无机材料学报 ›› 2016, Vol. 31 ›› Issue (12): 1279-1288.DOI: 10.15541/jim20150652 CSTR: 32189.14.10.15541/jim20150652
王 雪1, 2, 张文强1, 于 波1, 陈 靖1
收稿日期:2015-12-28
修回日期:2016-02-09
出版日期:2016-12-16
网络出版日期:2016-11-23
作者简介:王 雪(1989–), 女, 博士研究生. E-mail: wxue225@163.com
基金资助:WANG Xue1, 2, ZHANG Wen-Qiang1, YU Bo1, CHEN Jing1
Received:2015-12-28
Revised:2016-02-09
Published:2016-12-16
Online:2016-11-23
About author:WANG Xue. E-mail: wxue225@163.com
Supported by:摘要:
固体氧化物燃料电池(SOFC)和固体氧化物电解池(SOEC)电堆的电化学分析和诊断是国际上的研究难点。明确多片电堆的本征电化学反应机理和性能规律, 是SOFC/SOEC技术实用化的关键。本研究采用弛豫时间分布法(DRT)耦合阻抗谱差异分析法(ADIS)对电堆在燃料电池模式和电解池模式下的复杂电化学行为进行了研究, 通过弛豫时间解析出各个过程对应的特征峰, 区分出不同的物理化学过程。研究表明, 电堆在SOFC模式运行时, 其氢电极含水量应大于20%, 而SOEC模式运行时, 含水量应小于80%, 以最小化电池的气体扩散阻抗。本方法可应用于SOFC/SOEC电堆的分析诊断, 简化电堆测量分析的复杂性, 有助于电堆衰减机理和原因的实时诊断, 并对电堆性能提高给出理论依据和指导建议。
中图分类号:
王 雪, 张文强, 于 波, 陈 靖. 基于DRT和ADIS的SOFC/SOEC电堆电化学阻抗谱研究[J]. 无机材料学报, 2016, 31(12): 1279-1288.
WANG Xue, ZHANG Wen-Qiang, YU Bo, CHEN Jing. SOC Stack Impedance Characterization and Identification Based on DRT and ADIS Methods[J]. Journal of Inorganic Materials, 2016, 31(12): 1279-1288.
| Item | Parameter |
|---|---|
| DC current/A | 5 |
| AC current/A | 2 |
| Frequency range /kHz | 10 |
| Number of test points | 10 |
表1 阻抗测试参数
