
Journal of Inorganic Materials ›› 2014, Vol. 29 ›› Issue (12): 1257-1264.DOI: 10.15541/jim20140047
• Orginal Article • Previous Articles Next Articles
LIU Qin, YUAN Wen, GAO Xue-Ping
Received:2014-01-22
Revised:2014-04-28
Published:2014-12-20
Online:2014-11-20
About author:LIU Qin. E-mail: lizzy1211@mail.nankai.edu.cn
CLC Number:
LIU Qin, YUAN Wen, GAO Xue-Ping. Surface Modification of Li-rich Layered Li(Li0.17Ni0.2Mn0.58Co0.05)O2 Oxide with TiO2(B) as the Cathode for Lithium-ion Batteries[J]. Journal of Inorganic Materials, 2014, 29(12): 1257-1264.
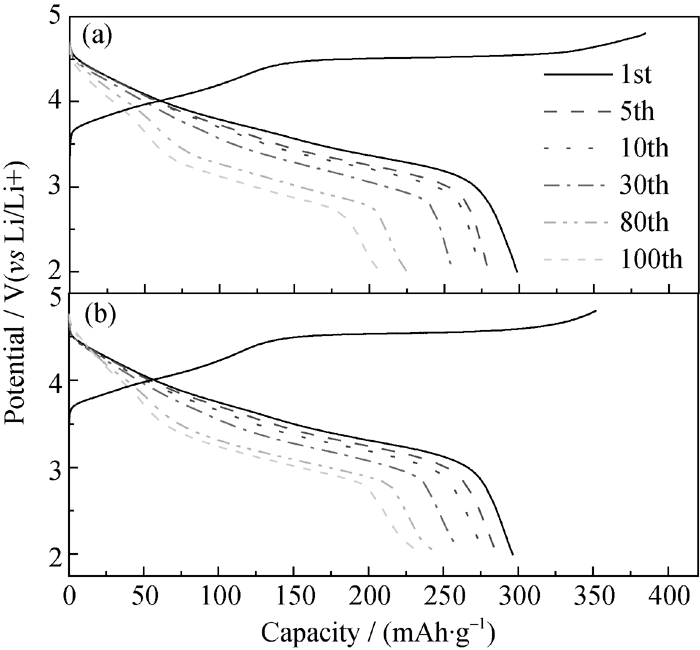
Fig. 6 Initial charge curves and subsequent discharge curves of the as-prepared sample (a) and the sample modified with 4wt% TiO2 (B) (b) at different cycles (0.1C rate)
| TiO2(B) content | Initial charge capacity / (mAh·g-1) | Initial discharge capacity / (mAh·g-1) | Initial irreversible capacity loss / (mAh·g-1) | Initial coulombic efficiency | Capacity retention after 100 cycles |
|---|---|---|---|---|---|
| 0 | 384.2 | 298.7 | 85.5 | 77.7% | 69.5% |
| 2wt% | 347.7 | 286.5 | 61.2 | 82.4% | 79.1% |
| 4wt% | 351.7 | 296.4 | 55.3 | 84.3% | 80.2% |
| 6wt% | 333.6 | 263.6 | 70.1 | 79.0% | 78.9% |
| 8wt% | 326.0 | 255.9 | 106.1 | 78.5% | 78.0% |
Table. 1 Electrochemical data of TiO2(B)-modified samples in the first cycle (0.1C rate)
| TiO2(B) content | Initial charge capacity / (mAh·g-1) | Initial discharge capacity / (mAh·g-1) | Initial irreversible capacity loss / (mAh·g-1) | Initial coulombic efficiency | Capacity retention after 100 cycles |
|---|---|---|---|---|---|
| 0 | 384.2 | 298.7 | 85.5 | 77.7% | 69.5% |
| 2wt% | 347.7 | 286.5 | 61.2 | 82.4% | 79.1% |
| 4wt% | 351.7 | 296.4 | 55.3 | 84.3% | 80.2% |
| 6wt% | 333.6 | 263.6 | 70.1 | 79.0% | 78.9% |
| 8wt% | 326.0 | 255.9 | 106.1 | 78.5% | 78.0% |
| [1] | LEE J S, TAI K S, CAO R, et al.Metal-air batteries with high energy density: Li-air versus Zn-air. Adv. Energy Mater., 2011, 1(1): 34-50. |
| [2] | GAO X P, YANG H X.Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci., 2010, 3(2): 174-189. |
| [3] | ZHAO Y J, FENG H L, ZHAO C S, et al.Progress of research on the Li-rich cathode materials xLi2MnO3·(1-x)LiMO2 (M=Co, Fe, Ni1/2Mn1/2…) for Li-ion batteries. Journal of Inorganic Materials, 2011, 26(7): 673-679. |
| [4] | LU Z, MACNEIL D D, DAHN J R.Layered cathode materials Li[NixLi1/3-2x/3Mn2/3-x/3]O2 for lithium-ion batteries. Electrochem. Solid-State Lett., 2001, 4(11): A191-A194. |
| [5] | NGALA J K, CHEMOVA N A, WHITTINGHAM M S, et al.The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound. J. Mater. Chem., 2004, 14(2): 214-220. |
| [6] | WU Y, MANTHIRAM A.Effect of surface modifications on the layered solid solution cathodes (1-z)Li[Li1/3Mn2/3]O2-(z)Li[Mn0.5-yNi0.5-yCo2y]O2. Solid State Ionics, 2009, 180(1): 50-56. |
