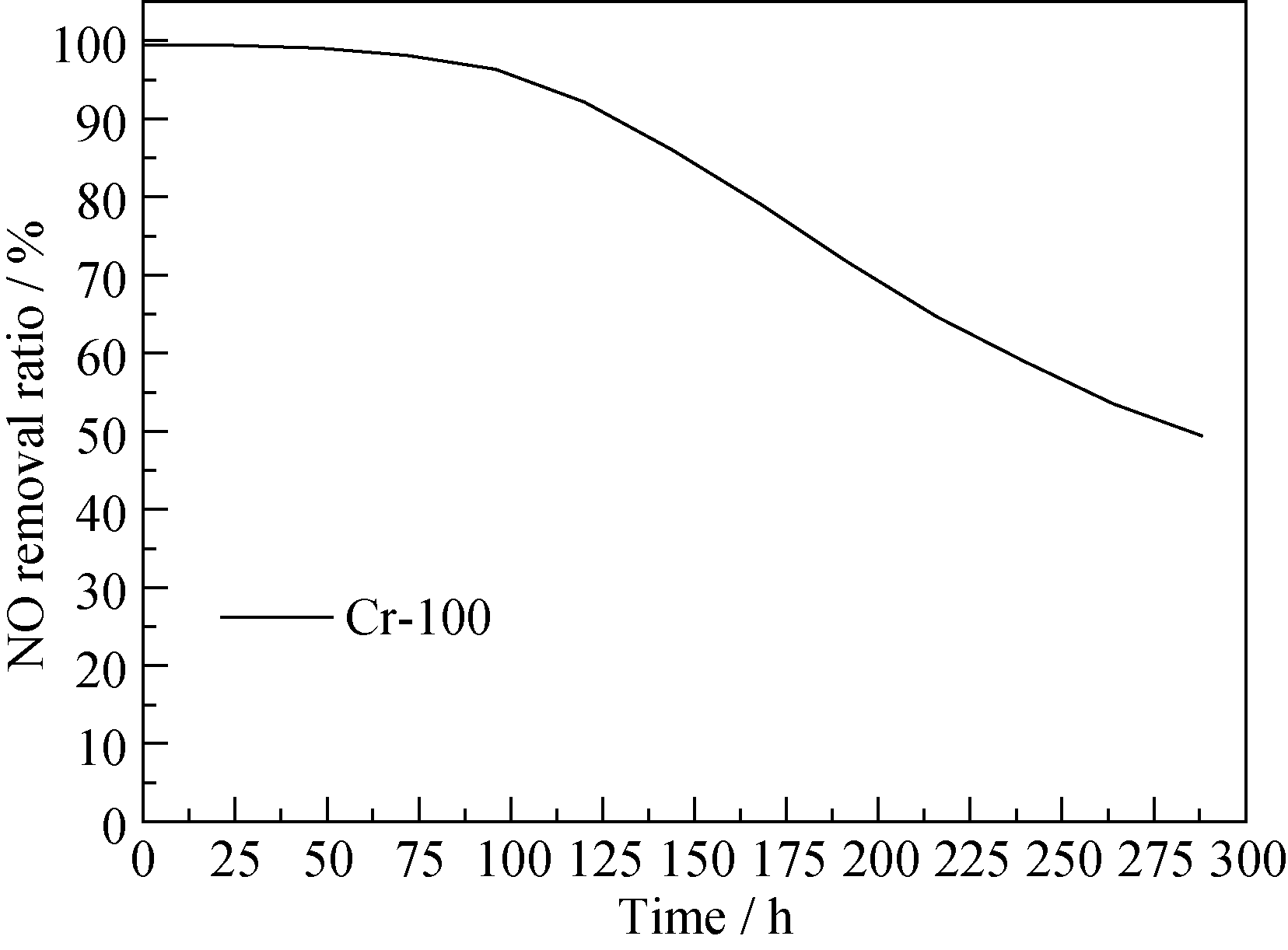
Fig. 8 The stability tests of NO removal over Cr-100 catalyst(Reaction conditions: mass of Cr-100 catalyst = 0.5 g, total flow rate: = 500 mL/min, GHSV = 60000 mL/(g·h), NO concentration = 1×10-6 , temperature = 30℃)
| [1] | WANG H K, CHEN C H, HUANG C, et al.On-road vehicle emission inventory and its uncertainty analysis for Shanghai, China.Science of The Total Environment, 2008, 398(1): 60-67. |
| [2] | KAMPA M, CASTANAS E.Human health effects of air pollution.Environmental Pollution, 2008, 151(2): 362-367. |
| [3] | LIU Z M, ZHANG S X, LI J H, et al.Promoting effect of MoO3 on the NOx reduction by NH3 over CeO2/TiO2 catalyst studied with in situ DRIFTS. Applied Catalysis B: Environmental, 2014, 144: 90-95. |
| [4] | WESTERDAHL D, WANG X, PAN X C, et al.Characterization of on-road vehicle emission factors and microenvironmental air quality in Beijing, China.Atmospheric Environment, 2009, 43(3): 697-705. |
| [5] | CHEN Y, ZHOU X X, WANG J, et al.Synthesis of mesoporous composite oxides for the catalysis of NH3-SCR.Journal of Inorganic Materials, 2015, 30(11): 1155-1160. |
| [6] | QIU L, MENG J J, PANG D D, et al.Reaction and characterization of Co and Ce doped Mn/TiO2 catalysts for low-temperature SCR of NO with NH3.Catalysis Letters, 2015, 145(7): 1500-1509. |
| [7] | SCHILL L, PUTLURU S S R, FEHRMANN R, et al. Low- temperature NH3-SCR of NO on mesoporous Mn0. 6Fe0.4/TiO2 prepared by a hydrothermal method.Catalysis Letters, 2014, 144(3): 395-402. |
| [8] | SCHILL L, PUTLURU S S R, JENSEN A D, et al. MnFe/Al2O3 catalyst synthesized by deposition precipitation for low-temperature selective catalytic reduction of NO with NH3.Catalysis Letters, 2015, 145(9): 1724-1732. |
| [9] | SOUSA J P, PEREIRA M F, FIGUEIREDO J L.NO oxidation over nitrogen doped carbon xerogels. Applied Catalysis B: Environmental, 2012, 125: 398-408. |
| [10] | WANG MX, HUANG Z H, SHEN K, et al.Catalytically oxidation of NO into NO2 at room temperature by graphitized porous nanofibers.Catalysis Today, 2013, 201: 109-114. |
| [11] | SHU Z, HUANG W M, HUA Z, et al.Template-free synthesis of mesoporous X-Mn (X = Co, Ni, Zn) bimetal oxides and catalytic application in the room temperature removal of low-concentration NO.Journal ofMaterials Chemistry A, 2013, 1(35): 10218-10227. |
| [12] | SHU Z, CHEN Y, HUANG W M, et al.Room-temperature catalytic removal of low-concentration NO over mesoporous Fe-Mn binary oxide synthesized using a template-free approach.Applied Catalysis B: Environmental, 2013, 140: 42-50. |