Table 1 EIS test parameters
| Item | Parameter |
|---|---|
| DC current/A | 5 |
| AC current/A | 2 |
| Frequency range /kHz | 10 |
| Number of test points | 10 |
| Measurement | Cell-1 | Cell-2 | ||
|---|---|---|---|---|
| SOFC | SOEC | SOFC | SOEC | |
| EIS/ (Ω·cm-2) | 0.20 | 0.20 | 0.18 | 0.18 |
| IV/ (Ω·cm-2) | 0.22 | 0.21 | 0.20 | 0.19 |
表2 阻抗谱和I-V曲线所得面电阻比较表
Table 2 Comparison of ASR tested from I-V curves & impedance spectra
| Measurement | Cell-1 | Cell-2 | ||
|---|---|---|---|---|
| SOFC | SOEC | SOFC | SOEC | |
| EIS/ (Ω·cm-2) | 0.20 | 0.20 | 0.18 | 0.18 |
| IV/ (Ω·cm-2) | 0.22 | 0.21 | 0.20 | 0.19 |
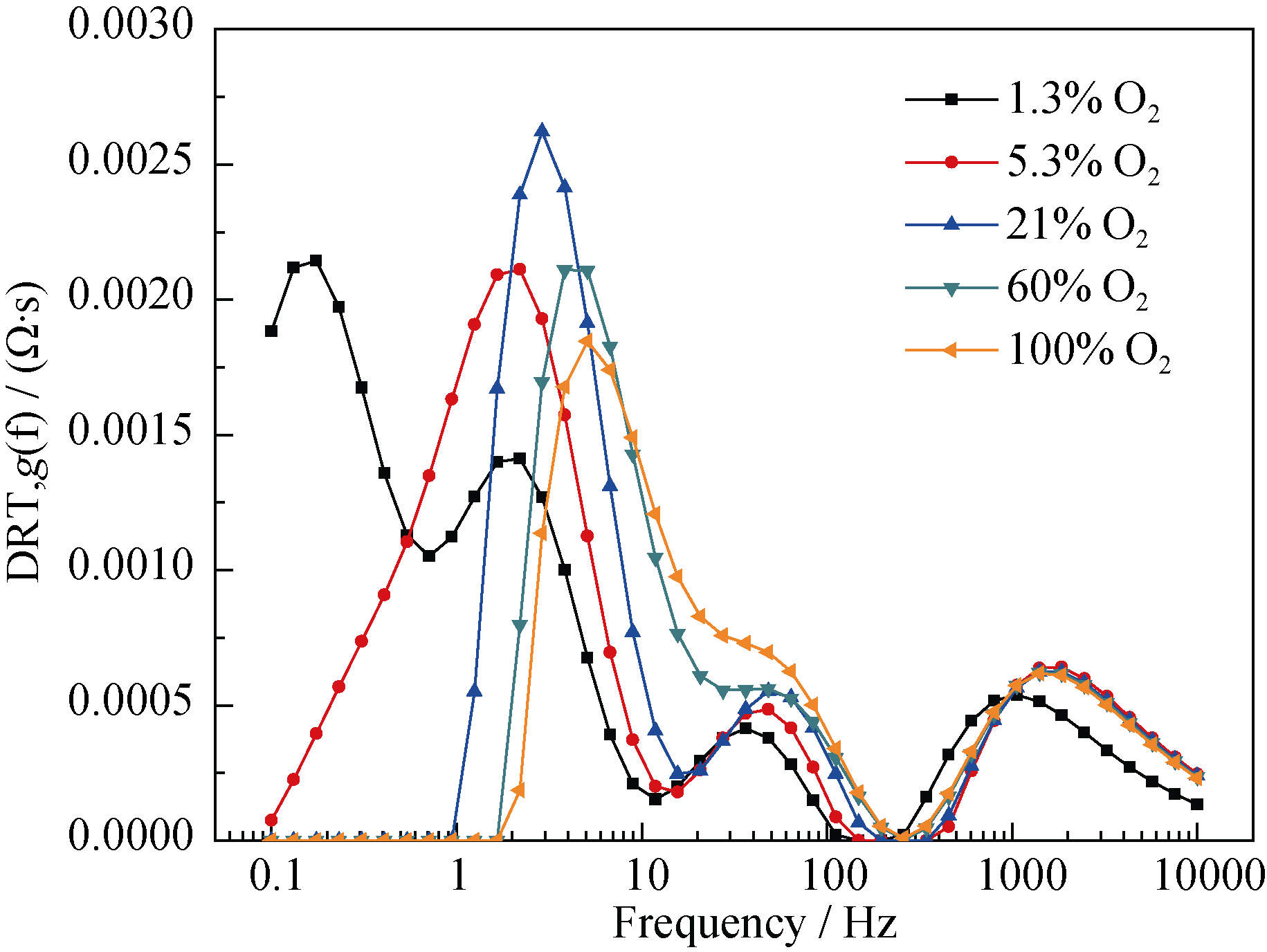
图8 阻抗与氧电极侧氧分压的关系DRT图
Fig. 8 Dependence of DRT plot on oxygen partial pressure of oxygen electrode^ (T = 700℃, SOFC mode, 80%H2 and 20% H2O in the hydrogen electrode)
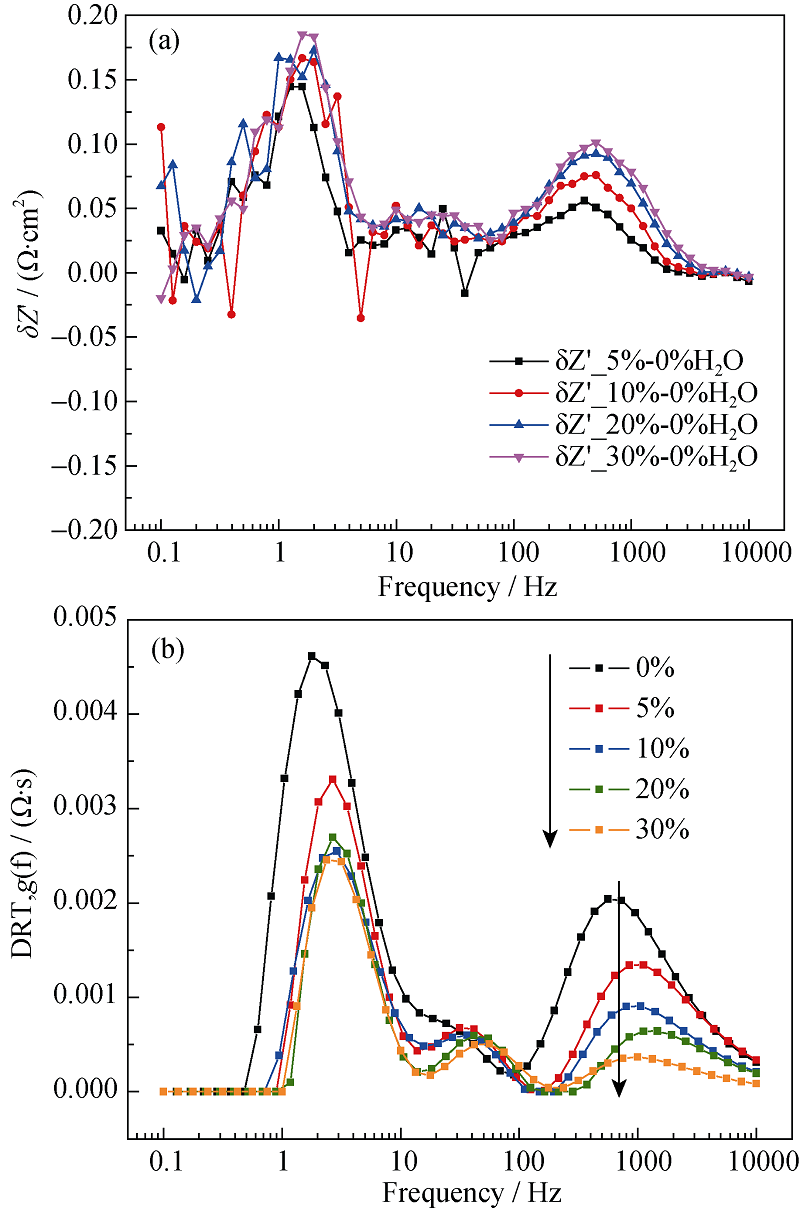
图9 阻抗与氢电极侧水含量的(a) ADIS图和(b) DRT图
Fig. 9 Dependence of (a) ADIS plot and (b) DRT plot on steam content of the hydrogen electrode^(T = 700℃, SOFC mode, 0~30% H2O in the hydrogen electrode)
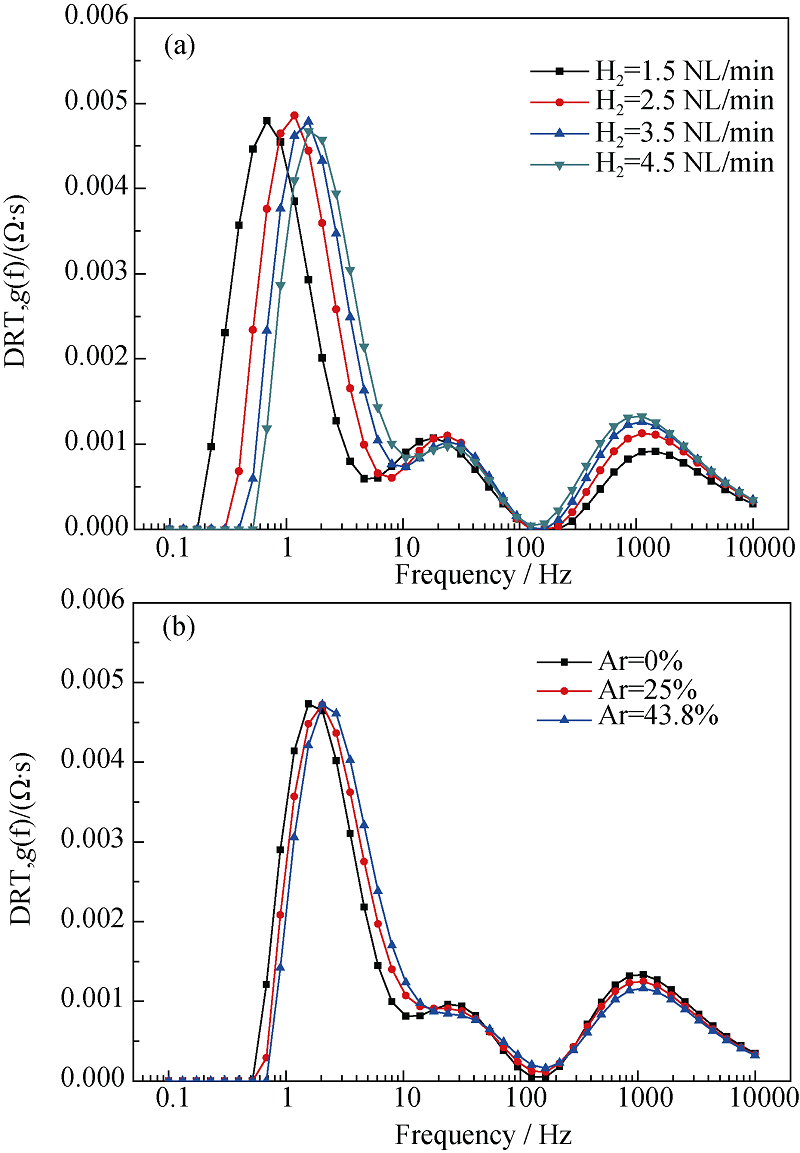
图10 阻抗与氢电极侧氢气流量(a)和与氢电极侧惰性载气流量(b)的DRT图