| [7] | WANG Q Y, LIU J, MURUGAN A V, et al.High capacity double-layer surface modified Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode with improved rate capability. J. Mater. Chem., 2009, 19(10): 4965-4972. |
| [8] | ZHANG WEN-HUA,HE WEI, PEI FENG, et al. Improved electrochemical properties of Al3+-doped 0.5Li2MnO3-0.5LiCo1/3Ni1/3Mn1/3O2 cathode for lithium ion batteries. Journal of Inorganic Materials, 2013, 28(11): 1261-1264. |
| [9] | JIAO L F, ZHANG M, YUAN H T, et al.Effect of Cr doping on the structural, electrochemical properties of Li[Li0.2Ni0.2-x/2Mn0.6-x/2 Crx]O2 (x = 0, 0.02, 0.04, 0.06, 0.08) as cathode materials for lithium secondary batteries. J. Power Sources, 2007, 167(1): 178-184. |
| [10] | ZHANG H Z, QIAO Q Q, LI G R, et al.PO43− polyanion-doping for stabilizing Li-rich layered oxides as cathode materials for advanced lithium-ion batteries. J. Mater. Chem. A, 2014, 2(20): 7454-7460. |
| [11] | POIZOT P, LARUELLE S, GRUGEON S, et al.Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature, 2000, 407: 496-499. |
| [12] | ZHANG Qian, LIU Wei-Wei, FANG Guo-Qing, et al.Structural and electrochemical performances of Li1+2xMn0.3+xNi0.3-3xCr0.4O2 synthesized by spray-dry method. Journal of Inorganic Materials, 2013, 28(06): 616-622. |
| [13] | ZHAO Y, ZHAO C, FENG H, et al.Enhanced electrochemical performance of LiNi0.2Li0.2Mn0.6O2 modified by manganese oxide coating for lithium-ion batteries. Electrochem. Solid-State Lett., 2011, 14(1): A1. |
| [14] | WU F, LI N, SU Y, et al.Can surface modification be more effective to enhance the electrochemical performance of lithium rich materials?J. Mater. Chem., 2012, 22: 1489-1497. |
| [15] | HE W, QIAN J F, AI X P.Improved electrochemical performances of nanocrystalline Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. RSC Adv., 2012, 2: 3423-3429. |
| [16] | MLADENOV M, STOYANOVA R, ZHECHEVA E, et al.Effect of Mg doping and MgO-surface modification on the cycling stability of LiCoO2 electrodes. Electrochem. Commun., 2001, 3(8): 410-416. |
| [17] | CHO M Y, ROH K C, PARK S M, et al.Effects of CeO2 coating uniformity on high temperature cycle life performance of LiMn2O4. Mater. Lett., 2011, 65: 2011-2014. |
| [18] | LI G R, FENG X, DING Y, et al.AlF3-coated Li(Li0.17Ni0.25Mn0.58) O2 as cathode material for Li-ion batteries. Electrochimica Acta, 2012, 78: 308-315. |
| [19] | WU Y, MURUGAN A V, MANTHIRAM A.Surface modification of high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes by AlPO4. J. Electrochem. Soc., 2008, 155(10): A635-A641. |
| [20] | KAN S H, THACKERAY M M.Enhancing the rate capability of high capacity xLi2MnO3·(1-x)LiMO2(M=Mn, Ni, Co) electrodes by Li-Ni-PO4 treatment. Electrochem. Commun., 2009, 11(4): 748-751. |
| [21] | LEE S H, KOO B K, KIM J C, et al.Effect of Co3(PO4)2 coating on Li[Co0.1Ni0.15Li0.2Mn0.55]O2 cathode material for lithium rechargeable batteries. J. Power Sources, 2008, 184(1): 276-283. |