| [13] | WANG J, ZHU J Z, ZHOU X X, et al.Nanoflower-like weak crystallization manganese oxide for efficient removal of low-concentration NO at room temperature.Journal of Material Chemistry A, 2015, 3(14): 7631-7638. |
| [14] | LIU S, ZHANG M T, HUANG Y Q, et al.A novel chromic oxide catalyst for NO oxidation at ambient temperature.RSC Advances, 2014, 4(55): 29180-29186. |
| [15] | HOANG D L, DITTMAR A, SCHNEIDER M, et al.Evidence of lanthanum-chromium mixed oxides formed in CrOx/La2O3 model catalysts.Thermochim Acta, 2003, 400(1): 153-163. |
| [16] | WANG F G, ZHAO K F, ZHANG H J, et al.Low temperature CO catalytic oxidation over supported Pd-Cu catalysts calcined at different temperatures.Chemical Engineering Journal. 2014, 242: 10-18. |
| [17] | FIOL N, ESCUDERO C, VILLAESCUSA I.Chromium sorption and Cr(VI) reduction to Cr(III) by grape stalks and yohimbe bark.Bioresource Technology, 2008, 99(11): 5030-5036. |
| [18] | YANG P, XUE X M, MENG Z H, et al.Enhanced catalytic activity and stability of Ce doping on Cr supported HZSM-5 catalysts for deep oxidation of chlorinated volatile organic compounds.Chemical Engineering Journal, 2013, 234: 203-210. |
| [19] | LIU Z M, HAO J M, FU L X, et al.Study of Ag/La0.6Ce0.4CoO3 catalysts for direct decomposition and reduction of nitrogen oxides with propene in the presence of oxygen.Applied Catalysis B: Environmental, 2003, 44(4): 355-370. |
| [20] | LI K, TANG X L, YI H H, et al.Low-temperature catalytic oxidation of NO over Mn-Co-Ce-Ox catalyst.Chemical Engineering Journal, 2012, 192: 99-104. |
| [1] | FENG Guanzheng, YANG Jian, ZHOU Du, CHEN Qiming, XU Wentao, ZHOU Youfu. Mechanism for Hydrothermal-carbothermal Synthesis of AlN Nanopowders [J]. Journal of Inorganic Materials, 2025, 40(1): 104-110. |
| [2] | XIAO Wenyan, FU Yan, YANG Shubin, ZHU Jie, CHENG Zhaoyang, WEN Xiaoxu, TANG Jiafan, YU Liang, ZHANG Qian. Seawater Electrolysis Performance of Self-supported Amorphous Ce-FeHPi/NF Electrode [J]. Journal of Inorganic Materials, 2024, 39(12): 1348-1356. |
| [3] | YUE Quanxin, GUO Ruihua, WANG Ruifen, AN Shengli, ZHANG Guofang, GUAN Lili. 3D Core-shell Structured NiMoO4@CoFe-LDH Nanorods: Performance of Efficient Oxygen Evolution Reaction and Overall Water Splitting [J]. Journal of Inorganic Materials, 2024, 39(11): 1254-1264. |
| [4] | XU Zhou, LIU Yuxuan, CHI Junlin, ZHANG Tingting, WANG Shuyue, LI Wei, MA Chunhui, LUO Sha, LIU Shouxin. Horseshoe-shaped Hollow Porous Carbon: Synthesis by Hydrothermal Carbonization with Dual-template and Electrochemical Property [J]. Journal of Inorganic Materials, 2023, 38(8): 954-962. |