Fig. 10 Dependence of DRT plot on hydrogen flow rate of the hydrogen electrode (a), and on nitrogen flow rate of the hydrogen electrode (b)^(T = 700℃, SOFC mode, 100%H2 or 100%N2 in the hydrogen electrode)
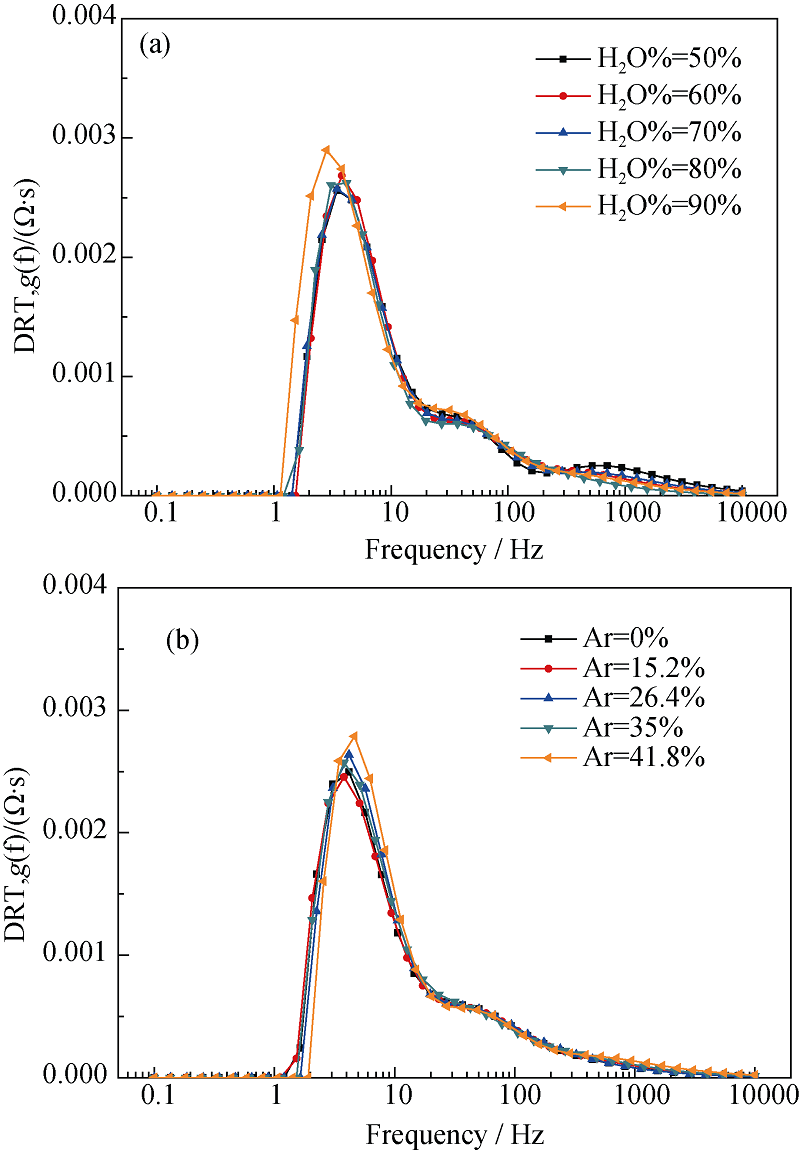
图11 (a)氢电极侧水含量和(b)氢电极侧惰性载气含量对阻抗的影响
Fig. 11 Dependence of DRT plot on steam content of the hydrogen electrode (a) and on nitrogen flow rate of the hydrogen electrode (b)
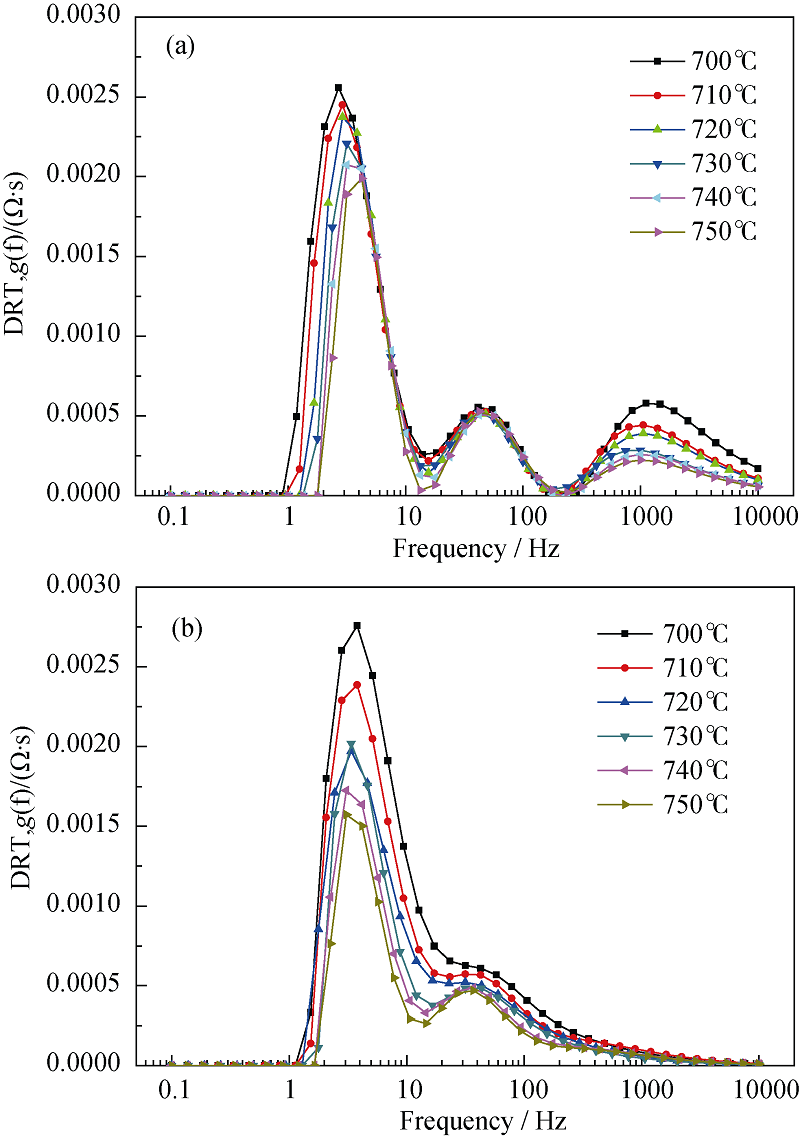
图12 阻抗与温度关系的DRT图
Fig. 12 Dependence of DRT plot on operating temperature^(a) SOFC mode, 20%H2O in the hydrogen electrode, air in the oxygen electrode; (b) SOEC mode, 80%H2O in the hydrogen electrode, air in the oxygen electrode
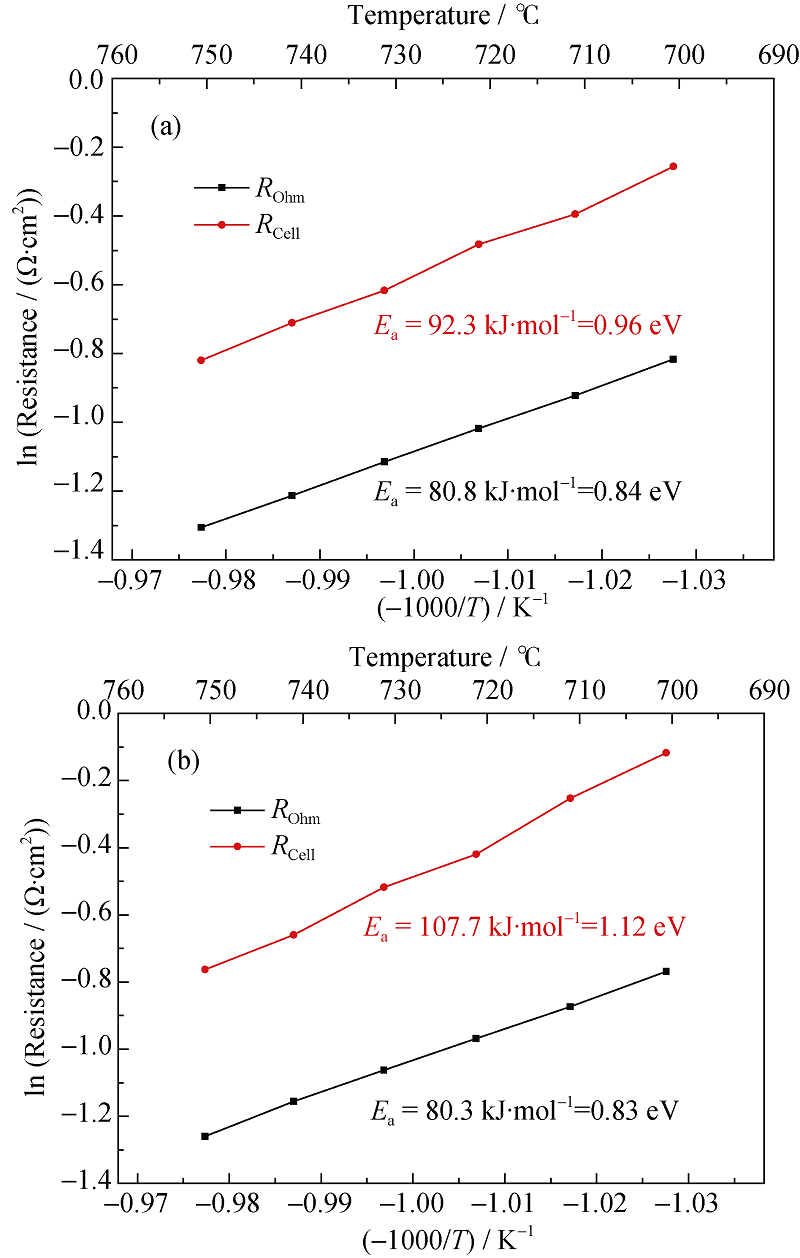
图13 可逆电池的Arrhenius曲线
Fig. 13 Arrhenius plots of the reversible SOC^(a) SOFC mode; (b) SOEC mode (50%H2+50%H2O in the hydrogen electrode, air in the oxygen electrode, Temperature range: 700℃~750℃)
| Process | Equivalent circuit | Frequency range /Hz | Dependencies | Physical process |
|---|---|---|---|---|
| P1 | RQ | 0.01-1 | | Gas diffusion in oxygen electrode |
| P2 | RQ | 1-10 | | Gas diffusion in substrate (fuel electrode) overlapped with gas conversion impedance |
| P3 | Gerischer | 10-100 | | Chemical surface exchange of O2 and O2- bulk diffusion in air electrode |