| [22] | QIAO Q Q, ZHANG H Z, LI G R, et al.Surface modification of Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide with Li-Mn-PO4 as the cathode for lithium-ion batteries. J. Mater. Chem. A, 2013, 1(17): 5262-5268. |
| [23] | ZHANG H Z, QIAO Q Q, LI G R, et al.Surface nitridation of Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as cathode material for lithium-ion battery. J. Mater. Chem., 2012, 22(26): 13104-13109. |
| [24] | ZHANG Z R, LI J, YANG Y.The effects of decomposition products of electrolytes on the thermal stability of bare and TiO2-coated delithiated Li1−xNi0.8Co0.2O2 cathode materials. Electrochimica Acta. 2006, 52: 1442-1450. |
| [25] | ZHENG J M, LI J, ZHANG Z R, et al.The effects of TiO2 coating on the electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium-ion battery. Solid State Ionics, 2008, 179(27-32): 1794-1799. |
| [26] | YIN S, FUJISHIRO Y, WU J H, et al.Synthesis and photocatalytic properties of fibrous titania by solvothermal reactions. J. Mater. Process Tech., 2003, 137: 45-48. |
| [27] | YANG D J, LIU H W, ZHENG Z F, et al.An efficient photocatalyst structure: TiO2(B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc., 2009, 131(49): 17885-17893. |
| [28] | JANOTTI A, VARLEY J B.Hybrid functional studies of the oxygen vacancy in TiO2,Phys. Rev. 2010, 81(8): 085212. |
| [29] | PAN X Y, YANG M Q.Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale, 2013, 5: 3601-3614. |
| [30] | ZHANG Z R, LI J, YANG Y.The effects of decomposition products of electrolytes on the thermal stability of bare and TiO2-coated delithiated Li1−xNi0.8Co0.2O2 cathode materials. Electrochimica Acta, 2006, 52(4): 1442-1450. |
| [31] | WANG J, YUAN G X, ZHANG M H, et al.The structure, morphology, and electrochemical properties of Li1+xNi1/6Co1/6Mn4/6 O2.25+x/2(0.1≤x≤0.7) cathode materials. Electrochimica Acta, 2012, 66: 61-66. |
| [32] | MACNEIL D D, LU Z, DAHN J R.Structure and electrochemistry of Li[NixCo1-2xMnx]O2(0<x<1). J. Electrochem. Soc., 2002, 149(10): A1332-A1336. |
| [33] | ARMSTRONG A R, HOLZAPEFEL M, NOVAK P, et al.Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li(Ni0.2Li0.2Mn0.6)O2. J. Am. Chem. Soc., 2006, 26(128): 8694-8698. |
| [1] | SUN Jing, LI Xiang, MAO Xiaojian, ZHANG Jian, WANG Shiwei. Effect of Lauric Acid Modifier on the Hydrolysis Resistance of Aluminum Nitride Powders [J]. Journal of Inorganic Materials, 2025, 40(7): 826-832. |
| [2] | XUE Ke, CAI Changkun, XIE Manyi, LI Shuting, AN Shengli. Pr1+xBa1-xFe2O5+δ Cathode Materials for Solid Oxide Fuel Cells: Preparation and Electrochemical Performance [J]. Journal of Inorganic Materials, 2025, 40(4): 363-371. |
| [3] | ZHU Zhijie, SHEN Mingyuan, WU Tao, LI Wencui. Inhibition of P2-O2 Phase Transition for P2-Na2/3Ni1/3Mn2/3O2 as Cathode of Sodium-ion Battery via Synergetic Substitution of Cu and Mg [J]. Journal of Inorganic Materials, 2025, 40(2): 184-195. |