| [5] | LI Yuejun, CAO Tieping, SUN Dawei. Bi4O5Br2/CeO2 Composite with S-scheme Heterojunction: Construction and CO2 Reduction Performance [J]. Journal of Inorganic Materials, 2023, 38(8): 963-970. |
| [6] | NIU Haibin, HUANG Jiahui, LI Qianwen, MA Dongyun, WANG Jinmin. Directly Hydrothermal Growth and Electrochromic Properties of Porous NiMoO4 Nanosheet Films [J]. Journal of Inorganic Materials, 2023, 38(12): 1427-1433. |
| [7] | YAO Yishuai, GUO Ruihua, AN Shengli, ZHANG Jieyu, CHOU Kuochih, ZHANG Guofang, HUANG Yarong, PAN Gaofei. In-situ Loaded Pt-Co High Index Facets Catalysts: Preparation and Electrocatalytic Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 71-78. |
| [8] | XUE Hongyun, WANG Congyu, MAHMOOD Asad, YU Jiajun, WANG Yan, XIE Xiaofeng, SUN Jing. Two-dimensional g-C3N4 Compositing with Ag-TiO2 as Deactivation Resistant Photocatalyst for Degradation of Gaseous Acetaldehyde [J]. Journal of Inorganic Materials, 2022, 37(8): 865-872. |
| [9] | ZHANG Xian, ZHANG Ce, JIANG Wenjun, FENG Deqiang, YAO Wei. Synthesis, Electronic Structure and Visible Light Photocatalytic Performance of Quaternary BiMnVO5 [J]. Journal of Inorganic Materials, 2022, 37(1): 58-64. |
| [10] | WANG Tingting, SHI Shumei, LIU Chenyuan, ZHU Wancheng, ZHANG Heng. Synthesis of Hierarchical Porous Nickel Phyllosilicate Microspheres as Efficient Adsorbents for Removal of Basic Fuchsin [J]. Journal of Inorganic Materials, 2021, 36(12): 1330-1336. |
| [11] | SONG Keke, HUANG Hao, LU Mengjie, YANG Anchun, WENG Jie, DUAN Ke. Hydrothermal Preparation and Characterization of Zn, Si, Mg, Fe Doped Hydroxyapatite [J]. Journal of Inorganic Materials, 2021, 36(10): 1091-1096. |
| [12] | XIAO Yumin, Li Bin, QIN Lizhao, LIN Hua, LI Qing, LIAO Bin. Efficient Preparation of CuGeO3 with Controllable Morphology Using CuCl2 as Copper Source [J]. Journal of Inorganic Materials, 2021, 36(1): 69-74. |
| [13] | WANG Juhan,WEN Xiong,LIU Chengchao,ZHANG Yuhua,ZHAO Yanxi,LI Jinlin. Preparation and Fischer-Tropsch Synthesis Performance of Hierarchical Co/Al-SiO2 Catalyst [J]. Journal of Inorganic Materials, 2020, 35(9): 999-1004. |
| [14] | ZHANG Dongshuo,CAI Hao,GAO Kaiyin,MA Zichuan. Preparation and Visible-light Photocatalytic Degradation on Metronidazole of Zn2SiO4-ZnO-biochar Composites [J]. Journal of Inorganic Materials, 2020, 35(8): 923-930. |
| [15] | WANG Zhihu,ZHANG Jumei,BAI Lijing,ZHANG Guojun. Mg(OH)2 Film on Micro-arc Oxidation Ceramic Coating of AZ31 Magnesium Alloy: Preparation and Corrosion Resistance [J]. Journal of Inorganic Materials, 2020, 35(6): 709-716. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||