| P4 | RQ | 100-10000 | | Charge transfer reactions and ionic transport in YSZ and TPB |
表3 DRT特征峰对照表
Table 3 Processes identified by DRT analysis
| Process | Equivalent circuit | Frequency range /Hz | Dependencies | Physical process |
|---|---|---|---|---|
| P1 | RQ | 0.01-1 | | Gas diffusion in oxygen electrode |
| P2 | RQ | 1-10 | | Gas diffusion in substrate (fuel electrode) overlapped with gas conversion impedance |
| P3 | Gerischer | 10-100 | | Chemical surface exchange of O2 and O2- bulk diffusion in air electrode |
| P4 | RQ | 100-10000 | | Charge transfer reactions and ionic transport in YSZ and TPB |
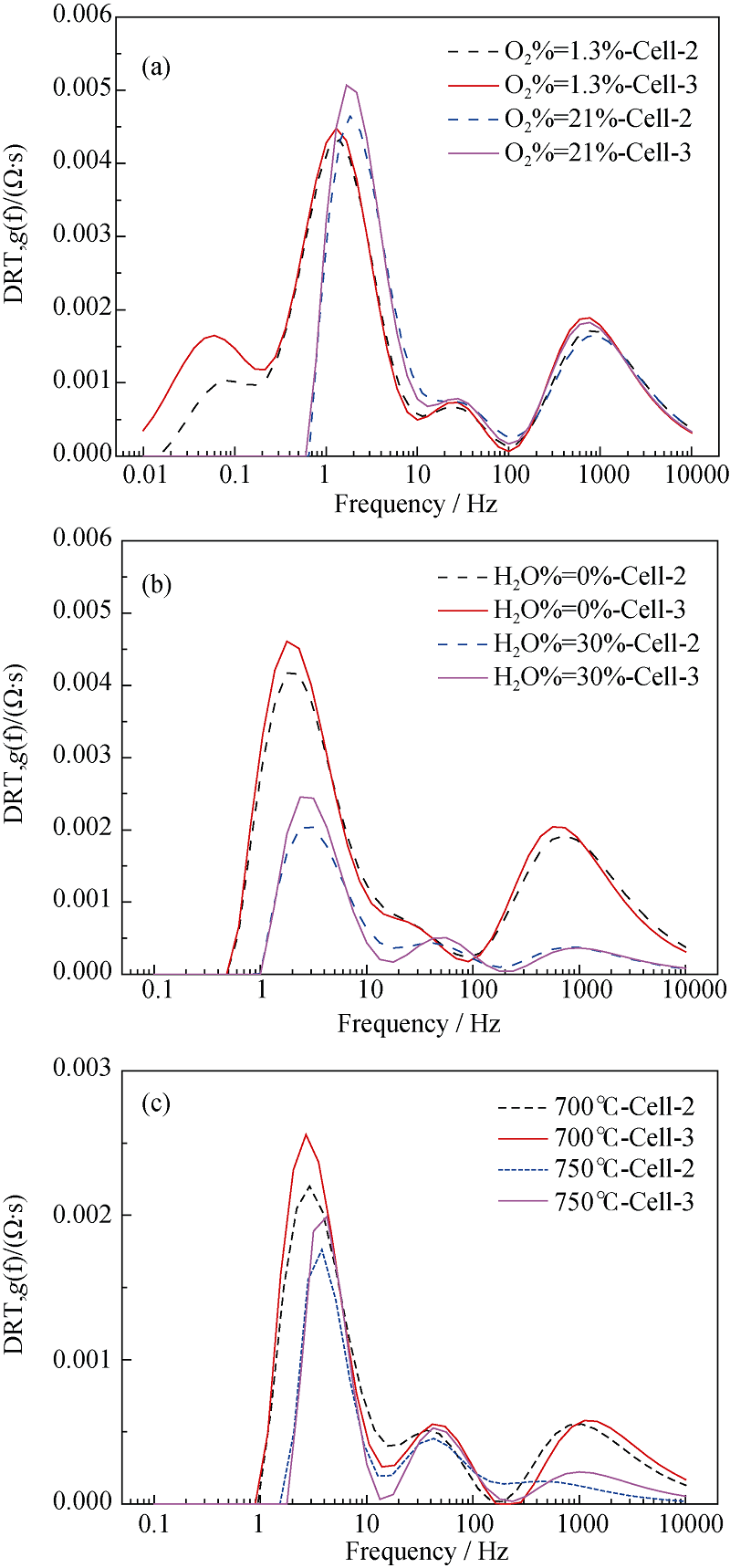
图14 SOFC模式下Cell-2和Cell-3在不同条件下的DRT曲线
Fig. 14 DRT plots of the Cell-2 and Cell-3 under different conditions in SOFC mode Change of gas composition of hydrogen electrode (a), gas composition of oxygen electrode (b) and operating temperature (c)
| Before degradation | After degradation | Impedance growth rate | ||||
|---|---|---|---|---|---|---|
| Cell-2 | Cell-3 | Cell-2 | Cell-3 | Cell-2 | Cell-3 | |
| Ohmic resistance/ (Ω·cm-2) | 0.612 | 0.471 | 1.010 | 0.485 | 65% | 3% |
| Polarization impedance /(Ω·cm-2) | 1.029 | 1.066 | 2.521 | 1.511 | 145% | 42% |
表4 Cell-2和Cell-3衰减前后阻抗对比