| [4] | WANG Kunpeng, LIU Zhaolin, LIN Cunsheng, WANG Zhiyu. Development of Quasi-solid-state Na-ion Battery Based on Water-minimal Prussian Blue Cathode [J]. Journal of Inorganic Materials, 2024, 39(9): 1005-1012. |
| [5] | CAI Heqing, HAN Lu, YANG Songsong, XUE Xinyu, ZHANG Kou, SUN Zhicheng, LIU Ruping, HU Kun, WEI Yan. Fe3O4-DMSA-PEI Magnetic Nanoparticles with Small Particle Size: Preparation and Gene Loading [J]. Journal of Inorganic Materials, 2024, 39(5): 517-524. |
| [6] | ZHANG Kun, WANG Yu, ZHU Tenglong, SUN Kaihua, HAN Minfang, ZHONG Qin. LaNi0.6Fe0.4O3 Cathode Contact Material: Electrical Conducting Property Manipulation and Its Effect on SOFC Electrochemical Performance [J]. Journal of Inorganic Materials, 2024, 39(4): 367-373. |
| [7] | CHENG Jie, ZHOU Yue, LUO Xintao, GAO Meiting, LUO Sifei, CAI Danmin, WU Xueyin, ZHU Licai, YUAN Zhongzhi. Construction and Electrochemical Properties of Yolk-shell Structured FeF3·0.33H2O@N-doped Graphene Nanoboxes [J]. Journal of Inorganic Materials, 2024, 39(3): 299-305. |
| [8] | CHEN Zhengpeng, JIN Fangjun, LI Mingfei, DONG Jiangbo, XU Renci, XU Hanzhao, XIONG Kai, RAO Muming, CHEN Chuangting, LI Xiaowei, LING Yihan. Double Perovskite Sr2CoFeO5+δ: Preparation and Performance as Cathode Material for Intermediate-temperature Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2024, 39(3): 337-344. |
| [9] | ZHOU Jingyu, LI Xingyu, ZHAO Xiaolin, WANG Youwei, SONG Erhong, LIU Jianjun. Rate and Cycling Performance of Ti and Cu Doped β-NaMnO2 as Cathode of Sodium-ion Battery [J]. Journal of Inorganic Materials, 2024, 39(12): 1404-1412. |
| [10] | HU Mengfei, HUANG Liping, LI He, ZHANG Guojun, WU Houzheng. Research Progress on Hard Carbon Anode for Li/Na-ion Batteries [J]. Journal of Inorganic Materials, 2024, 39(1): 32-44. |
| [11] | KONG Guoqiang, LENG Mingzhe, ZHOU Zhanrong, XIA Chi, SHEN Xiaofang. Sb Doped O3 Type Na0.9Ni0.5Mn0.3Ti0.2O2 Cathode Material for Na-ion Battery [J]. Journal of Inorganic Materials, 2023, 38(6): 656-662. |
| [12] | GUO Tianmin, DONG Jiangbo, CHEN Zhengpeng, RAO Mumin, LI Mingfei, LI Tian, LING Yihan. Enhanced Compatibility and Activity of High-entropy Double Perovskite Cathode Material for IT-SOFC [J]. Journal of Inorganic Materials, 2023, 38(6): 693-700. |
| [13] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [14] | TAN Shuyu, LIU Xiaoning, BI Zhijie, WAN Yong, GUO Xiangxin. Jointing of Cathode Coating and Interface Modification for Stabilizing Poly(ethylene oxide) Electrolytes Against High-voltage Cathodes [J]. Journal of Inorganic Materials, 2023, 38(12): 1466-1474. |
| [15] | LI Tao, CAO Pengfei, HU Litao, XIA Yong, CHEN Yi, LIU Yuejun, SUN Aokui. NH4+ Assisted Interlayer-expansion of MoS2: Preparation and Its Zinc Storage Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 79-86. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||