Table 4 Comparison of the resistances of cell-2 and cell-3 before and after degradation
| Before degradation | After degradation | Impedance growth rate | ||||
|---|---|---|---|---|---|---|
| Cell-2 | Cell-3 | Cell-2 | Cell-3 | Cell-2 | Cell-3 | |
| Ohmic resistance/ (Ω·cm-2) | 0.612 | 0.471 | 1.010 | 0.485 | 65% | 3% |
| Polarization impedance /(Ω·cm-2) | 1.029 | 1.066 | 2.521 | 1.511 | 145% | 42% |
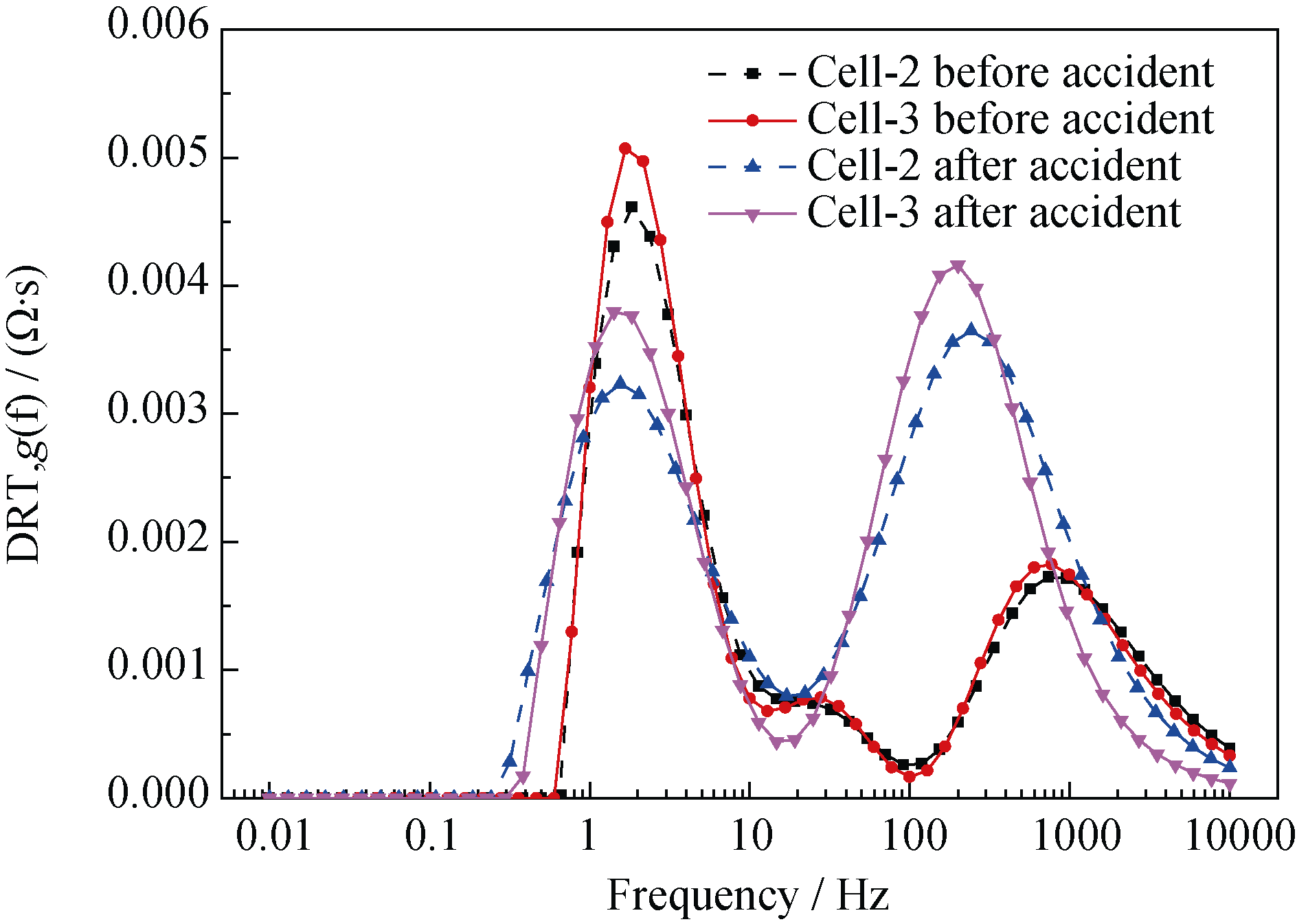
图17 衰减前后DRT图
Fig. 17 DRT plots of the cell-2 and cell-3 before and after degradation^(T=700℃, SOECmode, 100%H2 in the hydrogen electrode, air in the oxygen electrode)
| [1] | WACHSMAN E D, LEE K T.Lowering the temperature of solid oxide fuel cells.Science, 2011, 334(6058): 935-939. |
| [2] | EBBESEN S D, JENSEN S H, HAUCH A,et al. High temperature electrolysis in alkaline cells, solid proton conducting cells, and solid oxide cells. Chemical Reviews, 2014, 114: 10697-10734. |
| [3] | YU B, ZHANG W Q, XU J M,et al. Status and research of highly efficient hydrogen production through high temperature steam electrolysis at INET. International Journal of Hydrogen Energy, 2009, 35(7): 2829-2835. |
| [4] | MAWDSLEY J R, CARTER J D, KROPF A J,et al.Post-test evaluation of oxygen electrodes from solid oxide electrolysis stacks. International Journal of Hydrogen Energy, 2009, 34(9): 4198-4207. |
| [5] | MENZLER N H, BATFALSKY P, GROß S,et al. Post-Test characterization of an SOFC short-stack after 17, 000 hours of steady operation. ECS Transactions, 2011, 35(1): 195-206. |
| [6] | NECHACHE A, CASSIR M, RINGUEDÉ A.Solid oxide electrolysis cell analysis by means of electrochemical impedance spectroscopy: review. Journal of Power Sources, 2014, 258(15): 164-181. |
| [7] | ORAZEM M E, TRIBOLLET B. Electrochemical Impedance Spectroscopy. J. Wiley & Sons, Hobo-ken,New Jersey, 2008, 153-162. |
| [8] | VIRKAR A V.Transport through mixed proton, oxygen ion and electron (hole) conductors: Goldman-Hodgkin-Katz type equation.Journal of Power Sources , 2009, 194(2): 753-762. |
| [9] | YU B, LIU M Y, ZHANG W Q,et al. Polarization loss of single solid oxide electrolysis cells and microstructural optimization of the cathode. Acta Physico-Chimica Sinica, 2011, 27(2): 395-402. |
| [10] | SCHICHLEIN H,MÜLLER A C, VOIGTS M,et al. Deconvolution of electrochemical impedance spectra for the identification of electrode reaction mechanisms in solid oxide fuel cells. Journal of Applied Electrochemistry, 2002, 32: 875-882. |
| [11] | LEONIDE A, SONN V, WEBER A,et al. Evaluation and modeling of the cell resistance in anode-supported solid oxide fuel cells. Journal of The Electrochemical Society, 2008, 155(1): B36-B41. |
| [12] | SCHONLEBER M, IVERS-TIFFEE E.Approximability of impedance spectra by RC elements and implications for impedance analysis.Electrochemistry Communications, 2015, 58(9): 15-19. |
| [13] | JENSEN S H, HAUCH A, HENDRIKSEN P V,et al. A method to separate process contributions in impedance spectra by variation of test conditions. Journal of The Electrochemical Society, 2007, 154(12): B1325-B1330. |
| [14] | EBBESEN S D, GRAVES C, HAUCH A,et al. Poisoning of solid oxide electrolysis cells by impurities. Journal of the Electrochemical Society, 2010, 157(10): B1419-B1429. |
| [15] | KUZNECOV M, OTSCHIK P, OBENAUS P,et al. Diffusion controlled oxygen transport and stability at the perovskite/ electrolyte interface. Solid State Ionics, 2003, 157(1-4): 371-378. |
| [16] | WEESE J.A reliable and fast method for the solution of Fredhol integral equations of the first kind based on Tikhonov regularization.Computer Physics Communications, 1992, 69(1): 99-111. |
| [17] | BARFOD R, HAGEN A, RAMOUSSE S,et al. Break down of losses in thin electrolyte SOFCs. Fuel Cells, 2006, 6(2): 141-145. |
| [18] | JORGENSEN M J, MOGENSEN M.Impedance of solid oxide fuel cell LSM/YSZ composite cathodes.Journal of The Electrochemical Society, 2001, 148(5): A433-A442. |
| [19] | FU Y.Theoretical and Experimental Study of Solid Oxide Fuel Cell (SOFC) Using Impedance Spectra. Massachusetts Institute of Technology, 2014. |
| [1] | 王玥, 崔常松, 王士维, 占忠亮. LaxSr2-xFe1.5Ni0.1Mo0.4O6-δ对称电池电解CO2研究[J]. 无机材料学报, 2021, 36(12): 1323-1329. |
| [2] | 李 想, 葛武杰, 王 昊, 瞿美臻. 高镍系三元层状氧化物正极材料容量衰减机理的研究进展[J]. 无机材料学报, 2017, 32(2): 113-121. |
| [3] | 苏 婧, 吴兴隆, 郭玉国. LiMn0.8Fe0.2PO4/C纳米复合材料的制备与电化学性能研究[J]. 无机材料学报, 2013, 28(11): 1248-1254. |
| [4] | 于 波, 张文强, 梁明德, 张 平, 徐景明. PMMA造孔剂对固体氧化物电解池制氢性能的影响[J]. 无机材料学报, 2011, 26(8): 807-812. |
| [5] | 孔江榕, 周 涛, 刘 鹏, 张 勇, 徐景明. La0.6Sr0.4Co0.2Fe0.8O3-δ基固体氧化物电解池复合阳极的制备及性能[J]. 无机材料学报, 2011, 26(10): 1049-1052. |
| [6] | 张雪林,姜兆华,姚忠平,吴振东. 等离子体电解氧化陶瓷膜生长过程的电化学阻抗谱研究[J]. 无机材料学报, 2009, 24(1): 111-116. |
| [7] | 关永军,夏原. 铝合金表面等离子体电解氧化陶瓷涂层在NaCl溶液中的电化学阻抗谱研究[J]. 无机材料学报, 2008, 23(4): 784-788. |
| [8] | 王贵领,张密林,赵辉,霍丽华,孙丽萍. La9(SiO4)6-x(VO4)xO1.5+0.5x的合成及其导电性能的研究[J]. 无机材料学报, 2006, 21(5): 1258-1262. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||